Agenda for CWG Meeting January 6 2002 1










- Slides: 15

Agenda for CWG Meeting January 6, 2002 1. Update on Commons V 2. 0 schedule 2. Close Out/FSR Interface Requirements 3. Discussion of Competitive Application § § § Current process for receipt and referral of paper applications Benefits of datastreams for data/process validation through business rules Opportunities for reengineering receipt, 1 referral, and the research plan

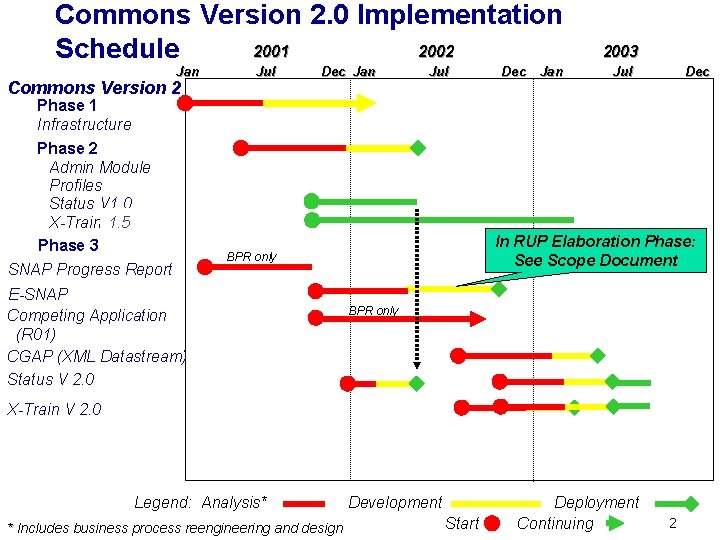
Commons Version 2. 0 Implementation 2001 2002 Schedule Jan Commons Version 2 Phase 1 Infrastructure Phase 2 Admin Module Profiles Status V 1. 0 1. 5 X-Train 2. 0 Phase 3 SNAP Progress Report Jul Dec Jan Jul Dec In RUP Elaboration Phase: See Scope Document BPR only E-SNAP Competing Application (R 01) CGAP (XML Datastream) Status V 2. 0 Dec 2003 BPR only X-Train V 2. 0 Legend: Analysis* * Includes business process reengineering and design Development Start Deployment Continuing 2

Status on X-Train V 1. 5 Deployment n n 12 Grantee Organizations Participating BAYLOR COLLEGE OF MEDICINE UNIVERSITY OF ALABAMA AT BIRMINGHAM CORNELL UNIVERSITY, ITHACA UNIVERSITY OF CALIFORNIA, LOS ANGELES DUKE UNIVERSITY OF MEDICINE & DENTISTRY OF NJ NORTHWESTERN UNIVERSITY OF TEXAS MEDICAL BR, GALVESTON PENNSYLVANIA STATE UNIVERSITY OF TEXAS SW MED CTR, DALLAS DARTMOUTH COLLEGE UNIVERSITY OF WISCONSIN, MADISON n 35 trainee appointments processed since October 1, 2001 n Proceed to Production Deployment of V 1. 5 by May 2002 Issues/Feedback n n n Delegation – Alter “AA” Role to allow X-Train data entry and submission: interim until Commons V 2. 0 Include notification/warning prior to submission to minimize unauthorized submissions Other avenues for feedback? 3

Other Commons V 2. 0 Functionality n Status V 1. 0 n n n New Role/Rights Model n n Feedback to be requested soon… Commons GUI Standards n n Summary Statements in PDF – February 2002 Complete NIH Staff contact information – February 2002 Final Standards Document due in February V 2. 0 User Interface Survey now in your hands… Need feedback by February 1, 2002 SBIR Initiative n n Funding committed by NIH ICs to meet scope RFA nearing completion, proposed publication by end of January 4

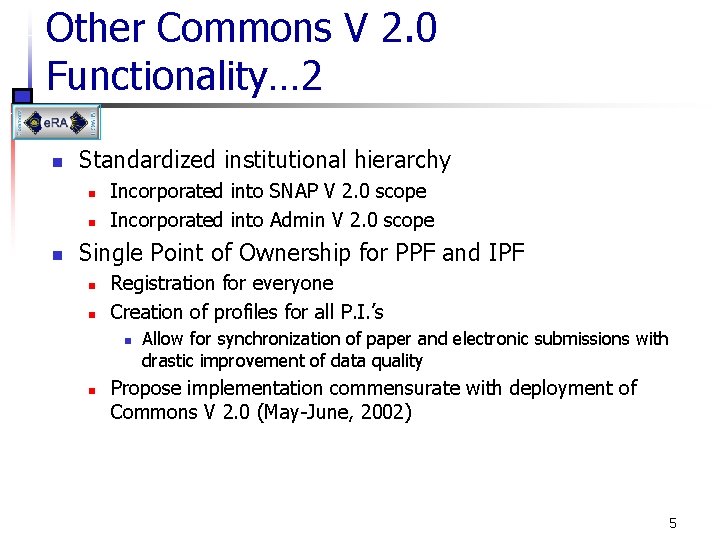
Other Commons V 2. 0 Functionality… 2 n Standardized institutional hierarchy n n n Incorporated into SNAP V 2. 0 scope Incorporated into Admin V 2. 0 scope Single Point of Ownership for PPF and IPF n n Registration for everyone Creation of profiles for all P. I. ’s n n Allow for synchronization of paper and electronic submissions with drastic improvement of data quality Propose implementation commensurate with deployment of Commons V 2. 0 (May-June, 2002) 5

