AGENDA BELL WORK 1 2 3 4 5

AGENDA BELL WORK 1. 2. 3. 4. 5. What goes up and down stairs without moving? Give it food and it will live; give it water and it will die. What can you catch but not throw? I run, yet I have no legs. What am I? Take one out and scratch my head, I am now black but once was red. NOTES 8. 1 HOMEWORK: WORKBOOK SECTION 8. 1

CHAPTER 8 COVALENT BONDING MRS. RAGSDALE CHEMISTRY I
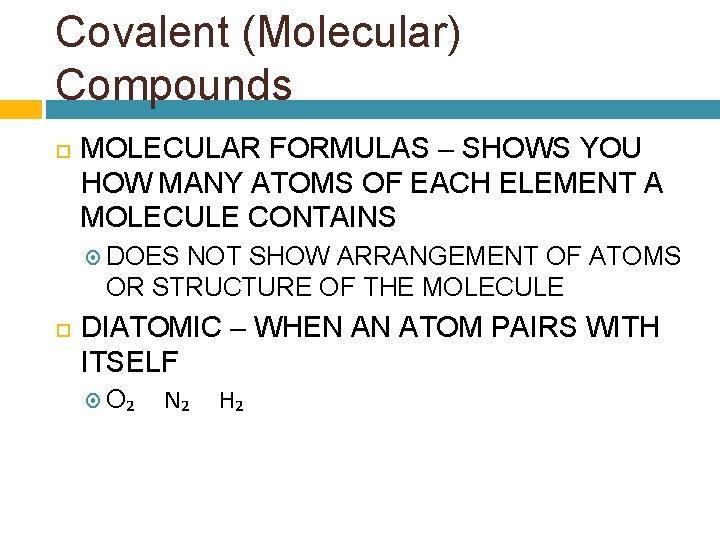
Covalent (Molecular) Compounds MOLECULAR FORMULAS – SHOWS YOU HOW MANY ATOMS OF EACH ELEMENT A MOLECULE CONTAINS DOES NOT SHOW ARRANGEMENT OF ATOMS OR STRUCTURE OF THE MOLECULE DIATOMIC – WHEN AN ATOM PAIRS WITH ITSELF O₂ N₂ H₂
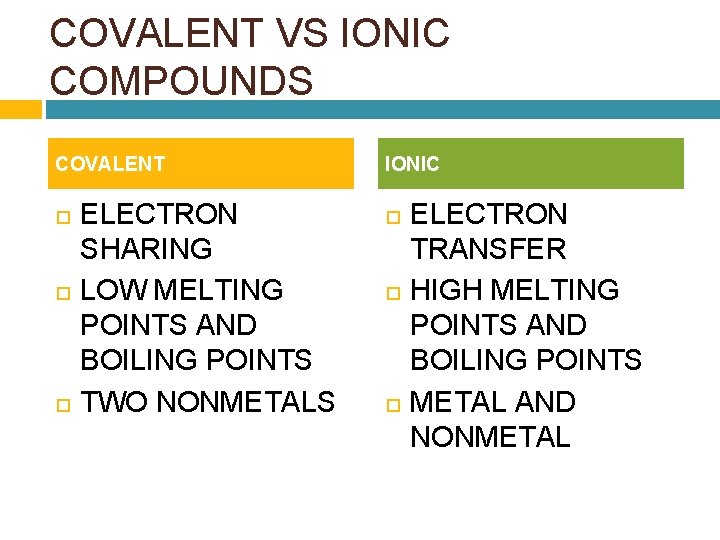
COVALENT VS IONIC COMPOUNDS COVALENT ELECTRON SHARING LOW MELTING POINTS AND BOILING POINTS TWO NONMETALS IONIC ELECTRON TRANSFER HIGH MELTING POINTS AND BOILING POINTS METAL AND NONMETAL
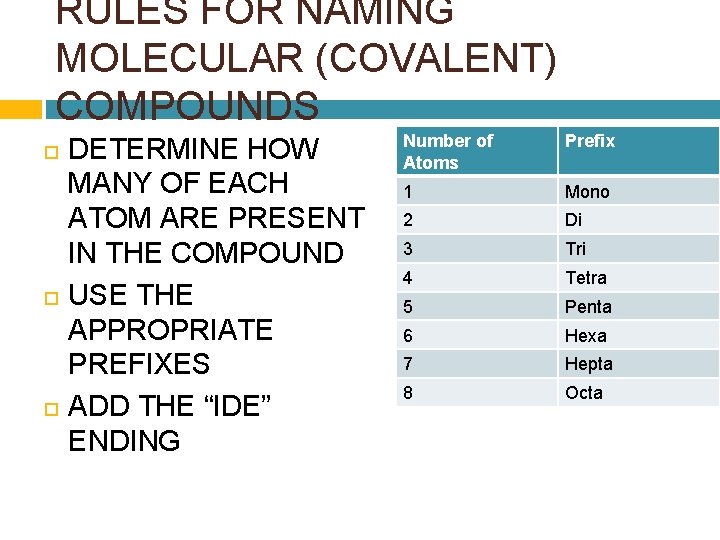
RULES FOR NAMING MOLECULAR (COVALENT) COMPOUNDS DETERMINE HOW MANY OF EACH ATOM ARE PRESENT IN THE COMPOUND USE THE APPROPRIATE PREFIXES ADD THE “IDE” ENDING Number of Atoms Prefix 1 Mono 2 Di 3 Tri 4 Tetra 5 Penta 6 Hexa 7 Hepta 8 Octa

