Agar sterilization time temperature and expiry dates for















- Slides: 26

Agar sterilization time, temperature and expiry dates for prepared media Janine Coetzer Integral Labs

No matter how prepared you are for your next audit, there will always be something you missed, there is always something you can improve on and there always to make your life easier and to work more efficient. Working under conditions which does not allow for the preparation of media every day, it is necessary to prepare bigger batches of media when high volume of samples are expected. Even though well maintained and calibrated equipment are being used, time and temperature limits are sometimes exceeded. This can become very expensive if this media has to be discarded. • • These questions popped up in an Internal Audit 1. How long can you keep your prepared agar in the fridge. 2. How many degrees can you go above 121°C while sterilizing agar. 3. How much extra time can you allow at the required temperature with no effect to agar. • And off course there were no answers. • The idea behind this exercise was be to establish expiry dates for prepared agar, • Minimum/maximum time and temperatures for sterilization of agar that would not affect the integrity of the agar. • •

Question 1. How long can we store prepared agar: There is no data available under the manufacturers preparation procedures regarding the time prepared media can be stored. • The idea is to prepare a batch, and over a period of time perform: 1. Positive and negative controls 2. p. H 3. Sterilities • to try and establish a suitable time media can be stored. • LAB M Harlequin e. coli/coliform Medium 1. Soak for 10 min, swirl to mix. 2. Autoclave at 121°c for 15 min. 3. p. H 7. 2 ± 0. 2 Biolab Plate Count Agar for HPCs 1. Boil until completely dissolved. 2. Autoclave at 121°c for 15 min. 3. p. H 7. 0 ± 0. 2

Harlequin coliform agar • Agar was also poured into 4 sterile containers to allow for p. H’s to be performed over a period of 4 weeks. • Every week a p. H would be run • Plates was poured into Petri dishes and stored in a sealed container at 28°C these were to be used as Positive and Negative controls. Plate Count Agar for HPCs • Agar were stored in separate containers at 2 -8°C, allowing it to be melted every week in order to perform positive controls, sterilities and p. H

LAB M Harlequin e. coli/coliform Medium 1. Soak for 10 min, swirl to mix. 2. Autoclave at 121°C for 15 min. 3. p. H 7. 2 ± 0. 2 Date Prep Date Run Steri Temp 2016/09/14 123°c 2016/09/14 2016/09/20 123°c 2016/09/14 2016/09/27 123°c 2016/09/14 2016/10/03 123°c 2016/09/14 2016/10/11 123°c Av time at Max temp 12 min at 123°C 12 min at 123°C Steri Time 121 ± 5°c Agar/Media p. H range. Positive Negative 26 min Harlequin 7. 25 7. 0 ± 0. 2 Blue colonies. Pink colonies 26 min Harlequin 7. 21 7. 0 ± 0. 2 Blue colonies. Pink colonies 26 min Harlequin 7. 23 7. 0 ± 0. 2 Blue colonies. Pink colonies 26 min Harlequin 7. 16 7. 0 ± 0. 2 Blue colonies. Pink colonies Blank No Growth No Growth Logger position External External

