Afscheidsrede prof dr R A Sheldon hoogleraar Biokatalyse
Afscheidsrede prof. dr. R. A. Sheldon hoogleraar Biokatalyse en Organische Chemie December 7, 2007 ‘E Factors, Green Chemistry and Catalysis: Records of the Travelling Chemist’ 7 december 2007 R. A. Sheldon, Rec. Trav. Chem. 2007, 1, 1 1

Green Chemistry Green chemistry efficiently utilises (preferably renewable) raw materials, eliminates waste and avoids the use of toxic and/or hazardous solvents and reagents in the manufacture and application of chemical products. 2 Anastas and Warner, Eds, Green Chemistry. Theory & Practice, Oxford U. Press, 1998

Sustainability Meeting the needs of the present generation without compromising the needs of future generations to meet their own needs is the goal 3 “What we need is more imagination not more knowledge” Albert Einstein

Do politicians understand the issues? It’s not pollution that is the problem it’s the impurities in our air and water. Dan Quayle 4
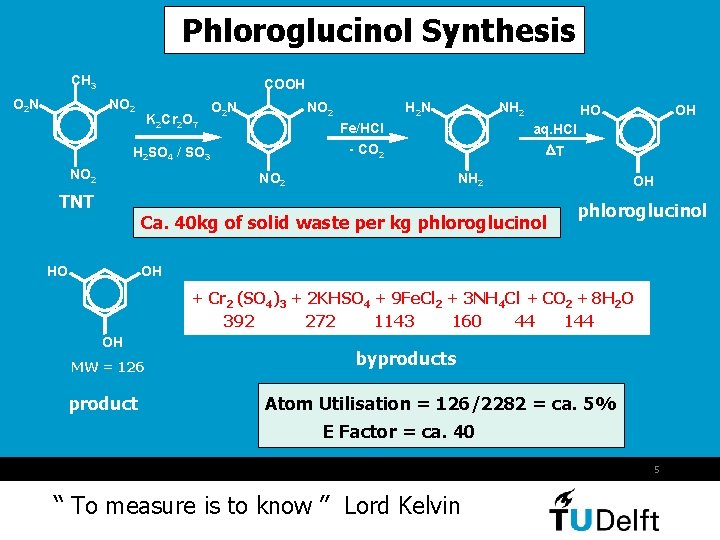
Phloroglucinol Synthesis CH 3 O 2 N COOH NO 2 K 2 Cr 2 O 7 O 2 N NO 2 HO NH 2 Ca. 40 kg of solid waste per kg phloroglucinol HO OH aq. HCl DT - CO 2 NO 2 TNT NH 2 Fe/HCl H 2 SO 4 / SO 3 NO 2 H 2 N OH phloroglucinol OH + Cr 2 (SO 4)3 + 2 KHSO 4 + 9 Fe. Cl 2 + 3 NH 4 Cl + CO 2 + 8 H 2 O 392 272 1143 160 44 144 OH MW = 126 product byproducts Atom Utilisation = 126/2282 = ca. 5% E Factor = ca. 40 5 “ To measure is to know ” Lord Kelvin
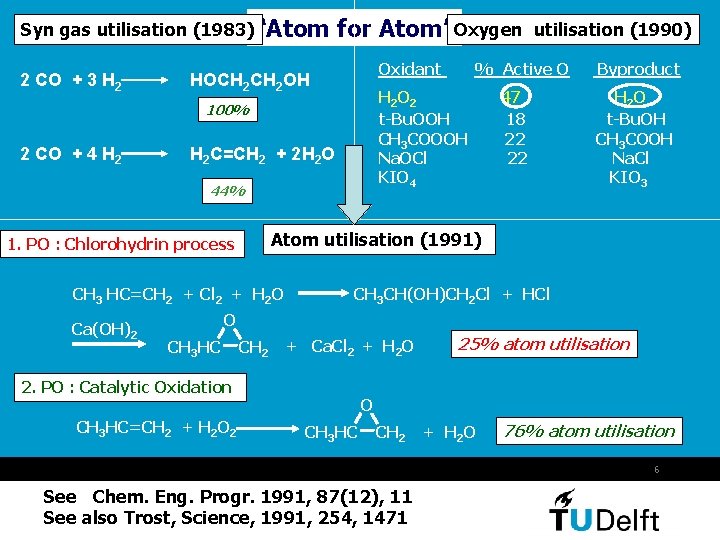
Syn gas utilisation (1983)“Atom 2 CO + 3 H 2 for Atom”Oxygen Oxidant HOCH 2 OH 2 CO + 4 H 2 C=CH 2 + 2 H 2 O 44% 47 18 22 22 Byproduct H 2 O t-Bu. OH CH 3 COOH Na. Cl KIO 3 Atom utilisation (1991) 1. PO : Chlorohydrin process CH 3 HC=CH 2 + Cl 2 + H 2 O Ca(OH)2 % Active O H 2 O 2 t-Bu. OOH CH 3 COOOH Na. OCl KIO 4 100% utilisation (1990) CH 3 CH(OH)CH 2 Cl + HCl O CH 3 HC CH 2 + Ca. Cl 2 + H 2 O 2. PO : Catalytic Oxidation CH 3 HC=CH 2 + H 2 O 2 25% atom utilisation O CH 3 HC CH 2 + H 2 O 76% atom utilisation 6 See Chem. Eng. Progr. 