Agenda Clinical Research within an EMR What the










- Slides: 31

Agenda • Clinical Research within an EMR • What the difference between an EMR and an EHR • Define Clinical Research • Practical Clinical Networks (Single Source) DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

Why do we want to do this • Recruitment • Patient are recruited from existing local sites • Lower Start Up Time and Cost • Trails use existing networks and infrastructure • Allows for simple trials in clinical setting • Patients are more representative of general population • Unique information for Longitudinal Data, Registries • Lower Cost of Data Collections • Eliminates Source Document Verification • Eliminates redundant data entry • Lower Monitoring Costs • Reduces queries and time spent on query resolution • Builds in quality checks DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

Agenda • Clinical Research within an EMR • What the difference between an EMR and an EHR. • EMR. = Used by a clinician to provide care • EHR. = Synopsis of individual health related information • Define Clinical Research • Practical Clinical Networks DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

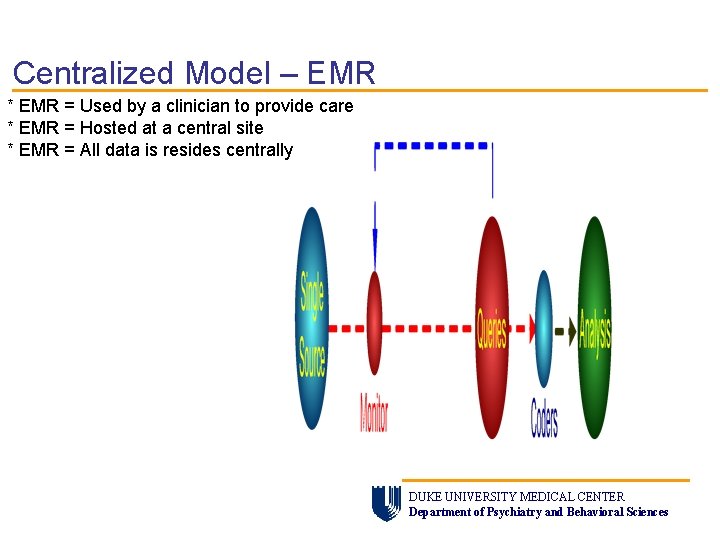
Centralized Model – EMR * EMR = Used by a clinician to provide care * EMR = Hosted at a central site * EMR = All data is resides centrally DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

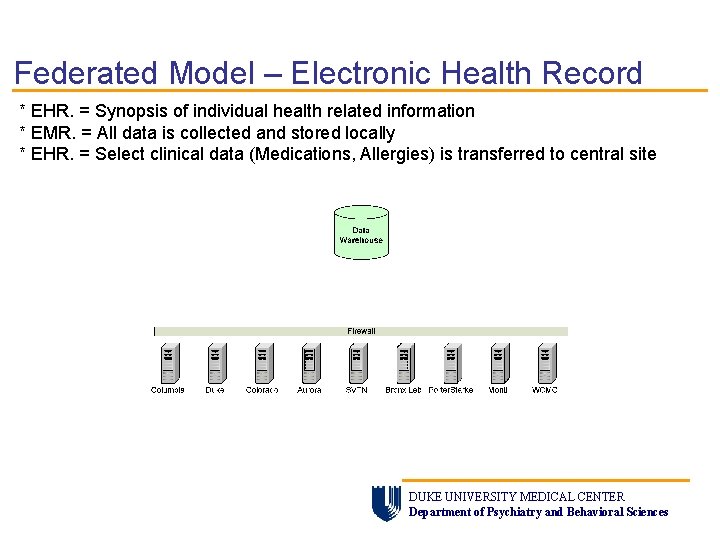
Federated Model – Electronic Health Record * EHR. = Synopsis of individual health related information * EMR. = All data is collected and stored locally * EHR. = Select clinical data (Medications, Allergies) is transferred to central site DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

Federated Model – Electronic Health Record * EHR. = Synopsis of individual health related information * EMR. = All data is collected and stored locally * EHR. = Select clinical data (medications, Allergies) is transferred to central site DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

Agenda • Clinical Research within an EMR • Define Clinical Research • e. PRO, EDC, • Practical Clinical Networks DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

Research - Definitions Paper Source Medical Record Case Report Form Patient Rated Scales Electronic Equivalent Electronic Health Record (‘EHR’) Electronic Data Capture (EDC) Electronic Patient Reported Outcomes (e. Pro) DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