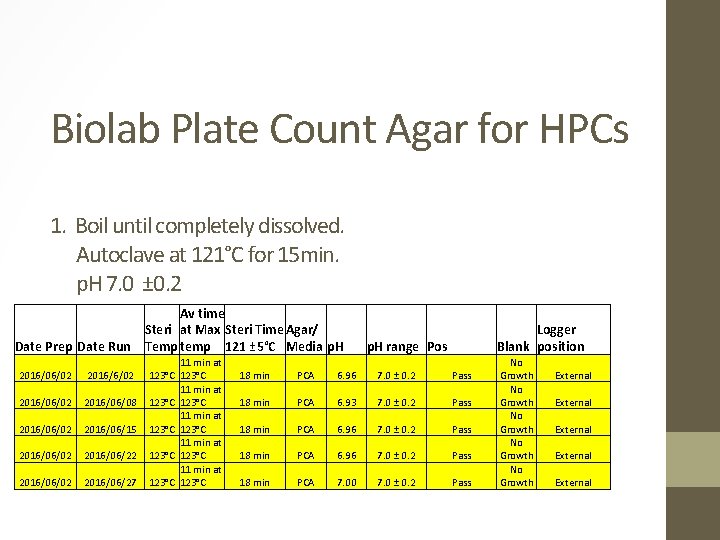
Biolab Plate Count Agar for HPCs 1. Boil until completely dissolved. Autoclave at 121°C for 15 min. p. H 7. 0 ± 0. 2 Date Prep Date Run 2016/06/02 2016/06/08 2016/06/02 2016/06/15 2016/06/02 2016/06/22 2016/06/02 2016/06/27 Av time Steri at Max Steri Time Agar/ Temp temp 121 ± 5°C Media p. H 11 min at 123°C 123°C 11 min at 123°C Logger Blank position p. H range Pos 18 min PCA 6. 96 7. 0 ± 0. 2 Pass 18 min PCA 6. 93 7. 0 ± 0. 2 Pass 18 min PCA 6. 96 7. 0 ± 0. 2 Pass 18 min PCA 7. 00 7. 0 ± 0. 2 Pass No Growth No Growth External External

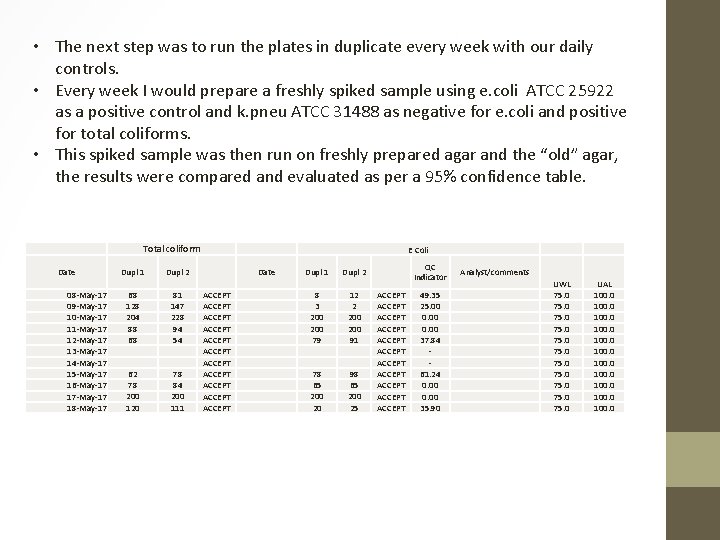
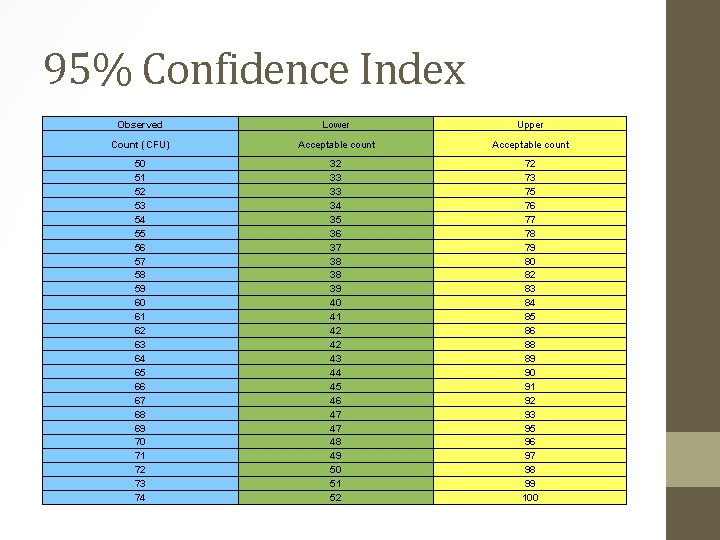
• The next step was to run the plates in duplicate every week with our daily controls. • Every week I would prepare a freshly spiked sample using e. coli ATCC 25922 as a positive control and k. pneu ATCC 31488 as negative for e. coli and positive for total coliforms. • This spiked sample was then run on freshly prepared agar and the “old” agar, the results were compared and evaluated as per a 95% confidence table. Total coliform Date 08 -May-17 09 -May-17 10 -May-17 11 -May-17 12 -May-17 13 -May-17 14 -May-17 15 -May-17 16 -May-17 17 -May-17 18 -May-17 Dupl 1 Dupl 2 68 128 204 88 68 81 147 228 94 54 62 78 200 120 78 84 200 111 E Coli Date ACCEPT ACCEPT ACCEPT Dupl 1 Dupl 2 8 3 200 79 12 2 200 91 78 65 200 20 98 65 200 25 QC Indicator ACCEPT ACCEPT ACCEPT 49. 35 25. 00 0. 00 37. 84 61. 24 0. 00 35. 90 Analyst/comments UWL 75. 0 75. 0 UAL 100. 0 100. 0