1991, 87(12), 11 See also Trost, Science, 1991, 254, 1471
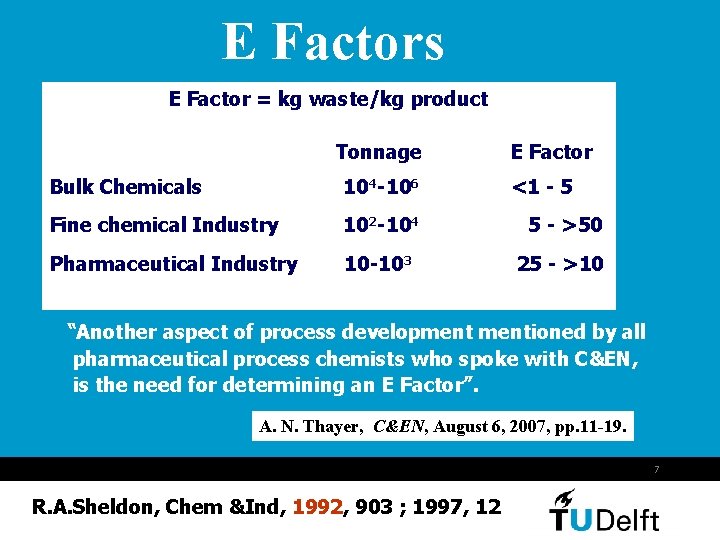
E Factors E Factor = kg waste/kg product Tonnage E Factor Bulk Chemicals 104 -106 <1 - 5 Fine chemical Industry 102 -104 5 - >50 Pharmaceutical Industry 10 -103 25 - >10 “Another aspect of process developmentioned by all pharmaceutical process chemists who spoke with C&EN, is the need for determining an E Factor”. A. N. Thayer, C&EN, August 6, 2007, pp. 11 -19. 7 R. A. Sheldon, Chem &Ind, 1992, 903 ; 1997, 12

I Factor = 4. 19 8 R. A. Sheldon, Green Chem, 2007, 9, 1273 -1283
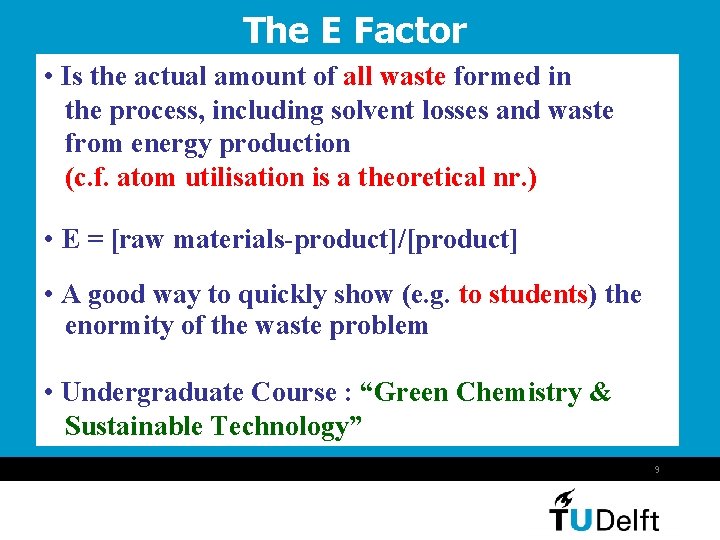
The E Factor • Is the actual amount of all waste formed in the process, including solvent losses and waste from energy production (c. f. atom utilisation is a theoretical nr. ) • E = [raw materials-product]/[product] • A good way to quickly show (e. g. to students) the enormity of the waste problem • Undergraduate Course : “Green Chemistry & Sustainable Technology” 9
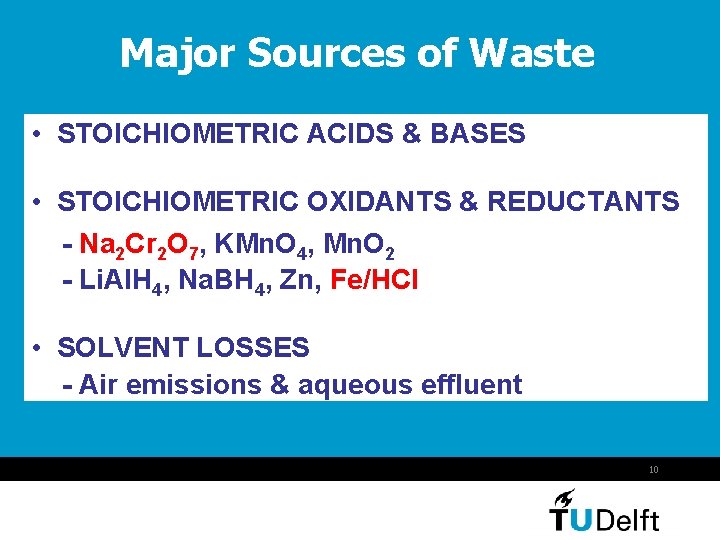
Major Sources of Waste • STOICHIOMETRIC ACIDS & BASES • STOICHIOMETRIC OXIDANTS & REDUCTANTS - Na 2 Cr 2 O 7, KMn. O 4, Mn. O 2 - Li. Al. H 4, Na. BH 4, Zn, Fe/HCl • SOLVENT LOSSES - Air emissions & aqueous effluent 10
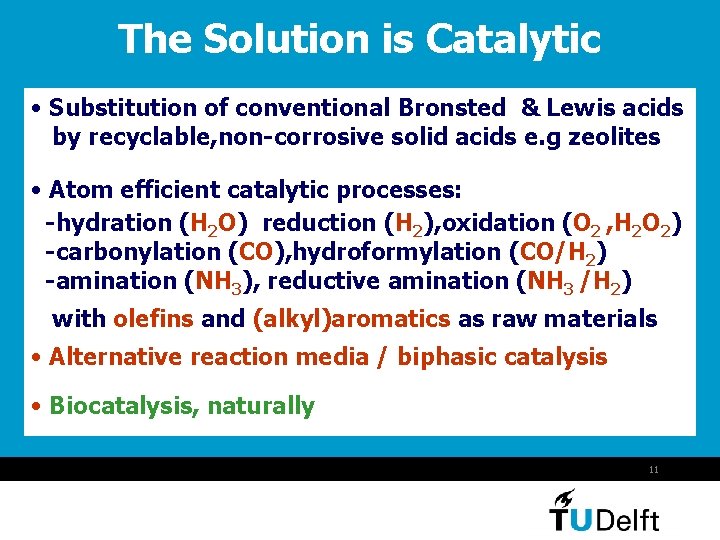