Case Report Forms DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

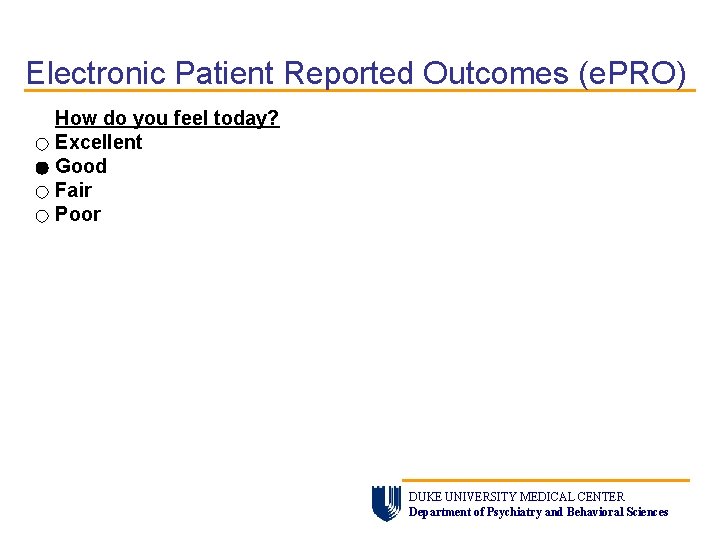
Electronic Patient Reported Outcomes (e. PRO) How do you feel today? Excellent Good Fair Poor DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

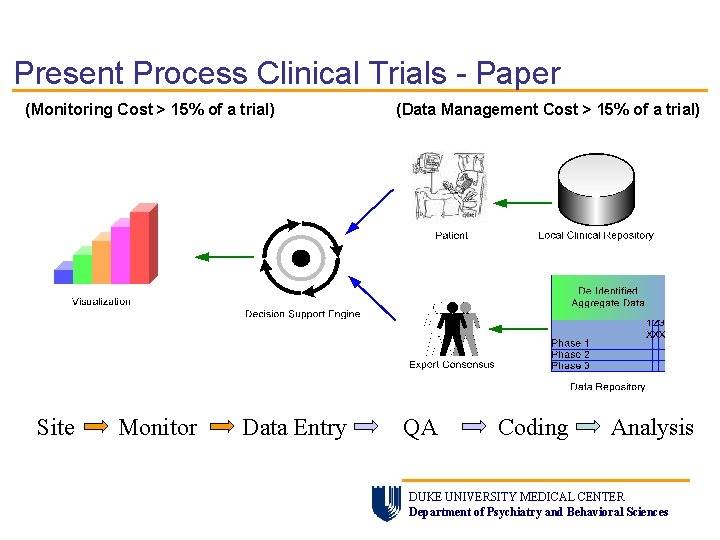
Present Process Clinical Trials - Paper (Monitoring Cost > 15% of a trial) Site Monitor Data Entry (Data Management Cost > 15% of a trial) QA Coding Analysis DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

Clinical Trials – EDC (Electronic CRFs) Site Monitor Data Entry QA Coding Analysis DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

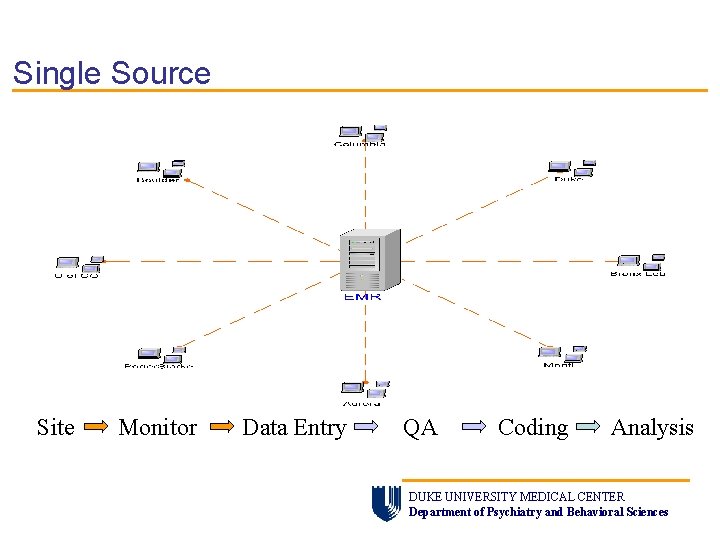
Single Source Site Monitor Data Entry QA Coding Analysis DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