Harlequin Duplicates Fresh vs “Old” Prepared: 14/9/2016 Run: 20/9/16 e. Coli ATCC 25922 Date 20 -Sep-16 Dupl 1 Dupl 2 >200 #VALUE! k. Variicola ATCC 31488 Date 20 -Sep-16 Dupl 1 Dupl 2 >200 #VALUE!

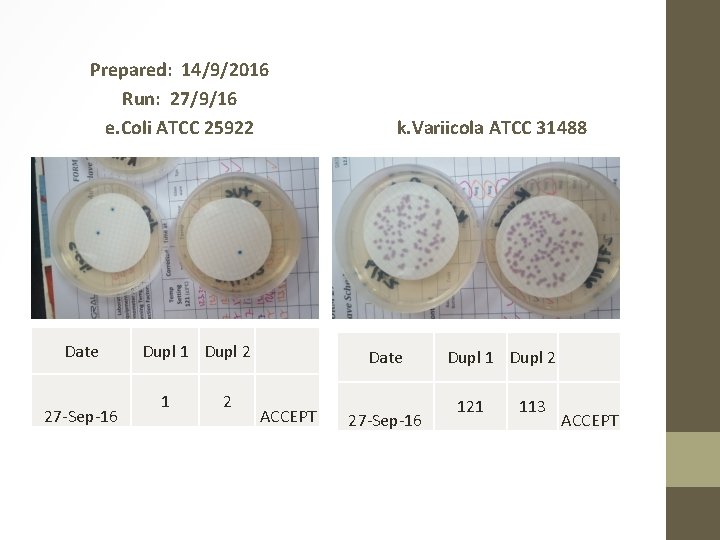
Prepared: 14/9/2016 Run: 27/9/16 e. Coli ATCC 25922 Date 27 -Sep-16 Dupl 1 Dupl 2 1 2 k. Variicola ATCC 31488 Date ACCEPT 27 -Sep-16 Dupl 1 Dupl 2 121 113 ACCEPT

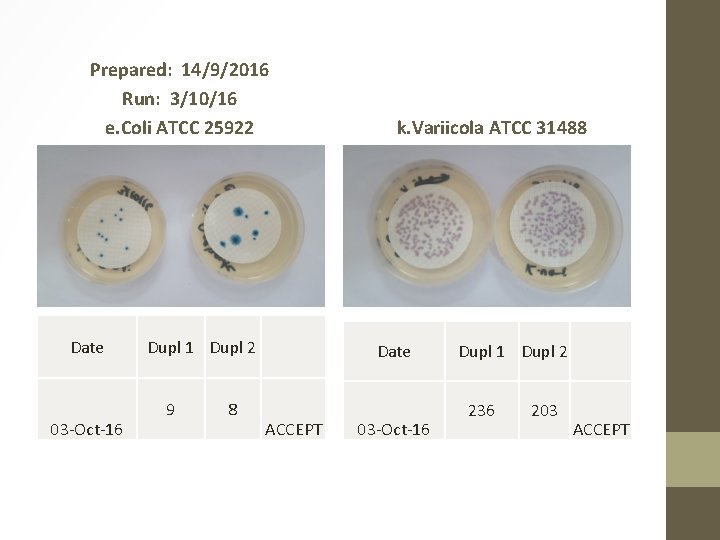
Prepared: 14/9/2016 Run: 3/10/16 e. Coli ATCC 25922 Date 03 -Oct-16 Dupl 1 Dupl 2 9 8 k. Variicola ATCC 31488 Date ACCEPT 03 -Oct-16 Dupl 1 Dupl 2 236 203 ACCEPT