The Solution is Catalytic • Substitution of conventional Bronsted & Lewis acids by recyclable, non-corrosive solid acids e. g zeolites • Atom efficient catalytic processes: -hydration (H 2 O) reduction (H 2), oxidation (O 2 , H 2 O 2) -carbonylation (CO), hydroformylation (CO/H 2) -amination (NH 3), reductive amination (NH 3 /H 2) with olefins and (alkyl)aromatics as raw materials • Alternative reaction media / biphasic catalysis • Biocatalysis, naturally 11
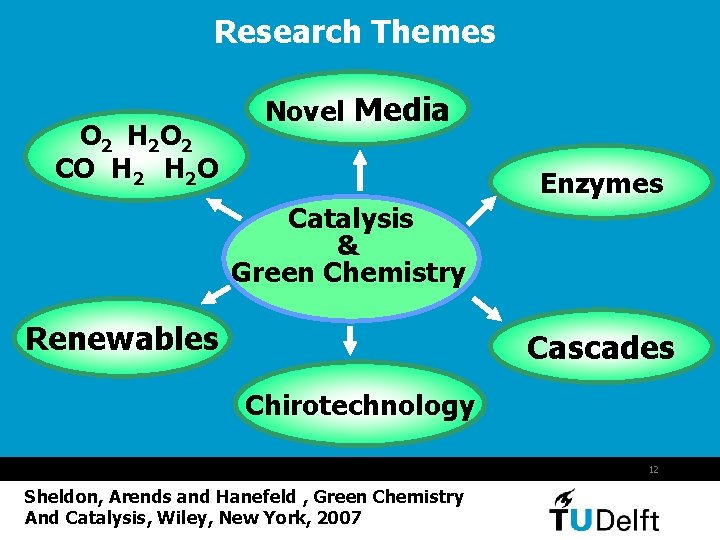
Research Themes O 2 H 2 O 2 CO H 2 O Novel Media Enzymes Catalysis & Green Chemistry Renewables Cascades Chirotechnology 12 Sheldon, Arends and Hanefeld , Green Chemistry And Catalysis, Wiley, New York, 2007

Heterogeneous Catalysis 13
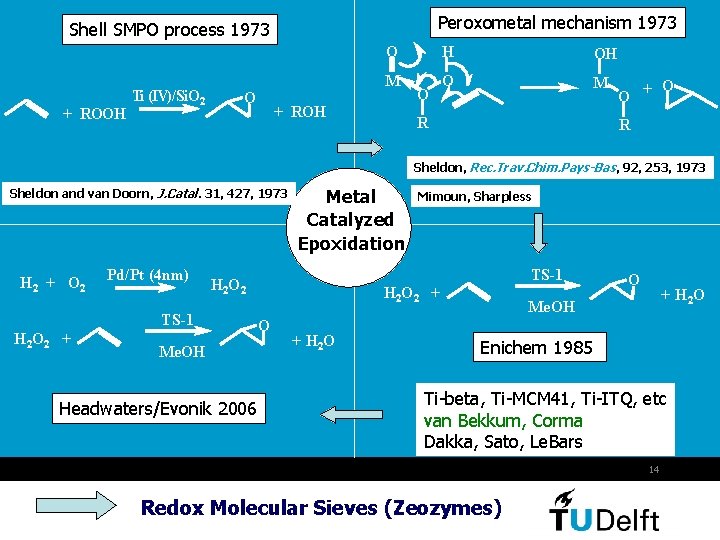
Peroxometal mechanism 1973 Shell SMPO process 1973 O + ROOH Ti (IV)/Si. O 2 M O + ROH O H OH O M R O O + R Sheldon, Rec. Trav. Chim. Pays-Bas, 92, 253, 1973 Sheldon and van Doorn, J. Catal. 31, 427, 1973 H 2 + O 2 Pd/Pt (4 nm) Me. OH Headwaters/Evonik 2006 Mimoun, Sharpless TS-1 H 2 O 2 + Metal Catalyzed Epoxidation H 2 O 2 + O + H 2 O Me. OH Enichem 1985 Ti-beta, Ti-MCM 41, Ti-ITQ, etc van Bekkum, Corma Dakka, Sato, Le. Bars 14 Redox Molecular Sieves (Zeozymes)
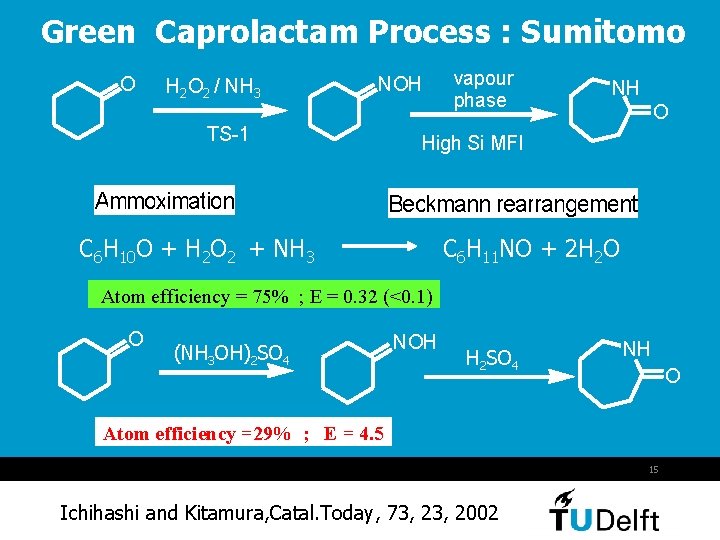
Green Caprolactam Process : Sumitomo O H 2 O 2 / NH 3 NOH TS-1 Ammoximation vapour phase NH O High Si MFI Beckmann rearrangement C 6 H 10 O + H 2 O 2 + NH 3 C 6 H 11 NO + 2 H 2 O Atom efficiency = 75% ; E = 0. 32 (<0. 1) O (NH 3 OH)2 SO 4 NOH H 2 SO 4 NH O Atom efficiency =29% ; E = 4. 5 15 Ichihashi and Kitamura, Catal. Today, 73, 2002
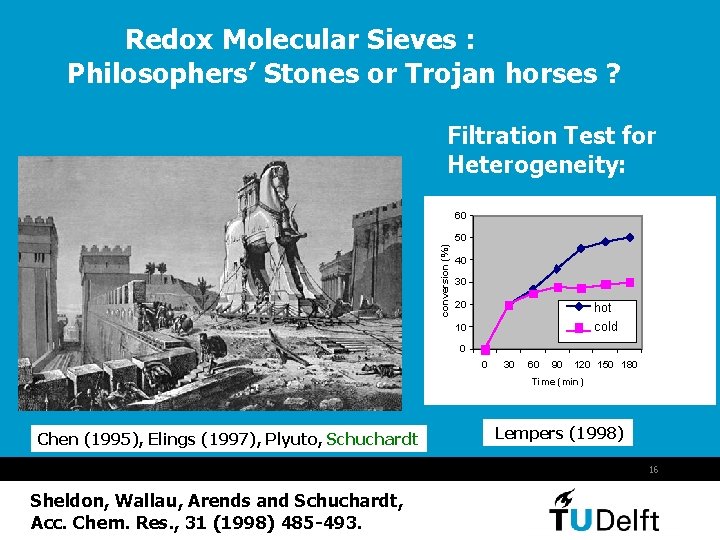
Redox Molecular Sieves : Philosophers’ Stones or Trojan horses ? Filtration Test for Heterogeneity: 60 conversion (%) 50 40 30 20 hot cold 10 0 0 30 60 90 120 150 180 Time (min) Chen (1995), Elings (1997), Plyuto, Schuchardt Lempers (1998) 16 Sheldon, Wallau, Arends and Schuchardt, Acc. Chem. Res. , 31 (1998) 485 -493.