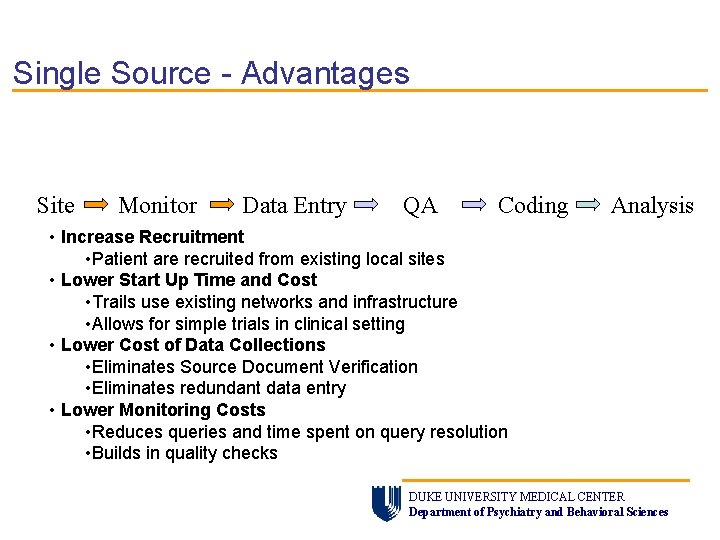
Single Source - Advantages Site Monitor Data Entry QA Coding Analysis • Increase Recruitment • Patient are recruited from existing local sites • Lower Start Up Time and Cost • Trails use existing networks and infrastructure • Allows for simple trials in clinical setting • Lower Cost of Data Collections • Eliminates Source Document Verification • Eliminates redundant data entry • Lower Monitoring Costs • Reduces queries and time spent on query resolution • Builds in quality checks DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

Agenda • Clinical Research within an EMR • What the difference between an EMR and an EHR. • Define Clinical Research • Practical Clinical Networks • Barriers to Implementation DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

Barriers to Implementation The limitations to creating a single source platform are formidable. • Technical limitations include: • Security issues of traversing both inbound and outbound firewalls at different institutions • EMRs are not interoperability • EMRs are not research ready, don’t understand concepts like a visit schedule, and ODM protocol • Evolving software standards form both HL 7, CDISC IHE, CCHIT and other organizations • Regularity barriers including meeting both • Cost to HIPPA, CCHIT, IHE and • Cost to be FDA compliance - 21 Part 11 compliant. • Workflow barriers include the need to function both as a • EMR – not structured, information based, work flow • Case Report Forms are a defined subset of elements • Loss of efficiency for busy clinicians who see research patient. • Functionality who should build this • Unique data requirements of a clinical record and a clinical trail. • EDC does not do billing, treatment plans, state and local requirements • EDC does not what to host an EMR with liability • EDC do not know EMR • EMR don’t care about research and market is very small

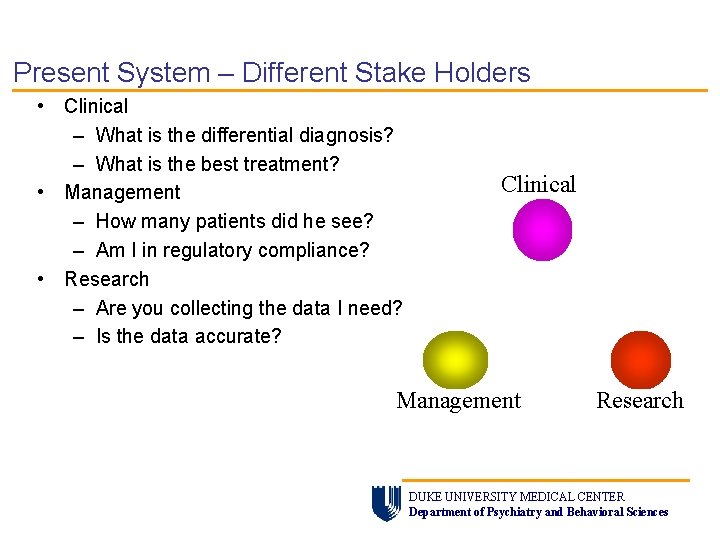
Present System – Different Stake Holders • Clinical – What is the differential diagnosis? – What is the best treatment? • Management – How many patients did he see? – Am I in regulatory compliance? • Research – Are you collecting the data I need? – Is the data accurate? Clinical Management Research DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

