Aerobic Plate Count Gram Stain and Isolation Food

Aerobic Plate Count, Gram Stain, and Isolation Food Microbiology Laboratory

Aerobic Plate Count • Provides general estimate of live, aerobic, bacteria • Excludes – Obligate Anaerobes – Microaerophiles
Plate Counts • Assumption – Each colonies arises from a single bacterial cell – Bacteria like to “clump” together so some colonies may arise from more than one cell • Report as – Colony Forming Unit (CFU)/gram or ml – NOT at total bacteria
APC Results • • Evaluate Sanitation of Product Predict Shelf-life “Safety” Indicator Monitor Environment
Limitations of APC • • • Only aerobic organisms are counted Bacteria Type not known Media may not support growth of certain bacteria Eye strain/Human Error Hard to Distinguish Between food particles and bacteria • Don’t Use on Fermented Foods • Colonies may be too small to see
Types of Samples • Liquid – Non-viscous Liquids can be measured with pipet – Viscous liquids should be weighed • Solid – Aseptically weigh Sample • Sponge/Swab Collect sample by swabbing a defined area • Environmental and Container – Rinse inside of Containers – Open Plate to Collect Air Samples – RODAC Plates
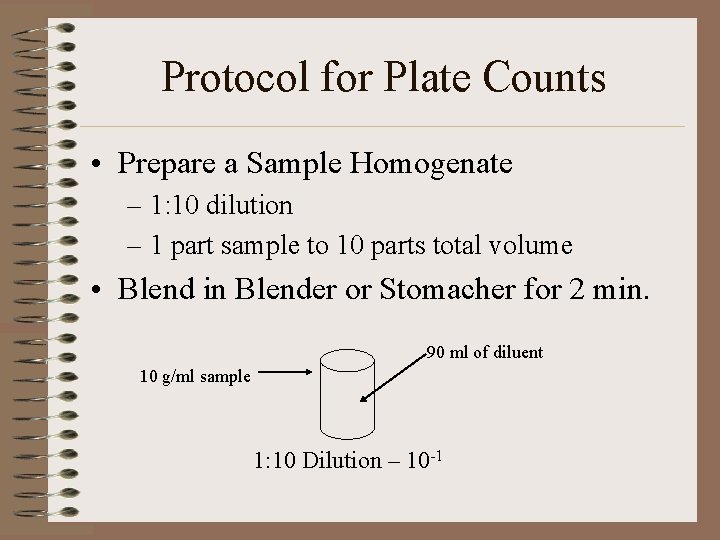
Protocol for Plate Counts • Prepare a Sample Homogenate – 1: 10 dilution – 1 part sample to 10 parts total volume • Blend in Blender or Stomacher for 2 min. 90 ml of diluent 10 g/ml sample 1: 10 Dilution – 10 -1

Formula • 10 ml/g sample, want 1: 100 dilution – 100 – 10 = 90 ml of diluent needed • Start with Different Sample Sizes – 50 g sample • Must have 500 g total volume for 1: 10 • 500 – 50 = 450 ml diluent needed – 95 ml sample • Must have 950 total volume for 1: 10 • 950 – 95 = 855 ml of diluent