Homogeneous Catalysis 17
Catalysis in Non-conventional Reaction Media Two challenges : • Toxicity and/or hazards of atmospheric and ground water pollution by conventional solvents • Separation/recycling of homogeneous catalysts • (Biphasic) catalysis in non-conventional media - water - supercritical carbon dioxide (Martyn Poliakoff) - ionic liquids (Ken Seddon) - fluorous biphasic (Istvan Horvath) 18 “The best solvent is no solvent”
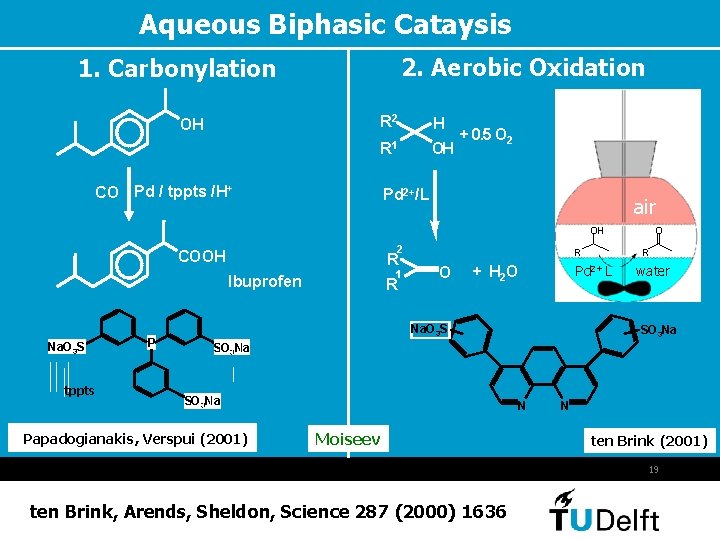
Aqueous Biphasic Cataysis 2. Aerobic Oxidation 1. Carbonylation OH R 2 H R 1 OH CO Pd / tppts /H+ + 0. 5 O 2 Pd 2+/L air OH 2 COOH R 1 Ibuprofen Na. O 3 S tppts P R R O R Pd 2+ L + H 2 O Na. O 3 S O water SO 3 Na Papadogianakis, Verspui (2001) N Moiseev N ten Brink (2001) 19 ten Brink, Arends, Sheldon, Science 287 (2000) 1636
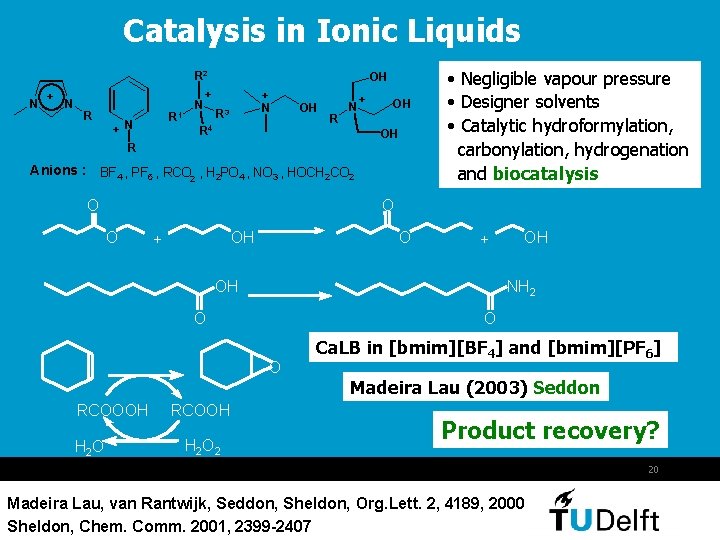
Catalysis in Ionic Liquids R 2 N + N R R 1 +N N OH + + N R 3 R 4 OH R N + OH OH R Anions : BF 4 , PF 6 , RCO 2 , H 2 PO 4 , NO 3 , HOCH 2 CO 2 O • Negligible vapour pressure • Designer solvents • Catalytic hydroformylation, carbonylation, hydrogenation and biocatalysis O O OH + OH NH 2 O O O RCOOOH H 2 O RCOOH H 2 OH Ca. LB in [bmim][BF 4] and [bmim][PF 6] Madeira Lau (2003) Seddon Product recovery? 20 Madeira Lau, van Rantwijk, Seddon, Sheldon, Org. Lett. 2, 4189, 2000 Sheldon, Chem. Comm. 2001, 2399 -2407
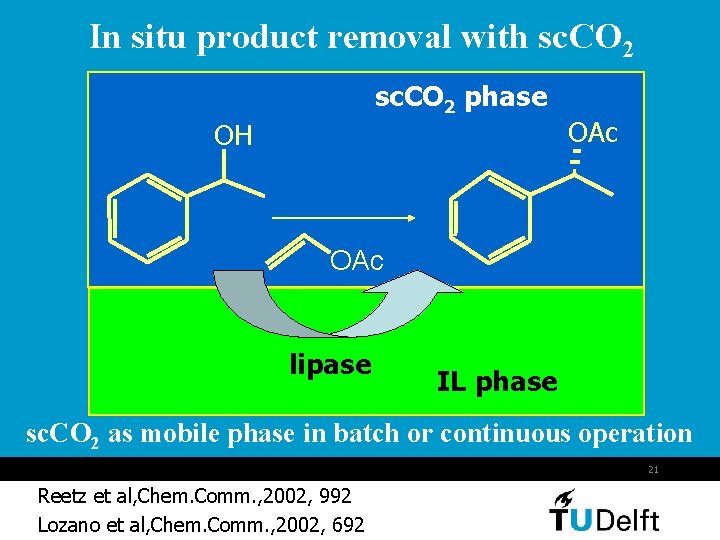
In situ product removal with sc. CO 2 phase OH OAc lipase IL phase sc. CO 2 as mobile phase in batch or continuous operation 21 Reetz et al, Chem. Comm. , 2002, 992 Lozano et al, Chem. Comm. , 2002, 692
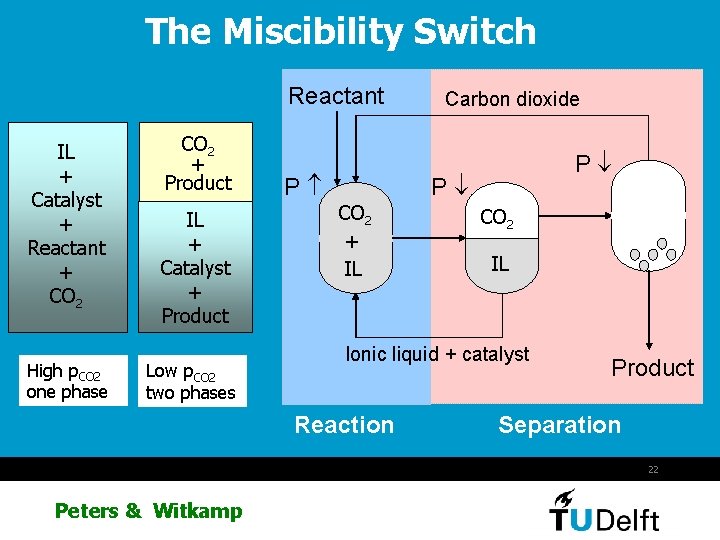
The Miscibility Switch Reactant IL + Catalyst + Reactant + CO 2 High p. CO 2 one phase CO 2 + Product IL + Catalyst + Product Low p. CO 2 two phases P CO 2 + IL Carbon dioxide P P CO 2 IL Ionic liquid + catalyst Reaction Product Separation 22 Peters & Witkamp

Organocatalysis 23
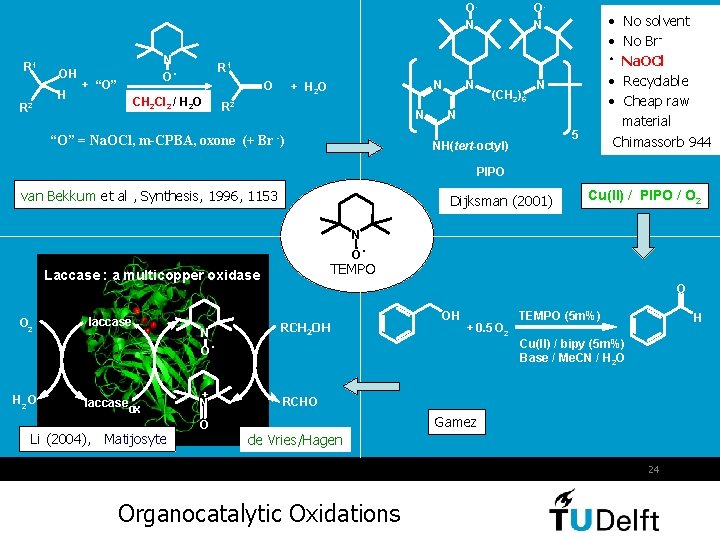