Planning the Close Out/FSR Module n n n Scope of Module n Close out = FSR, Final Invention Report, Final Progress Report n FSR = Stand alone interface Security authentication via Commons account/role. n EIN to be retained as data element of each FSR User Requirements n Interface to be interactive n Data entry/queries for reports on grant-by-grant basis n Requirement for datastream version? n System to include Work-in-Progress feature n 90 days prior to budget end date proposed n Will users actually start a WIP before the budget end date? 6

Planning the Close Out/FSR Module… 2 n FSR User Requirements…cont. n Data access requirements n n n Who at the grantee institution should be able to view submitted data? PI? Only authorized submitter? Requirement for others to view particular data items; e. g. , carryover balance or reported program income? Reporting requirements n n FSRs pending (due within 90 days)/due/overdue Historical reports # of reports submitted on time, late, revised Others? 7

Reengineering the Competitive Application n Streamlining data requirements n n Streamlining business process n n Advantages offered by implementation of profiles Advantages offered by recategorizing of information Opportunities to question need for information Receipt, referral, review Just-in-time submissions Application schedule and content Solicitation of CWG to formalize recommendations and seek consensus n n Yep…one of those wonderful Excel workbooks Discussion to follow at next CWG meeting 8

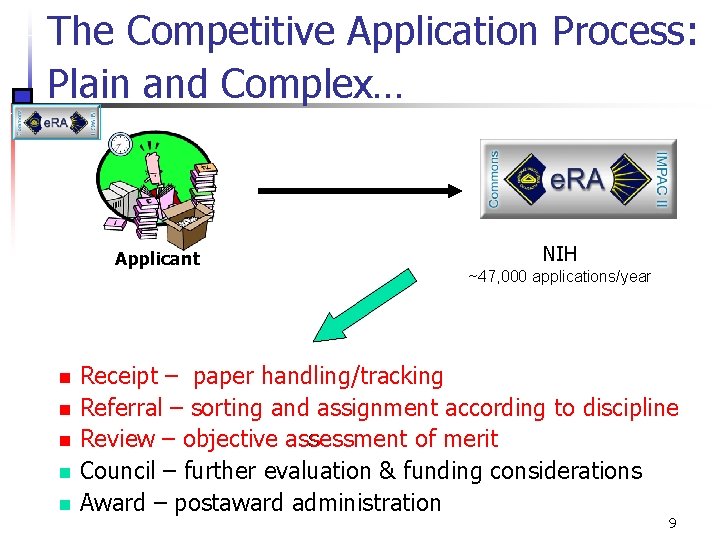
The Competitive Application Process: Plain and Complex… Applicant n n n NIH ~47, 000 applications/year Receipt – paper handling/tracking Referral – sorting and assignment according to discipline Review – objective assessment of merit Council – further evaluation & funding considerations Award – postaward administration 9

Application Receipt The current paper world… n n n Date stamp Accession number Open and count letters Separate bulky appendices Identify RFAs, other applications for special handling The electronic datastream submission… n Embedded business rules to automate n n n Date stamp Accession number Special handling considerations Letters/instructions included in datastream Potential for links to appendices 10

Project Control - Unit 1 & Referral Data and process validation afforded by datastream submission… n Form page data validated by NIH Commons system n n Special handling requests acknowledged as part of datastream receipt n n ARAs (Awaiting Receipt of Application) Eligibility controlled by datastream business rules n n n page 1, budget, Checklist, Personal Data page Budget limits, modular grant/budget formats A 2/2 year limit, Virtual A 3 s Other issues also controlled by datastream business rules and/or IMPAC II software n n n Duplicates, New vs. revised vs. supplements Text format, page limits Variation in paper form version Need to print PI application history Changes in policy 11