Plate Count Protocol • Prepare Serial Dilutions – Dilute to a level where you will get countable colonies on plates – Use a NEW STERILE PIPET between each dilution – Place pipet tip down in pipet tanks • Shake each dilution bottle 25 times in a 90 degree arc within 7 seconds. • Phosphate Buffer or Peptone Buffer to Dilute
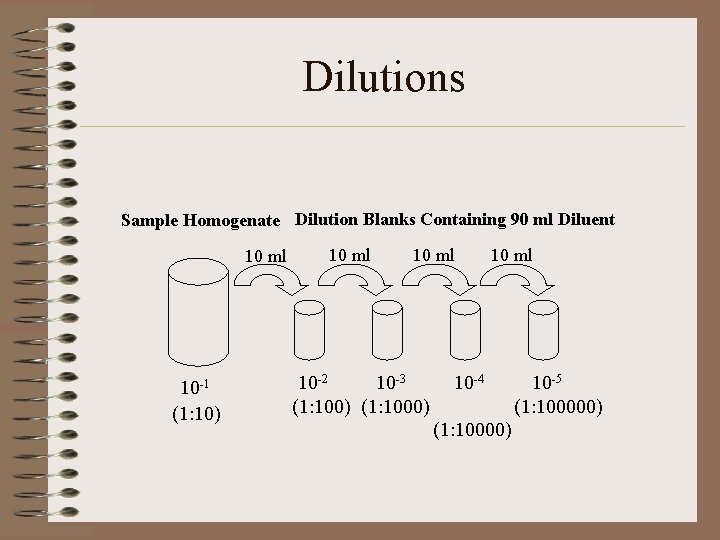
Dilutions Sample Homogenate Dilution Blanks Containing 90 ml Diluent 10 ml 10 -1 (1: 10) 10 ml 10 -2 10 -3 (1: 100) (1: 1000) 10 ml 10 -4 (1: 10000) 10 -5 (1: 100000)
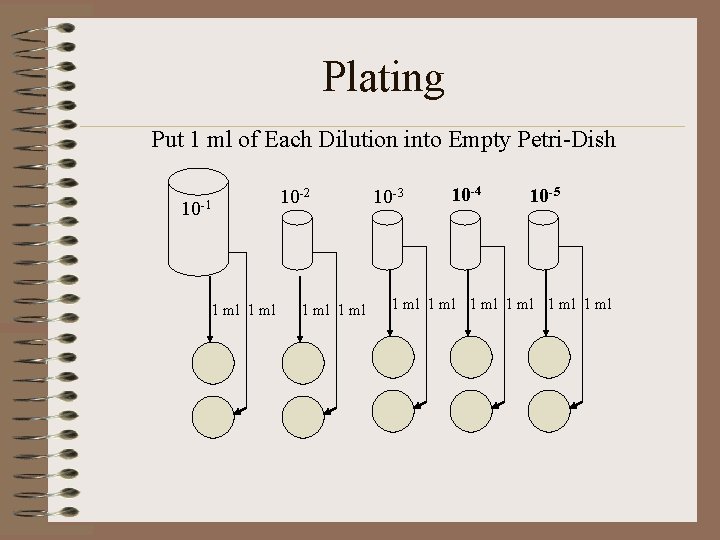
Plating Put 1 ml of Each Dilution into Empty Petri-Dish 10 -1 1 ml 10 -2 1 ml 10 -3 10 -4 10 -5 1 ml 1 ml
APC – Protocol • Add 18 -20 ml of tempered (45 -50 F), molten plate count agar to the petri dish. – Agar MUST be tempered or the bacteria will be killed by heat • • Standard Methods or Plate Count Agar Swirl 10 times in each direction Allow to Solidify Incubate inverted at 35 -37 C for 48 hours

Sterilization • Equipment and Media MUST be Sterile • Hot Air Sterilization – 170 C for 1 hour • Equipment Temperature • Put in oven for 2 hours • Wrap in paper, foil, etc. • Steam Sterilization – 121 C for 15 min. MUST have 15 psi pressure • Liquid Media or Equipment • Don’t Put Lids on tightly