O. N R 1 R 2 OH H N O. + “O” O. N • No solvent • No Br • Na. OCl • Recyclable • Cheap raw material Chimassorb 944 R 1 O CH 2 Cl 2 / H 2 O N + H 2 O R 2 N “O” = Na. OCl, m-CPBA, oxone (+ Br -) N (CH 2)6 N N 5 NH(tert-octyl) PIPO van Bekkum et al , Synthesis, 1996, 1153 Dijksman (2001) N O H 2 O laccase ox Li (2004), Matijosyte N. O + N O . TEMPO Laccase : a multicopper oxidase O 2 Cu(II) / PIPO / O 2 O RCH 2 OH OH + 0. 5 O 2 TEMPO (5 m%) H Cu(II) / bipy (5 m%) Base / Me. CN / H 2 O RCHO Gamez de Vries/Hagen 24 Organocatalytic Oxidations
subtilisin Ca. LB CPO phytase Biocatalysis laccase HNlase 25 Inventing New Enzymes & Enzymatic Reactions
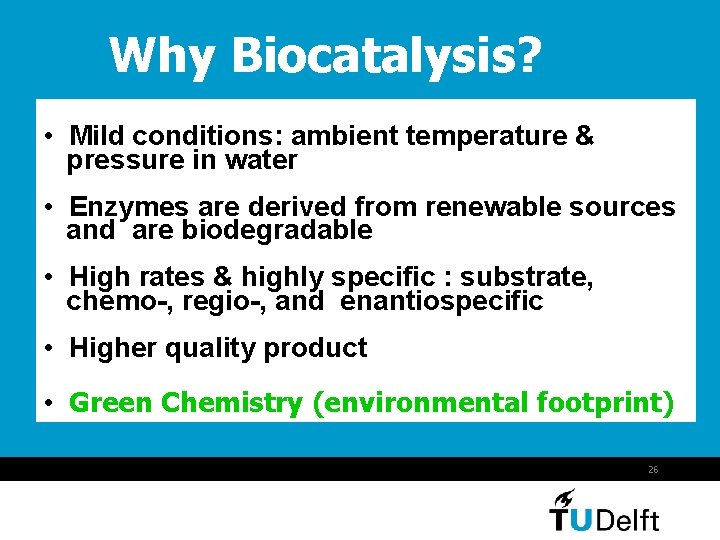
Why Biocatalysis? • Mild conditions: ambient temperature & pressure in water • Enzymes are derived from renewable sources and are biodegradable • High rates & highly specific : substrate, chemo-, regio-, and enantiospecific • Higher quality product • Green Chemistry (environmental footprint) 26
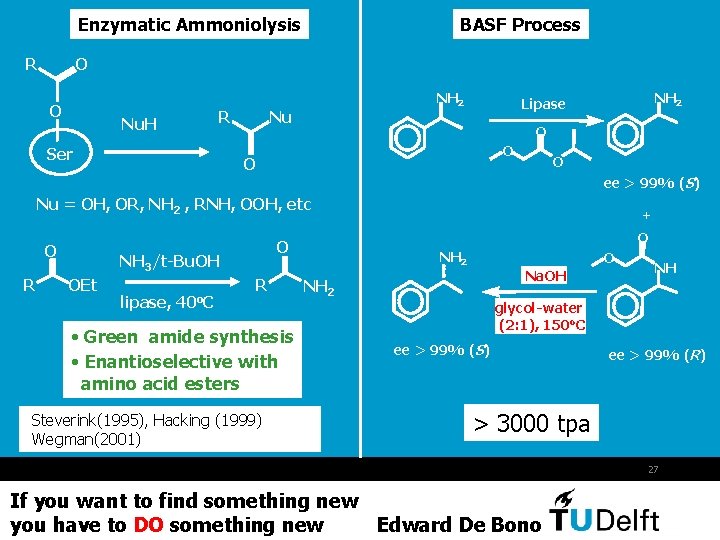
Enzymatic Ammoniolysis R BASF Process O O R Nu. H Ser NH 2 Nu NH 2 Lipase O O ee > 99% (S) Nu = OH, OR, NH 2 , RNH, OOH, etc O R OEt lipase, 40 o. C O O NH 3/t-Bu. OH R • Green amide synthesis • Enantioselective with amino acid esters Steverink(1995), Hacking (1999) Wegman(2001) + NH 2 O Na. OH NH 2 NH glycol-water (2: 1), 150 o. C ee > 99% (S) ee > 99% (R) > 3000 tpa 27 If you want to find something new you have to DO something new Edward De Bono
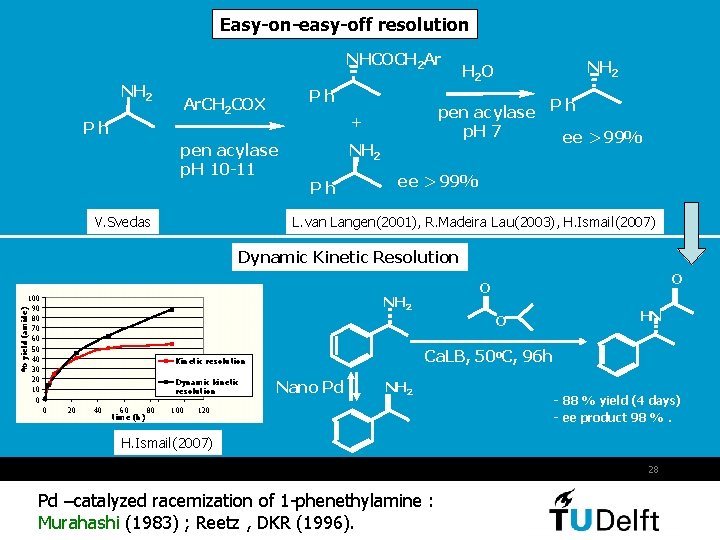
Easy-on-easy-off resolution NHCOCH 2 Ar NH 2 Ph Ar. CH 2 COX pen acylase p. H 10 -11 V. Svedas pen acylase P h p. H 7 ee > 99% + Ph NH 2 H 2 O ee > 99% L. van Langen(2001), R. Madeira Lau(2003), H. Ismail(2007) Dynamic Kinetic Resolution 100 90 80 70 60 50 40 30 20 10 0 % yield (amide) NH 2 0 20 40 60 80 time (h) 100 O HN Ca. LB, 50 o. C, 96 h Kinetic resolution Dynamic kinetic resolution O O Nano Pd NH 2 120 - 88 % yield (4 days) - ee product 98 %. H. Ismail(2007) 28 Pd –catalyzed racemization of 1 -phenethylamine : Murahashi (1983) ; Reetz , DKR (1996).