THE OCTET RULE – COVALENT COMPOUNDS IN COVALENT BONDS, EVERY ATOM WILL TRY TO OBTAIN 8 ELECTRONS BY SHARING BETWEEN ATOMS EXCEPTION!!! HYDROGEN WANTS TO BE LIKE He SO IT WILL ONLY TRY TO GET 2 ELECTRONS COVALENT BONDS CAN BE SINGLE, DOUBLE OR EVEN TRIPLE DEPENDING ON HOW MANY PAIRS OF ELECTRONS ARE SHARED
ELECTRON DOT STRUCTURES ALSO KNOWN AS “LEWIS STRUCTURES” REPRESENT THE SHARED PAIR OF ELECTRONS USING LINES AND DOTS
8. 2 LEWIS DOT STRUCTURES AND YOU MRS. RAGSDALE CHEMISTRY I
SINGLE, DOUBLE AND TRIPLE BONDS SINGLE BOND – 2 ELECTRONS SHARED (1 PAIR) DOUBLE – 4 ELECTRONS SHARED (2 PAIRS) TRIPLE – 6 ELECTRONS SHARED (3 PAIRS)

RULES FOR DOT STRUCTURES 1. 2. 3. 4. EVERY ATOM (EXCEPT FOR HYDROGEN) WANTS TO BE SURROUNDED BY 8 ELECTRONS – OCTET RULE ARANGE THE ELECTRONS SO THAT EVERY ATOM HAS 8 ELECTRONS ALWAYS MUST BE IN PAIRS. NO SINGLE ELECTRONS ARE ALLOWED FOR ADDITIONAL HELP, DRAW THE S AND P ORBITAL NOTATIONS FIRST

BOND DISSOCIATION ENERGY THE TOTAL ENERGY REQUIRED TO BREAK THE BOND BETWEEN TWO COVALENTLY CHARGED ATOMS THE STRONGER THE BOND, THE HIGHER THE DISSOCIATION ENERGY

RESONANCE STRUCTURES WHEN THERE IS MORE THAN ONE WAY TO WRITE AN ELECTRON DOT STRUCTURE RESONANCE STRUCTURES – THE POSSIBLITIES OF ELECTRON DOT STRUCTURES