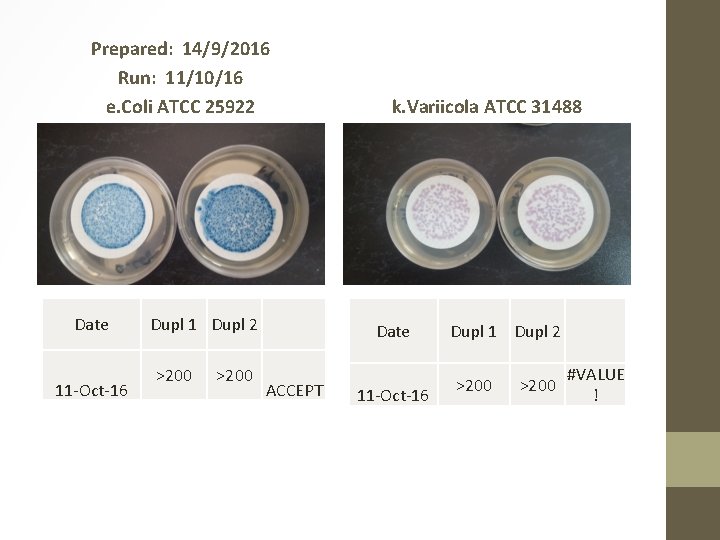
Prepared: 14/9/2016 Run: 11/10/16 e. Coli ATCC 25922 Date 11 -Oct-16 Dupl 1 Dupl 2 >200 k. Variicola ATCC 31488 Date ACCEPT 11 -Oct-16 Dupl 1 Dupl 2 >200 #VALUE !

PCA Duplicates Fresh vs “Old” Prepared: 2/6/2016 Run: 8/6/2016 p. H: 6. 93 e. Coli ATCC 25922 Fresh Agar e. Coli ATCC 25922 Date 08 -Jun-16 No. Dupl 1 Dupl 2 1 24 22

Prepared: 2/6/2016 Run: 15/6/2016 p. H: 6. 96 e. Coli ATCC 25922 Fresh Agar e. Coli ATCC 25922 Date No. Dupl 1 Dupl 2 08 -Jun-16 1 24 22 15 -Jun-16 2 1000

Prepared: 2/6/2016 Run: 22/06/2016 p. H: 6. 96 e. Coli ATCC 25922 Fresh Agar e. Coli ATCC 25922 Date No. 08 -Jun-16 15 -Jun-16 22 -Jun-16 1 2 3 Dupl 1 Dupl 2 24 1000 253 22 1000 274

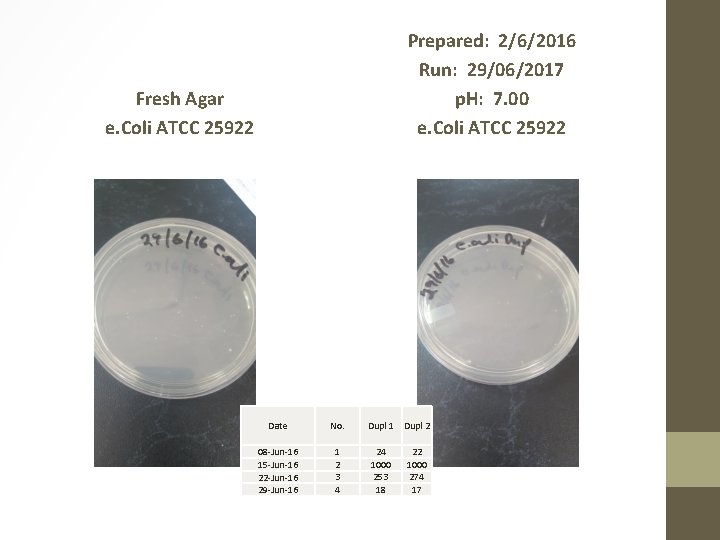
Prepared: 2/6/2016 Run: 29/06/2017 p. H: 7. 00 e. Coli ATCC 25922 Fresh Agar e. Coli ATCC 25922 Date No. 08 -Jun-16 15 -Jun-16 22 -Jun-16 29 -Jun-16 1 2 3 4 Dupl 1 Dupl 2 24 1000 253 18 22 1000 274 17