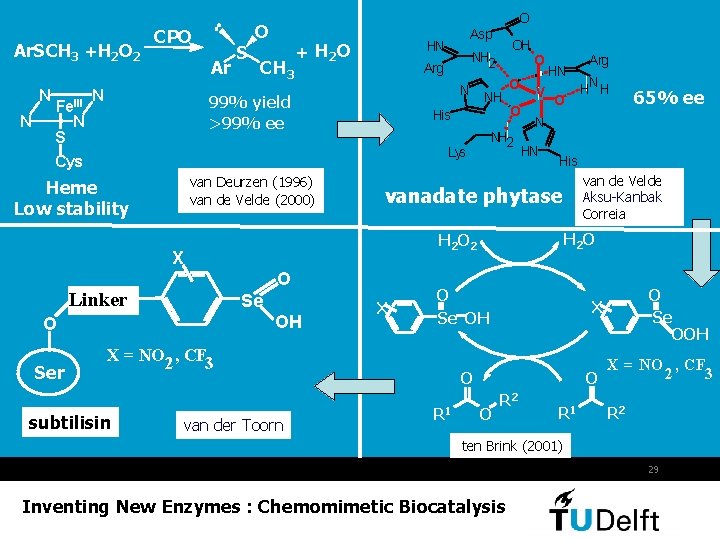
Ar. SCH 3 +H 2 O 2 N N Fe. III N S Cys CPO Ar N S O O CH 3 + H 2 O N NH His O V O HN Linker Se OH O X H 2 O van der Toorn O O O Se OOH X O R 1 65% ee van de Velde Aksu-Kanbak Correia O Se OH X = NO 2 , CF 3 N H H His H 2 O Arg N vanadate phytase X subtilisin O NH 2 Lys van Deurzen (1996) van de Velde (2000) OH NH 2 Arg 99% yield >99% ee Heme Low stability Ser Asp HN R 2 R 1 X = NO , CF 2 3 R 2 ten Brink (2001) 29 Inventing New Enzymes : Chemomimetic Biocatalysis
Historically: Adapt Process to fit Catalyst Available Catalyst Dream Process 30
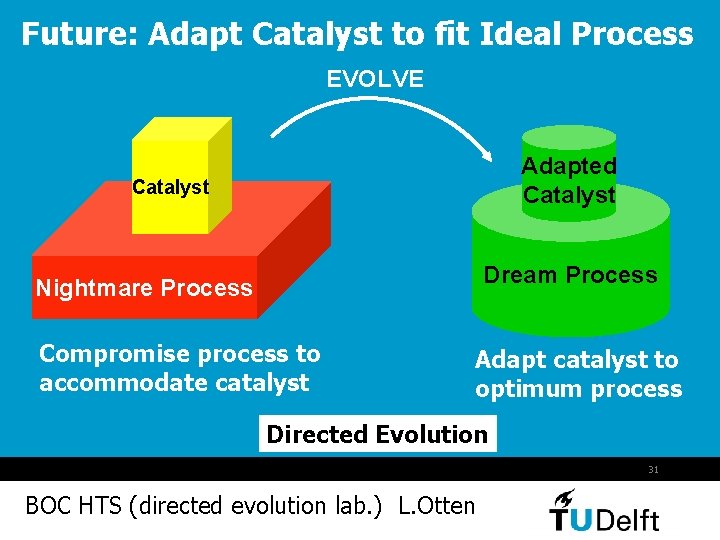
Future: Adapt Catalyst to fit Ideal Process EVOLVE Adapted Catalyst Dream Process Nightmare Process Compromise process to accommodate catalyst Adapt catalyst to optimum process Directed Evolution 31 BOC HTS (directed evolution lab. ) L. Otten
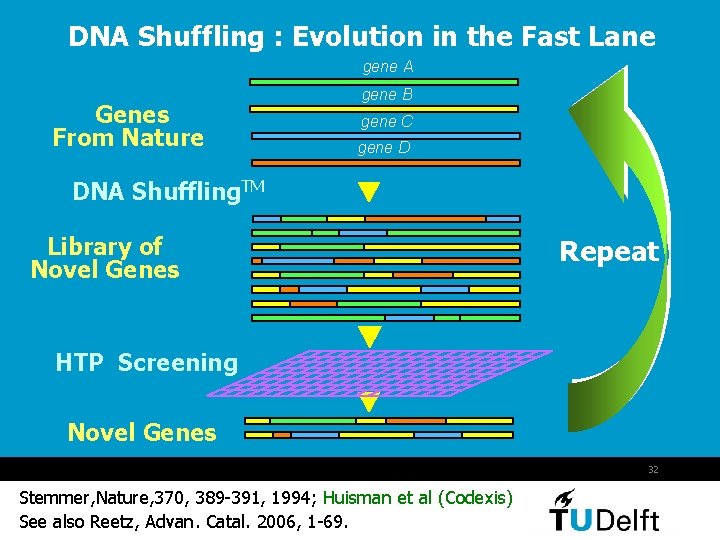
DNA Shuffling : Evolution in the Fast Lane gene A Genes From Nature gene B gene C gene D DNA Shuffling. TM Library of Novel Genes Repeat HTP Screening Novel Genes 32 Stemmer, Nature, 370, 389 -391, 1994; Huisman et al (Codexis) See also Reetz, Advan. Catal. 2006, 1 -69.