MRS. RAGSDALE CHEMISTRY I 8. 3 BONDING THEORIES
VSEPR THEORY REPULSION BETWEEN ELECTRON PAIRS CAUSES MOLECULAR SHAPES TO ADJUST SO THAT THE VALENCE ELECTRON PAIRS STAY AS FAR APART AS POSSIBLE 3 -D MODELS THAT SHOW THE PHYSICAL STRUCTURE OF MOLECULES
WHAT GIVES A MOLECULE “SHAPE”? UNSHARED ELECTRON PAIRS ARE JUST AS IMPORTANT AS BONDS! IN MOLECULAR GEOMETRY, SHAPES ARE DETERMINED BY THE NUMBER OF ATOMS, BONDS AND UNSHARED PAIRS OF ELECTRONS
STEPS FOR USING VSEPR 1. 2. 3. DETERMINE THE LEWIS DOT STRUCTURE COUNT HOW MANY ATOMS, BONDS AND UNSHARED ELECTRONS THERE ARE USE THE CHART ON PAGE 233 (FIGURE 8. 18) TO DETERMINE SHAPE
“AXE” METHOD A = CENTRAL ATOM X = OTHER ATOMS E = UNSHARED PAIRS OF ELECTRONS

8. 4 POLAR BONDS AND MOLECULES MRS. RAGSDALE CHEMISTRY I
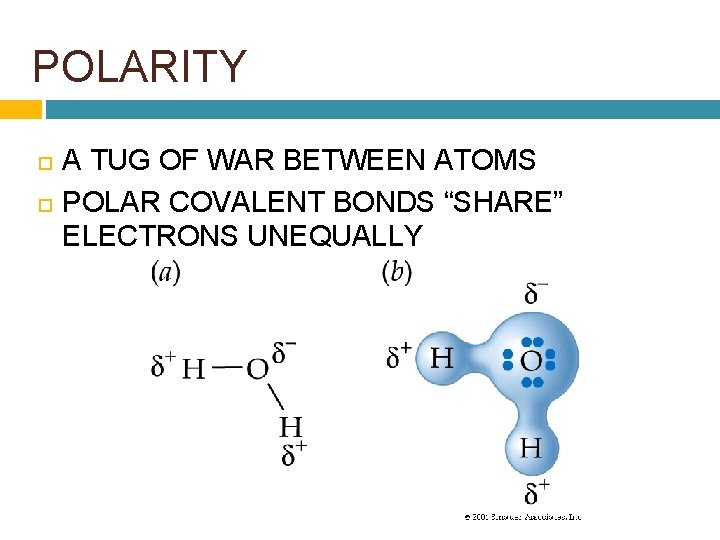
POLARITY A TUG OF WAR BETWEEN ATOMS POLAR COVALENT BONDS “SHARE” ELECTRONS UNEQUALLY
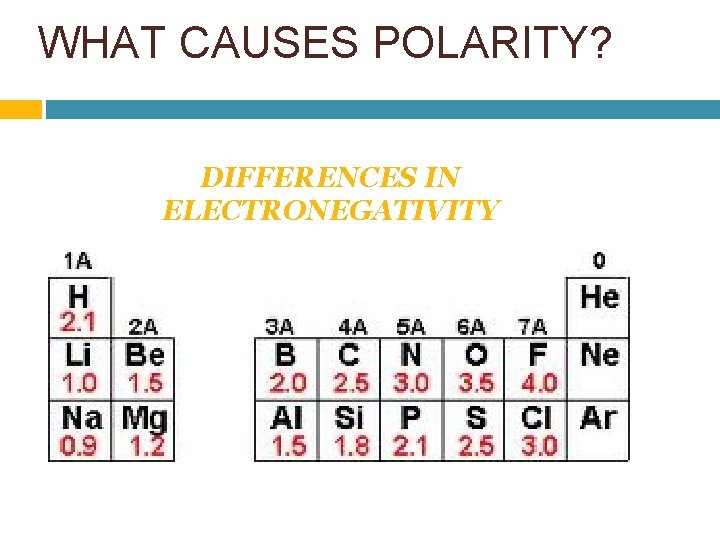
WHAT CAUSES POLARITY? DIFFERENCES IN ELECTRONEGATIVITY
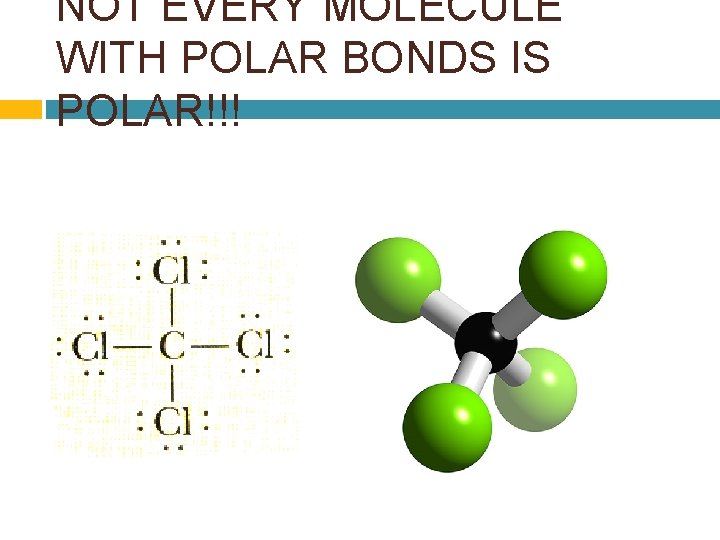
NOT EVERY MOLECULE WITH POLAR BONDS IS POLAR!!!
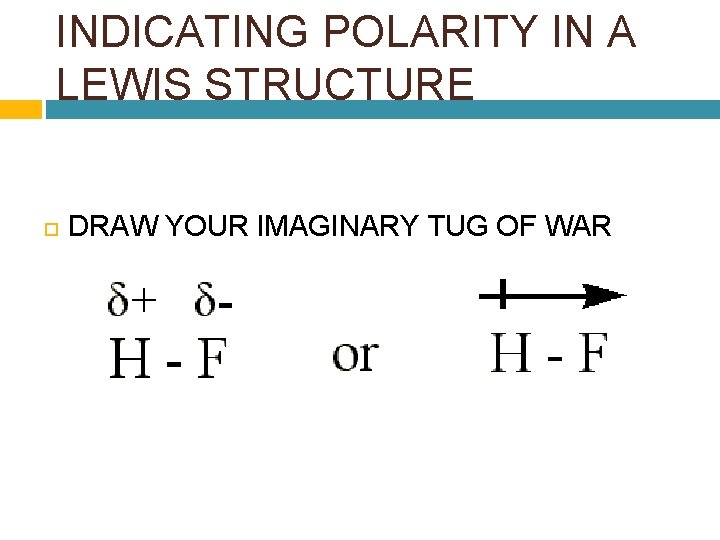
INDICATING POLARITY IN A LEWIS STRUCTURE DRAW YOUR IMAGINARY TUG OF WAR
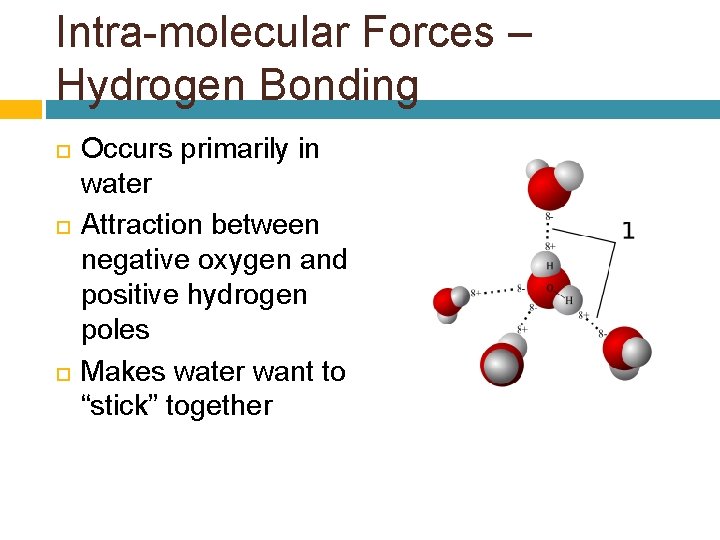
Intra-molecular Forces – Hydrogen Bonding Occurs primarily in water Attraction between negative oxygen and positive hydrogen poles Makes water want to “stick” together
- Slides: 23