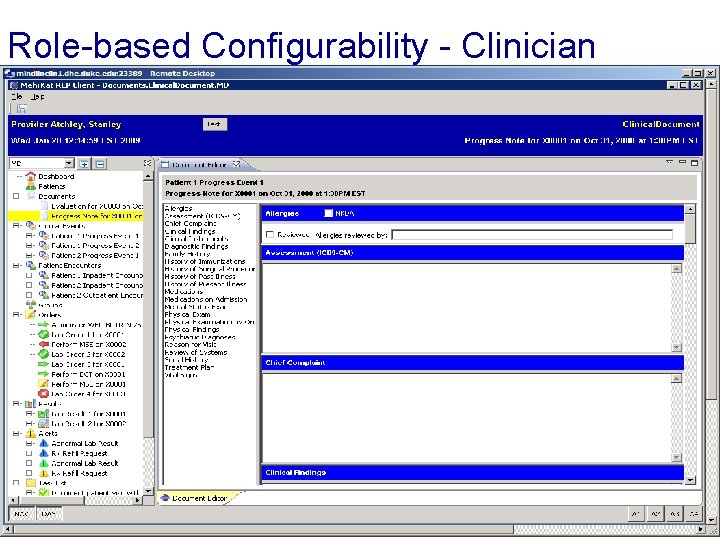
Role-based Configurability - Clinician End Slide

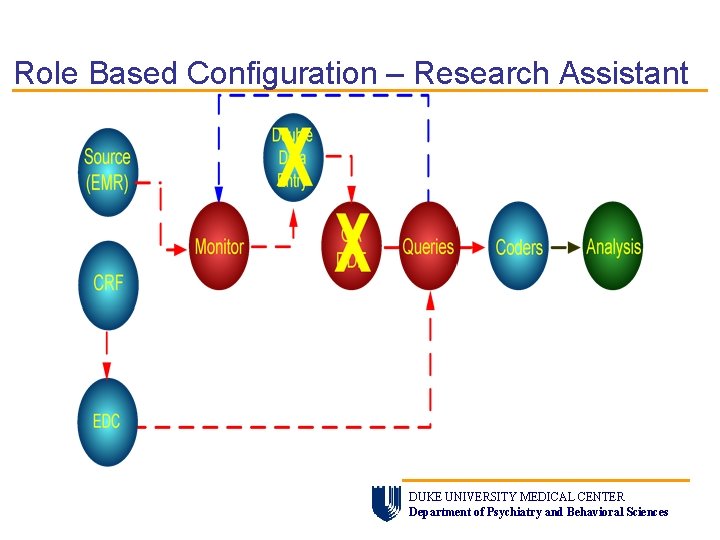
Role Based Configuration – Research Assistant DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

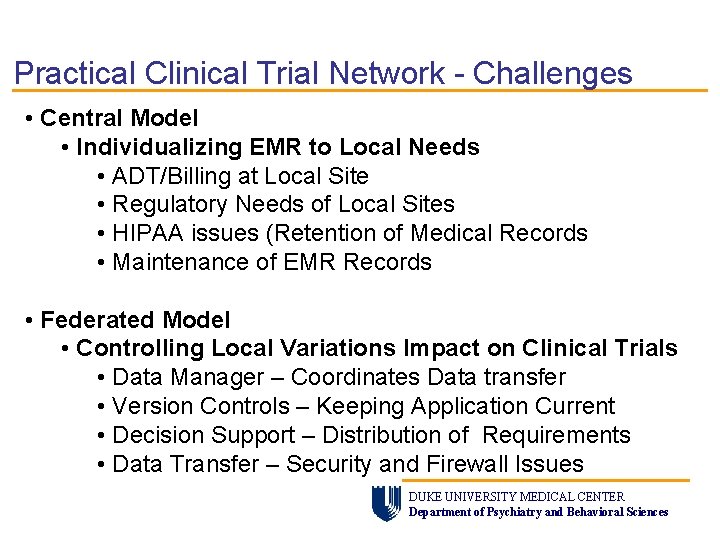
Practical Clinical Trial Network - Challenges • Central Model • Individualizing EMR to Local Needs • ADT/Billing at Local Site • Regulatory Needs of Local Sites • HIPAA issues (Retention of Medical Records • Maintenance of EMR Records • Federated Model • Controlling Local Variations Impact on Clinical Trials • Data Manager – Coordinates Data transfer • Version Controls – Keeping Application Current • Decision Support – Distribution of Requirements • Data Transfer – Security and Firewall Issues DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