Decision: Looking at the results obtained during the 4 weeks, all checks passed and the agar was still in good condition( no visible deterioration). • We’ve settled on a 4 week storage time for prepared plates, but prepared [plates hardly lasts longer than 1 week. At least this means business has picked up a lot. •

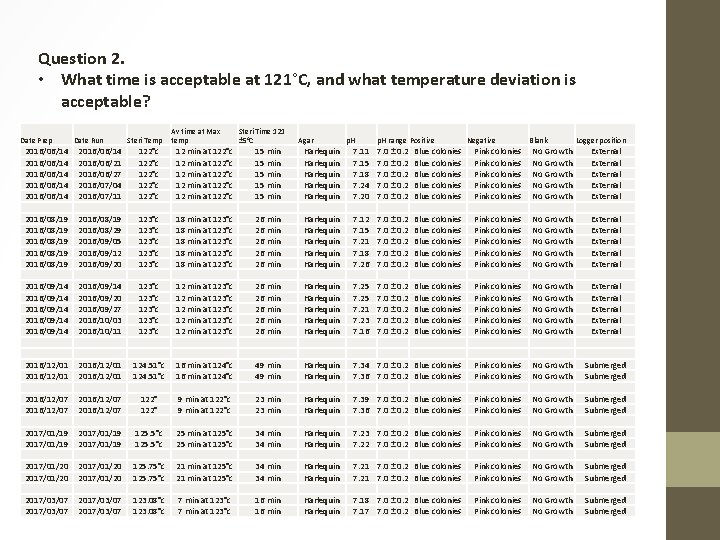
Question 2. • What time is acceptable at 121°C, and what temperature deviation would be tolerated by the media? • Sterilization Time and Temperature is still a bit of a Toffee. • On all the manufacturers preparation steps it states autocalve at 121°C for 15 minutes, no +/- this way or that. I’ve never seen a run this specific. • With a SANAS audit it was noted that the temperature logger was not submerged in the media during the sterilization process, and that different volumes were sterilized at the same time. • Subsequently all autoclaves have been re-calibrated with Loaded and Unloaded runs, and sterilizing of media and glassware etc. has been separated. • The temperatures on most of our runs are fairly regular and hardly ever exceeding 124°C. • On the odd occasion where the temperature was over 124°C no failures were noted on controls or p. H. • The average run time at 121°C after the last calibration sits roughly at 22 minutes, with no failures and very similar readings on controls and p. H. • We have also started to run a p. H with the daily controls with each batch of Plate Count Agar used.

• No failures were detected on any of these checks. • The p. H is very consistent and within range. • A duplicate was run on a weekly basis to compare freshly prepared agar with the stored plates. • All these duplicates passed as per the 95% Confidence range that we use to check our duplicates on.

95% Confidence Index Observed Lower Upper Count (CFU) Acceptable count 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 32 33 33 34 35 36 37 38 38 39 40 41 42 42 43 44 45 46 47 47 48 49 50 51 52 72 73 75 76 77 78 79 80 82 83 84 85 86 88 89 90 91 92 93 95 96 97 98 99 100