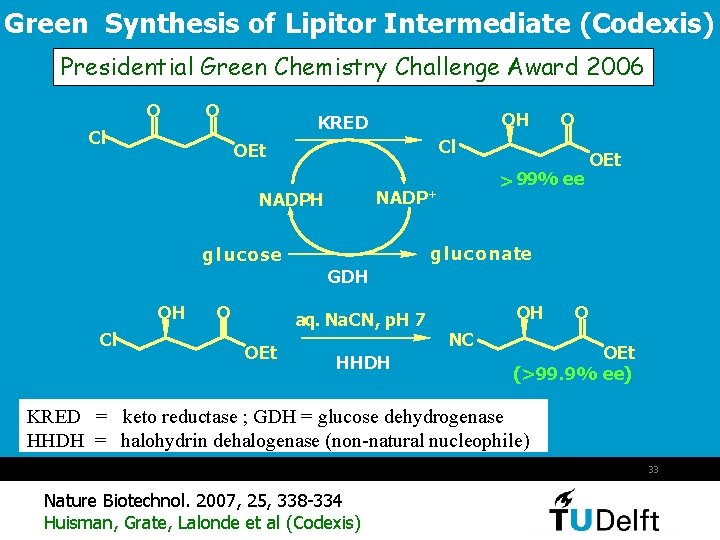
Green Synthesis of Lipitor Intermediate (Codexis) Presidential Green Chemistry Challenge Award 2006 O O Cl OH KRED O Cl OEt > 99% ee NADP+ NADPH OEt gluc o nate g luc o se GDH OH Cl O aq. Na. CN, p. H 7 OEt HHDH OH NC O OEt (>99. 9% ee) KRED = keto reductase ; GDH = glucose dehydrogenase HHDH = halohydrin dehalogenase (non-natural nucleophile) 33 Nature Biotechnol. 2007, 25, 338 -334 Huisman, Grate, Lalonde et al (Codexis)
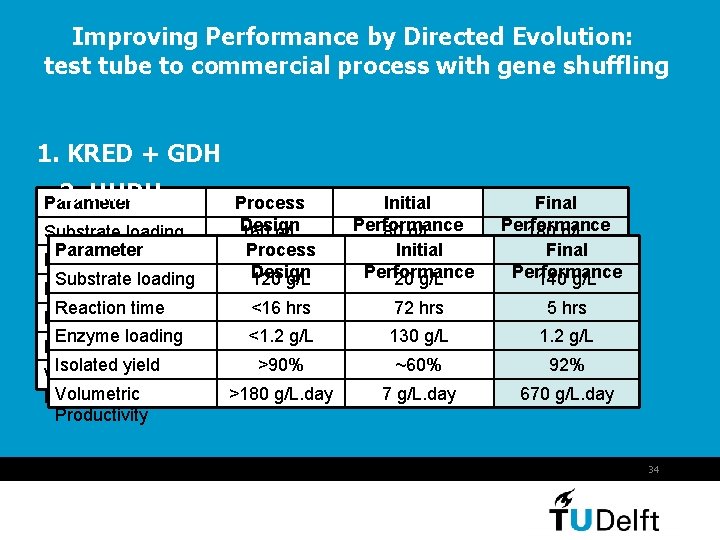
Improving Performance by Directed Evolution: test tube to commercial process with gene shuffling 1. KRED + GDH 2. HHDH Parameter Substrate loading Parameter Reaction time Substrate loading Enzyme loading Reaction time Isolated yield Enzyme loading Phase separation Isolated yield time Volumetric Productivity Process Design 160 g/L Process <16 hrs Design 120 g/L <16 hrs >90% <1. 2 g/L >90% >240 g/L. day >180 g/L. day Initial Performance 80 g/L Initial 24 hrs Performance 20 g/L 10 g/L 72 hrs ~80% 130 g/L >1 hr ~60% 80 g/L. day 7 g/L. day Final Performance 180 g/L Final 8 hrs Performance 140 g/L 0. 7 g/L 5 hrs 97% 1. 2 g/L ~1 min. 92% 540 g/L. day 670 g/L. day 34
Disadvantages of Enzymes • Low operational stability & shelf-life • Cumbersome recovery & re-use (batch vs continuous operation) • Product contamination Solution : Immobilization 35
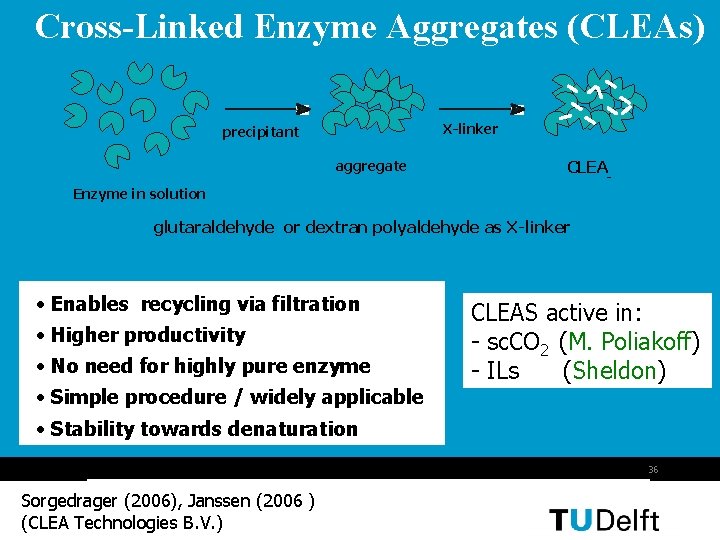
Cross-Linked Enzyme Aggregates (CLEAs) X-linker precipitant aggregate CLEA - Enzyme in solution glutaraldehyde or dextran polyaldehyde as X-linker • Enables recycling via filtration • Higher productivity • No need for highly pure enzyme CLEAS active in: - sc. CO 2 (M. Poliakoff) - ILs (Sheldon) • Simple procedure / widely applicable • Stability towards denaturation 36 Sorgedrager (2006), Janssen (2006 ) (CLEA Technologies B. V. )
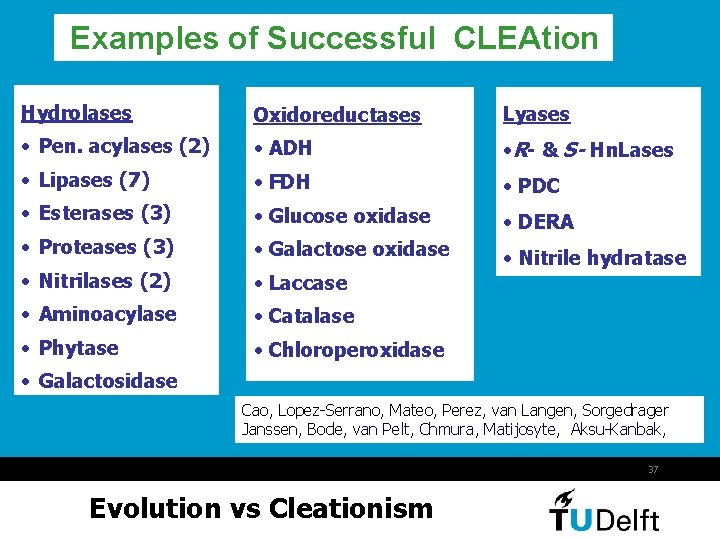