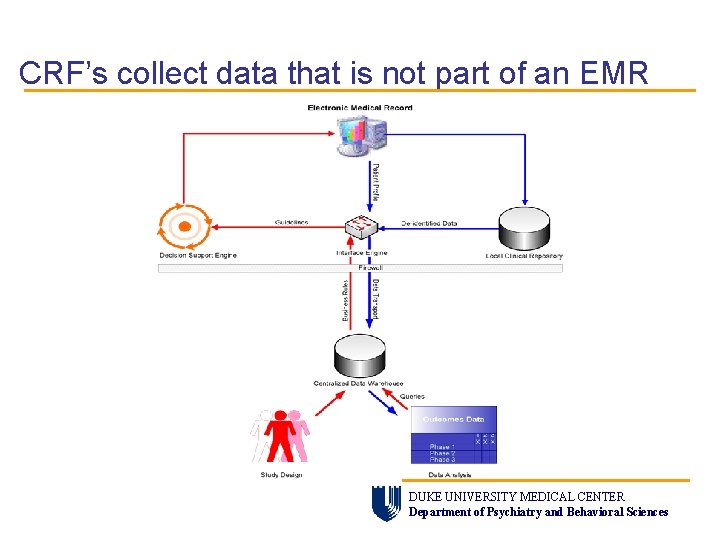
CRF’s collect data that is not part of an EMR DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

Integration of existing software End Slide

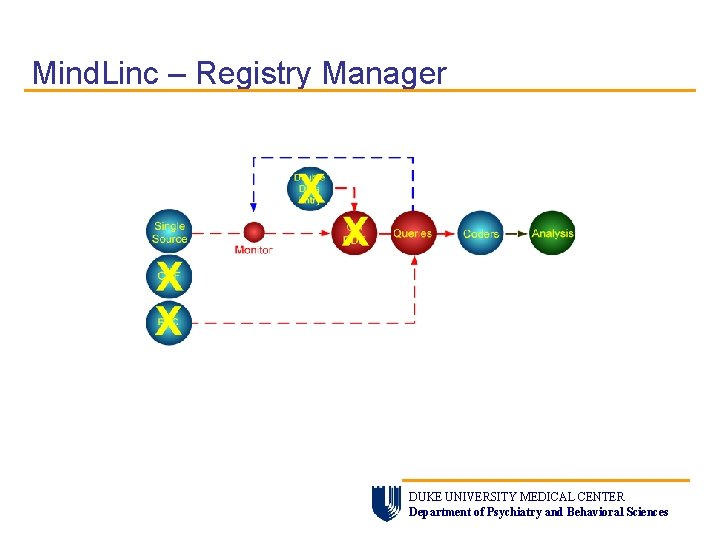
Mind. Linc – Registry Manager DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