Question 2. • What time is acceptable at 121°C, and what temperature deviation is acceptable? Date Prep Date Run Steri Temp Av time at Max temp Steri Time 121 ± 5°C Agar p. H range Positive Negative Blank Logger position 2016/06/14 2016/06/14 2016/06/21 2016/06/27 2016/07/04 2016/07/11 122°c 122°c 12 min at 122°c 12 min at 122°c 15 min 15 min Harlequin Harlequin 7. 11 7. 15 7. 18 7. 24 7. 20 7. 0 ± 0. 2 Blue colonies Blue colonies Pink colonies Pink colonies No Growth No Growth External External 2016/08/19 2016/08/19 2016/08/29 2016/09/05 2016/09/12 2016/09/20 123°c 123°c 18 min at 123°c 18 min at 123°c 26 min 26 min Harlequin Harlequin 7. 12 7. 15 7. 21 7. 18 7. 26 7. 0 ± 0. 2 Blue colonies Blue colonies Pink colonies Pink colonies No Growth No Growth External External 2016/09/14 2016/09/14 2016/09/20 2016/09/27 2016/10/03 2016/10/11 123°c 123°c 12 min at 123°c 12 min at 123°c 26 min 26 min Harlequin Harlequin 7. 25 7. 21 7. 23 7. 16 7. 0 ± 0. 2 Blue colonies Blue colonies Pink colonies Pink colonies No Growth No Growth External External 2016/12/01 124. 51°c 16 min at 124°c 49 min Harlequin 7. 34 7. 0 ± 0. 2 Blue colonies 7. 36 7. 0 ± 0. 2 Blue colonies Pink colonies No Growth Submerged 2016/12/07 122° 9 min at 122°c 23 min Harlequin 7. 39 7. 0 ± 0. 2 Blue colonies 7. 36 7. 0 ± 0. 2 Blue colonies Pink colonies No Growth Submerged 2017/01/19 125. 5°c 25 min at 125°c 34 min Harlequin 7. 23 7. 0 ± 0. 2 Blue colonies 7. 22 7. 0 ± 0. 2 Blue colonies Pink colonies No Growth Submerged 2017/01/20 125. 75°c 21 min at 125°c 34 min Harlequin 7. 21 7. 0 ± 0. 2 Blue colonies Pink colonies No Growth Submerged 2017/03/07 123. 08°c 7 min at 123°c 16 min Harlequin 7. 18 7. 0 ± 0. 2 Blue colonies 7. 17 7. 0 ± 0. 2 Blue colonies Pink colonies No Growth Submerged