Critical Participation by NIH Staff and Grantees post-datastream n Critical assessment/decisions by NIH Staff n n Study Section n n Determine if NIH or other agency application CSR or IC review Assignment Value-added by face-to-face discussion of applications Error Resolution n Interaction between NIH Staff and P. I. or Institutional Administrators n n Assignment changes Errors of omission in research plan, letters of reference, etc. Deadline issues Application outside scope of NIH funding 12

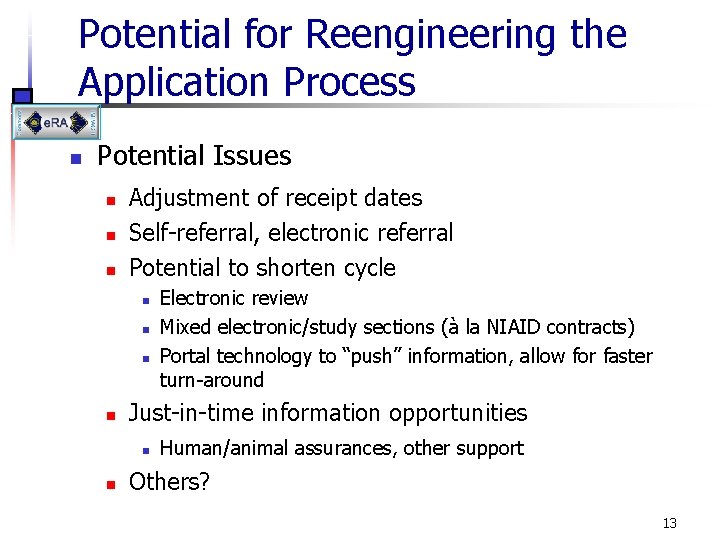
Potential for Reengineering the Application Process n Potential Issues n n n Adjustment of receipt dates Self-referral, electronic referral Potential to shorten cycle n n Just-in-time information opportunities n n Electronic review Mixed electronic/study sections (à la NIAID contracts) Portal technology to “push” information, allow for faster turn-around Human/animal assurances, other support Others? 13

Potential for Reengineering the Research Plan n Potential Issues n n Page limits Appendices n n Rich Text n n n Links Text format n n n PDF and other file formats (Word) XML Literature cited n n Just-in-time vs. embedded links size, font color considerations, viewing/printing Others? 14

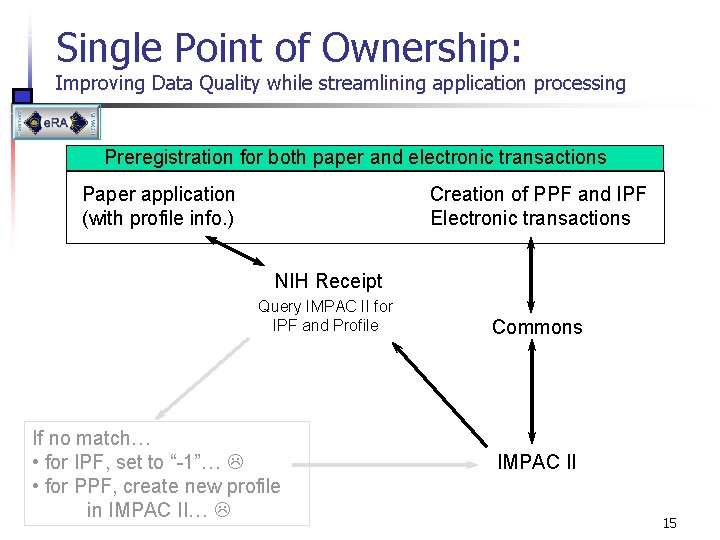
Single Point of Ownership: Improving Data Quality while streamlining application processing Preregistration for both paper and electronic transactions Paper application (with profile info. ) Creation of PPF and IPF Electronic transactions NIH Receipt Query IMPAC II for IPF and Profile If no match… • for IPF, set to “-1”… • for PPF, create new profile in IMPAC II… Commons IMPAC II 15
 Itu cwg fhr
Itu cwg fhr Agenda sistemica y agenda institucional
Agenda sistemica y agenda institucional Alco meeting agenda
Alco meeting agenda Agenda for parents meeting
Agenda for parents meeting Math department meeting agenda
Math department meeting agenda Replenishment meeting
Replenishment meeting Accounting meeting agenda
Accounting meeting agenda Graduation meeting agenda
Graduation meeting agenda The agenda communicates important information such as
The agenda communicates important information such as Volleyball parent meeting agenda
Volleyball parent meeting agenda Distributor meeting agenda
Distributor meeting agenda Chs lancers
Chs lancers Football coaches meeting agenda
Football coaches meeting agenda Architecture meeting agenda
Architecture meeting agenda Agenda welcome and introductions
Agenda welcome and introductions Football parent meeting agenda
Football parent meeting agenda