Mind. Linc DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

Single Source DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

Improving Clinical Care DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

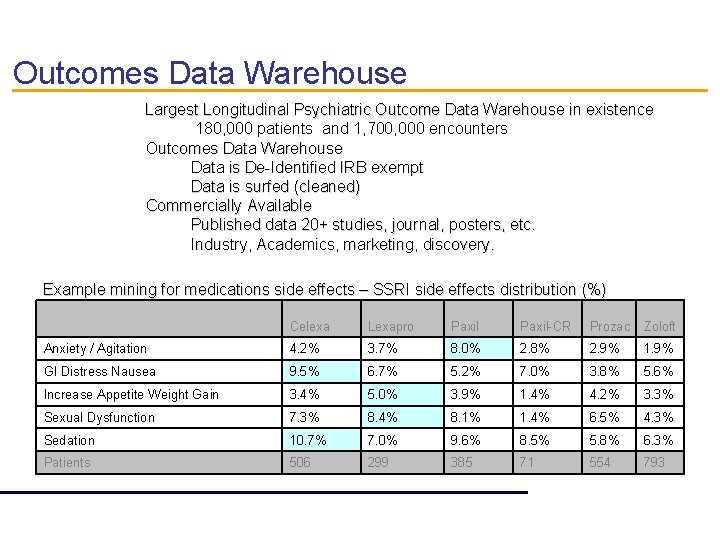
Outcomes Data Warehouse Largest Longitudinal Psychiatric Outcome Data Warehouse in existence 180, 000 patients and 1, 700, 000 encounters Outcomes Data Warehouse Data is De-Identified IRB exempt Data is surfed (cleaned) Commercially Available Published data 20+ studies, journal, posters, etc. Industry, Academics, marketing, discovery. Example mining for medications side effects – SSRI side effects distribution (%) Celexa Lexapro Paxil-CR Prozac Zoloft Anxiety / Agitation 4. 2% 3. 7% 8. 0% 2. 8% 2. 9% 1. 9% GI Distress Nausea 9. 5% 6. 7% 5. 2% 7. 0% 3. 8% 5. 6% Increase Appetite Weight Gain 3. 4% 5. 0% 3. 9% 1. 4% 4. 2% 3. 3% Sexual Dysfunction 7. 3% 8. 4% 8. 1% 1. 4% 6. 5% 4. 3% Sedation 10. 7% 7. 0% 9. 6% 8. 5% 5. 8% 6. 3% Patients 506 299 385 71 554 793

Visualization DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

Decision Support DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

The End DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences

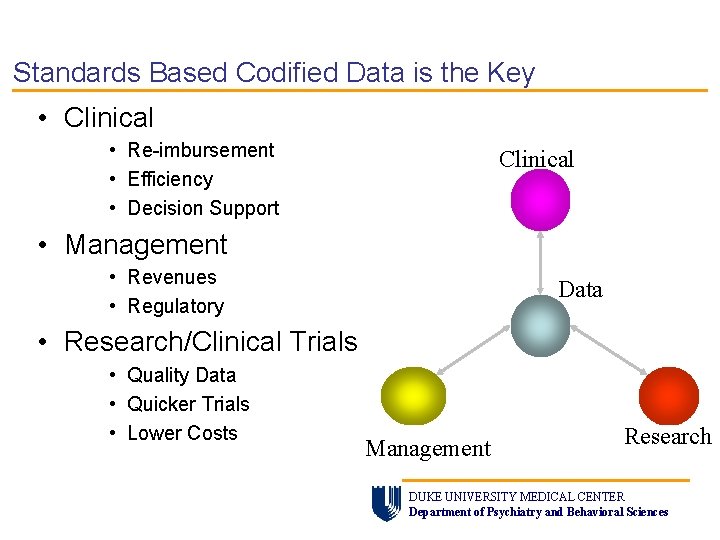
Standards Based Codified Data is the Key • Clinical • Re-imbursement • Efficiency • Decision Support Clinical • Management • Revenues • Regulatory Data • Research/Clinical Trials • Quality Data • Quicker Trials • Lower Costs Management Research DUKE UNIVERSITY MEDICAL CENTER Department of Psychiatry and Behavioral Sciences
 Agenda sistemica y agenda institucional
Agenda sistemica y agenda institucional Cprs emr
Cprs emr Acrendo
Acrendo Ps suite emr cost
Ps suite emr cost Nextgen electronic medical record
Nextgen electronic medical record Hadoop web services
Hadoop web services Emr data mining
Emr data mining Emr patient assessment
Emr patient assessment As an emr, your two primary extrication goals include:
As an emr, your two primary extrication goals include: Aws security group icon
Aws security group icon Medical record artinya
Medical record artinya Myhealth access network
Myhealth access network Gerimed emr
Gerimed emr Kaizers orchestra
Kaizers orchestra Ritefax
Ritefax Pathology emr
Pathology emr Amr medical records
Amr medical records Emr ehr phr
Emr ehr phr Holistic medicine ehr
Holistic medicine ehr Tribal health emr
Tribal health emr Tribal health emr
Tribal health emr Spectrum of waves
Spectrum of waves Open receivables
Open receivables Tribal health emr
Tribal health emr Service electronic
Service electronic Injuries to muscles and bones chapter 15
Injuries to muscles and bones chapter 15 Aws elb icon
Aws elb icon Society of clinical research associates
Society of clinical research associates Charlotte lemech
Charlotte lemech Research design in clinical psychology
Research design in clinical psychology Pi clinical research consultancy
Pi clinical research consultancy Good documentation practices error correction
Good documentation practices error correction























