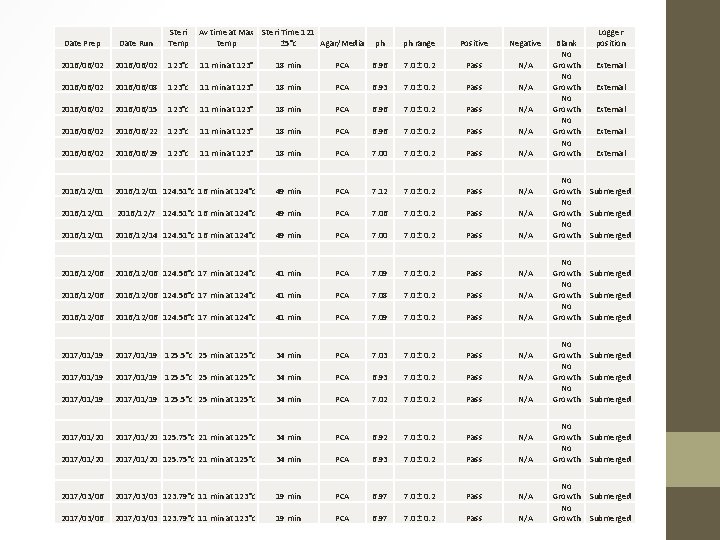
Date Prep Date Run Steri Temp Av time at Max Steri Time 121 temp ± 5°c Agar/Media 2016/06/02 123°c 11 min at 123° 18 min 2016/06/02 2016/06/08 123°c 11 min at 123° 2016/06/02 2016/06/15 123°c 2016/06/02 2016/06/22 2016/06/02 2016/06/29 ph ph range Positive Negative PCA 6. 96 7. 0 ± 0. 2 Pass N/A 18 min PCA 6. 93 7. 0 ± 0. 2 Pass N/A 11 min at 123° 18 min PCA 6. 96 7. 0 ± 0. 2 Pass N/A 123°c 11 min at 123° 18 min PCA 7. 00 7. 0 ± 0. 2 Pass N/A 2016/12/01 124. 51°c 16 min at 124°c 49 min PCA 7. 12 7. 0 ± 0. 2 Pass N/A 2016/12/01 2016/12/7 124. 51°c 16 min at 124°c 49 min PCA 7. 06 7. 0 ± 0. 2 Pass N/A 2016/12/01 2016/12/14 124. 51°c 16 min at 124°c 49 min PCA 7. 00 7. 0 ± 0. 2 Pass N/A 2016/12/06 124. 56°c 17 min at 124°c 41 min PCA 7. 09 7. 0 ± 0. 2 Pass N/A 2016/12/06 124. 56°c 17 min at 124°c 41 min PCA 7. 08 7. 0 ± 0. 2 Pass N/A 2016/12/06 124. 56°c 17 min at 124°c 41 min PCA 7. 09 7. 0 ± 0. 2 Pass N/A 2017/01/19 125. 5°c 25 min at 125°c 34 min PCA 7. 03 7. 0 ± 0. 2 Pass N/A 2017/01/19 125. 5°c 25 min at 125°c 34 min PCA 6. 93 7. 0 ± 0. 2 Pass N/A 2017/01/19 125. 5°c 25 min at 125°c 34 min PCA 7. 02 7. 0 ± 0. 2 Pass N/A 2017/01/20 125. 75°c 21 min at 125°c 34 min PCA 6. 93 7. 0 ± 0. 2 Pass N/A 2017/03/06 2017/03/03 123. 79°c 11 min at 123°c 19 min PCA 6. 97 7. 0 ± 0. 2 Pass N/A Blank No Growth No Growth No Growth No Growth No Growth Logger position External External Submerged Submerged Submerged Submerged

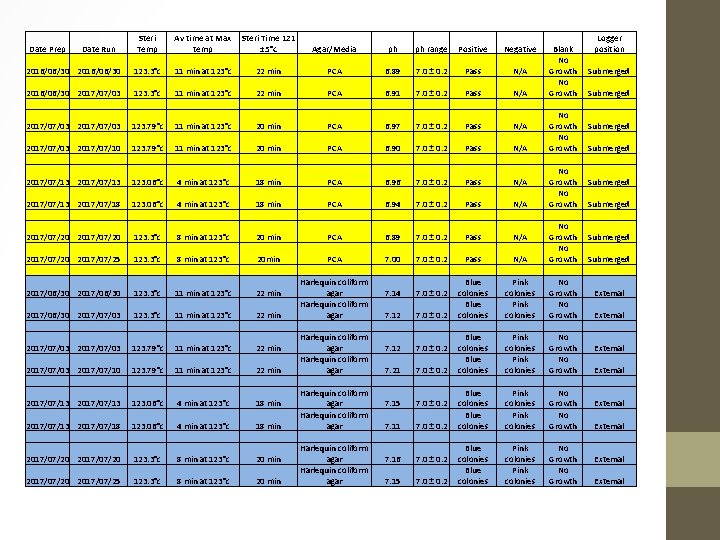
Steri Temp Av time at Max temp Steri Time 121 ± 5°c Agar/Media ph ph range Positive Negative 2016/06/30 123. 3°c 11 min at 123°c 22 min PCA 6. 89 7. 0 ± 0. 2 Pass N/A 2016/06/30 2017/07/03 123. 3°c 11 min at 123°c 22 min PCA 6. 91 7. 0 ± 0. 2 Pass N/A 2017/07/03 123. 79°c 11 min at 123°c 20 min PCA 6. 97 7. 0 ± 0. 2 Pass N/A 2017/07/03 2017/07/10 123. 79°c 11 min at 123°c 20 min PCA 6. 90 7. 0 ± 0. 2 Pass N/A 2017/07/13 123. 06°c 4 min at 123°c 18 min PCA 6. 96 7. 0 ± 0. 2 Pass N/A 2017/07/13 2017/07/18 123. 06°c 4 min at 123°c 18 min PCA 6. 94 7. 0 ± 0. 2 Pass N/A 2017/07/20 123. 3°c 8 min at 123°c 20 min PCA 6. 89 7. 0 ± 0. 2 Pass N/A 2017/07/20 2017/07/25 123. 3°c 8 min at 123°c 20 min PCA 7. 00 7. 0 ± 0. 2 Pass N/A No Growth 2017/06/30 123. 3°c 11 min at 123°c 22 min 7. 14 7. 0 ± 0. 2 2017/06/30 2017/07/03 123. 3°c 11 min at 123°c 22 min 7. 12 7. 0 ± 0. 2 Blue colonies Pink colonies No Growth 2017/07/03 123. 79°c 11 min at 123°c 22 min 7. 12 7. 0 ± 0. 2 2017/07/03 2017/07/10 123. 79°c 11 min at 123°c 22 min 7. 21 7. 0 ± 0. 2 Blue colonies Pink colonies No Growth 2017/07/13 123. 06°c 4 min at 123°c 18 min 7. 15 7. 0 ± 0. 2 2017/07/13 2017/07/18 123. 06°c 4 min at 123°c 18 min 7. 11 7. 0 ± 0. 2 Blue colonies Pink colonies No Growth 2017/07/20 123. 3°c 8 min at 123°c 20 min 7. 16 7. 0 ± 0. 2 2017/07/20 2017/07/25 123. 3°c 8 min at 123°c 20 min 7. 15 7. 0 ± 0. 2 Blue colonies Pink colonies No Growth Date Prep Date Run Harlequin coliform agar Harlequin coliform agar Blank No Growth No Growth Logger position Submerged Submerged External External