Examples of Successful CLEAtion Hydrolases Oxidoreductases Lyases • Pen. acylases (2) • ADH • R- & S- Hn. Lases • Lipases (7) • FDH • PDC • Esterases (3) • Glucose oxidase • DERA • Proteases (3) • Galactose oxidase • Nitrilases (2) • Nitrile hydratase • Laccase • Aminoacylase • Catalase • Phytase • Chloroperoxidase • Galactosidase Cao, Lopez-Serrano, Mateo, Perez, van Langen, Sorgedrager Janssen, Bode, van Pelt, Chmura, Matijosyte, Aksu-Kanbak, 37 Evolution vs Cleationism
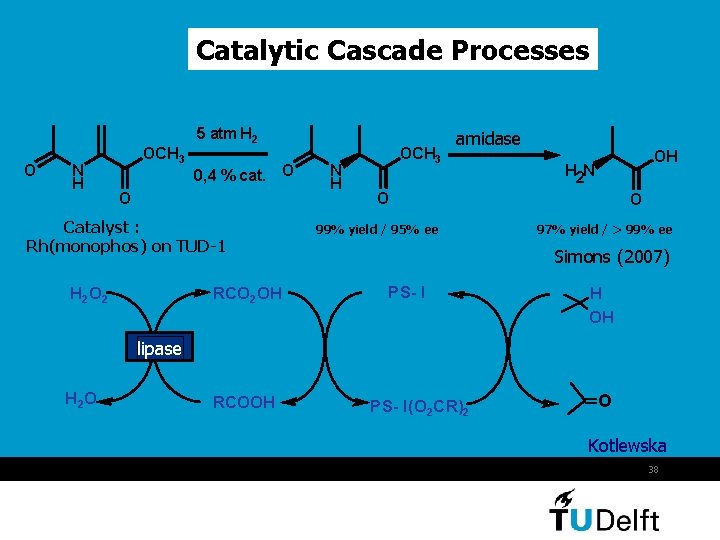
Catalytic Cascade Processes O N H OCH 3 5 atm H 2 0, 4 % cat. O O Catalyst : Rh(monophos) on TUD-1 H 2 O 2 RCO 2 OH N H OCH 3 amidase OH H 2 N O 99% yield / 95% ee O 97% yield / > 99% ee Simons (2007) PS- I H OH lipase H 2 O RCOOH PS- I(O 2 CR)2 O Kotlewska 38
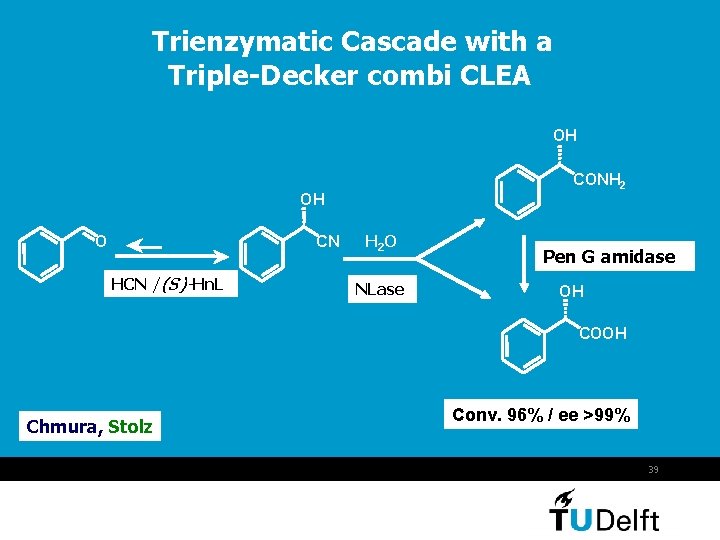
Trienzymatic Cascade with a Triple-Decker combi CLEA OH CONH 2 OH CN O HCN /(S)-Hn. L H 2 O NLase Pen G amidase OH COOH Chmura, Stolz Conv. 96% / ee >99% 39
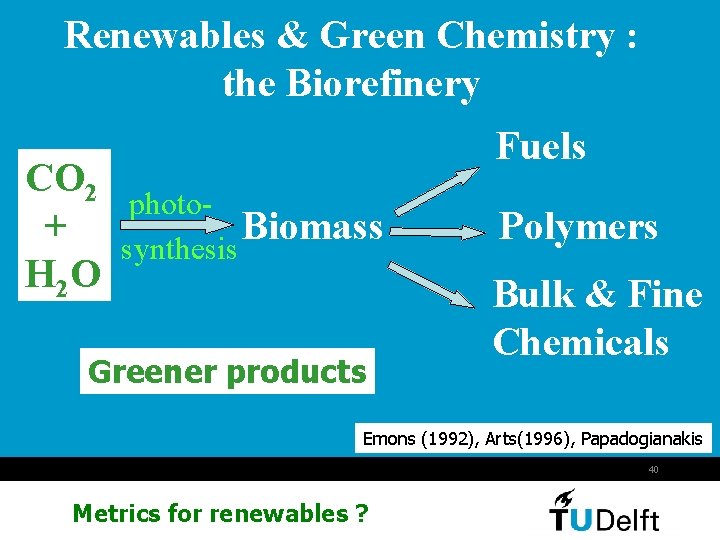
Renewables & Green Chemistry : the Biorefinery CO 2 + H 2 O Fuels photo. Biomass synthesis Greener products Polymers Bulk & Fine Chemicals Emons (1992), Arts(1996), Papadogianakis 40 Metrics for renewables ?
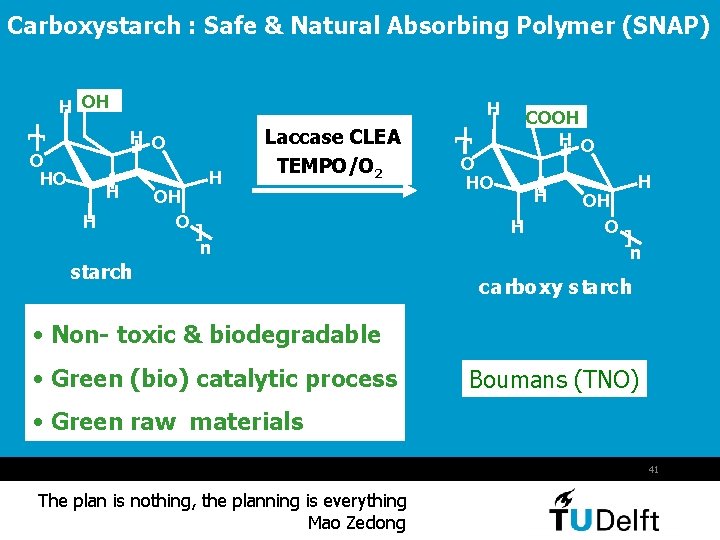
Carboxystarch : Safe & Natural Absorbing Polymer (SNAP) H OH H O HO H H OH O H Laccase CLEA TEMPO/O 2 ] n starch [ [ H O COOH H O O HO H H OH O H ] n carboxy starch • Non- toxic & biodegradable • Green (bio) catalytic process Boumans (TNO) • Green raw materials 41 The plan is nothing, the planning is everything Mao Zedong
Enantioselectivity Environment E The Think E Green Factor Elegance Economy 42 It’s better to travel one mile than to read a thousand books Confucius
43 So it goes…………

Collaboration & Funding EU IOP Katalyse IOP Koolhydraten Hoechst Celanese 44

behind every successful man there is an astonished woman 45

46

47
- Slides: 47