Decision: Temperature: • Looking at historical sterility checks we’ve always been working on a 121°C ± 3°C, and our recent data shows that we have been maintaining the required temperature. Sterilization time: • The data shows that we have times ranging from 18 -30 minutes at 121°C ± 3°C, and that the agar is still performing very well under these conditions. • No failures were detected on controls or p. H’s. • Sterilization time is set at 15 -25 minutes.

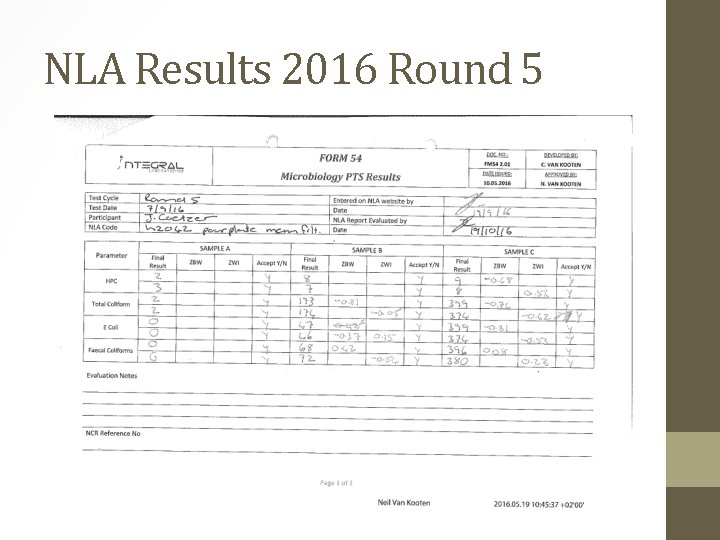
NLA Results • Looking at our NLA results, we’ve had very good results over the past couple of years. • Agar used in Round 5 2016: • Both Harlequin and PCA was prepared on the same day, 2/09/2016 and the NLA was run on 9/09/2016. • Temperature: 123. 39°C • Sterilization time @ 121°C ± 3°C: 26 minutes • Harlequin p. H: 7. 11 (7. 0 ± 0. 2) • PCA p. H: 6. 97 (7. 2 ± 0. 2)

NLA Results 2016 Round 5