Counting Plates • Only count plates with 25 -250 colonies • More than 250 – Too Numerous To Count – TNTC • Less than 25 – Too Few to Count - TFTC
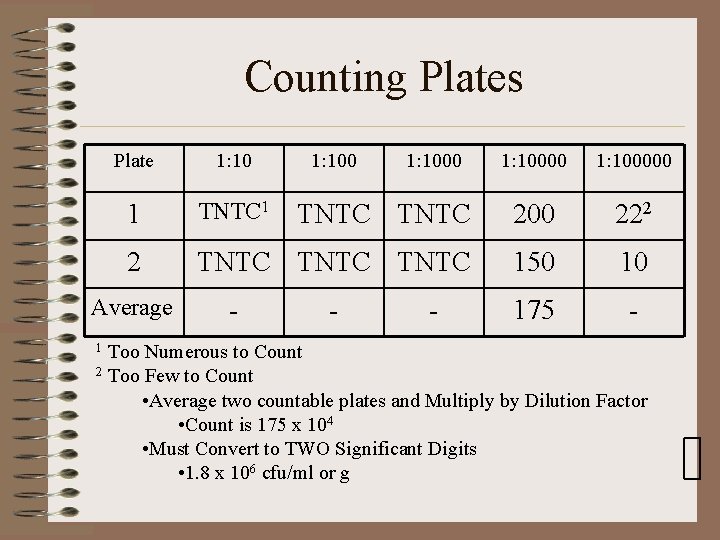
Counting Plates Plate 1: 10 1 TNTC 1 2 Average 1: 100000 TNTC 200 222 TNTC 150 10 175 - - 1: 1000 - Too Numerous to Count 2 Too Few to Count • Average two countable plates and Multiply by Dilution Factor • Count is 175 x 104 • Must Convert to TWO Significant Digits • 1. 8 x 106 cfu/ml or g 1
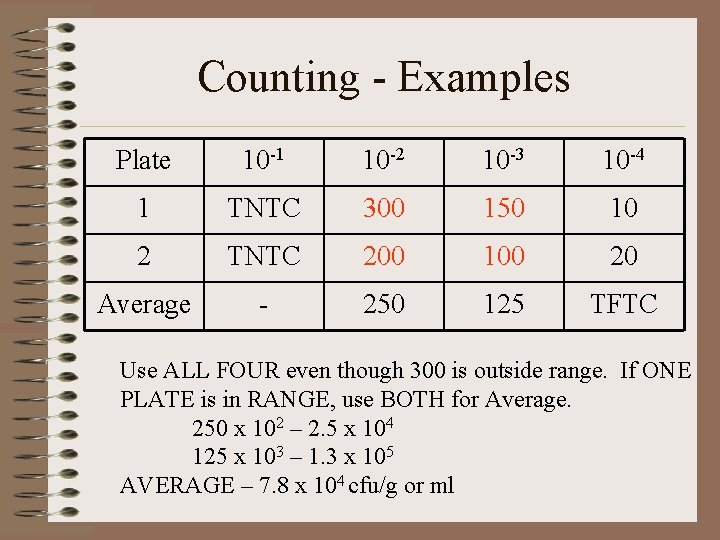
Counting - Examples Plate 10 -1 10 -2 10 -3 10 -4 1 TNTC 300 150 10 2 TNTC 200 100 20 Average - 250 125 TFTC Use ALL FOUR even though 300 is outside range. If ONE PLATE is in RANGE, use BOTH for Average. 250 x 102 – 2. 5 x 104 125 x 103 – 1. 3 x 105 AVERAGE – 7. 8 x 104 cfu/g or ml
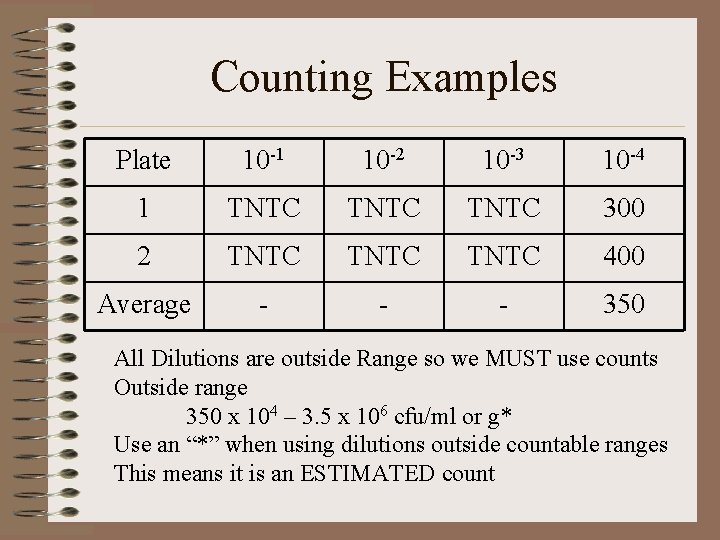
Counting Examples Plate 10 -1 10 -2 10 -3 10 -4 1 TNTC 300 2 TNTC 400 Average - - - 350 All Dilutions are outside Range so we MUST use counts Outside range 350 x 104 – 3. 5 x 106 cfu/ml or g* Use an “*” when using dilutions outside countable ranges This means it is an ESTIMATED count
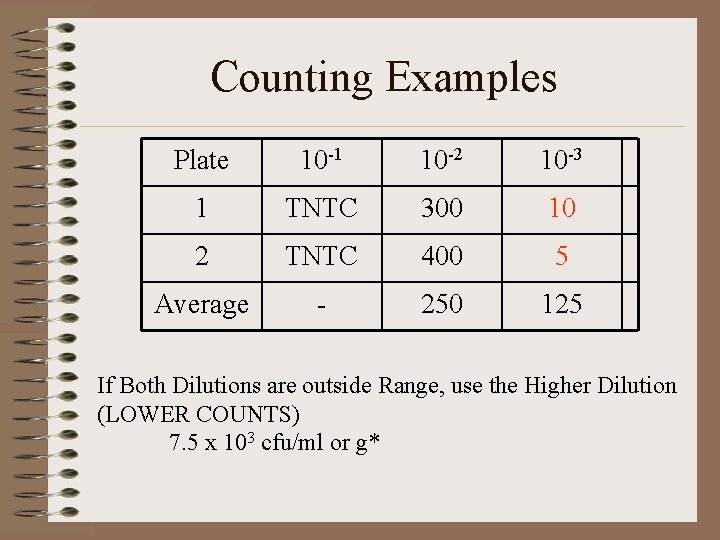
Counting Examples Plate 10 -1 10 -2 10 -3 1 TNTC 300 10 2 TNTC 400 5 Average - 250 125 If Both Dilutions are outside Range, use the Higher Dilution (LOWER COUNTS) 7. 5 x 103 cfu/ml or g*
Overloaded Plates • Use Highest Dilution and Use Grid on Colony Counter – 1 Grid = 1 cm 2 – A standard Plastic Plate has 56 cm 2 surface area • If <10 colonies/cm 2, count 12 squares (6 consecutive horizontally and 6 consecutive vertically) – Total and Divide by 12 (average). Multiply by 56 to get total colonies on plate. Report as Estimate • If >10 colonies/cm 2 – Count 4 squares, average and multiply by 56
APC Variations • Psychrotrophic – Incubate at 5 -7 C for 10 days – Use Pre-poured Plates • Thermoduric – Hold 5 ml liquid sample or 1: 10 diluent of solid sample in 60 -80 C water bath for 30 min – Cool on ice for 10 min – Plate and incubate
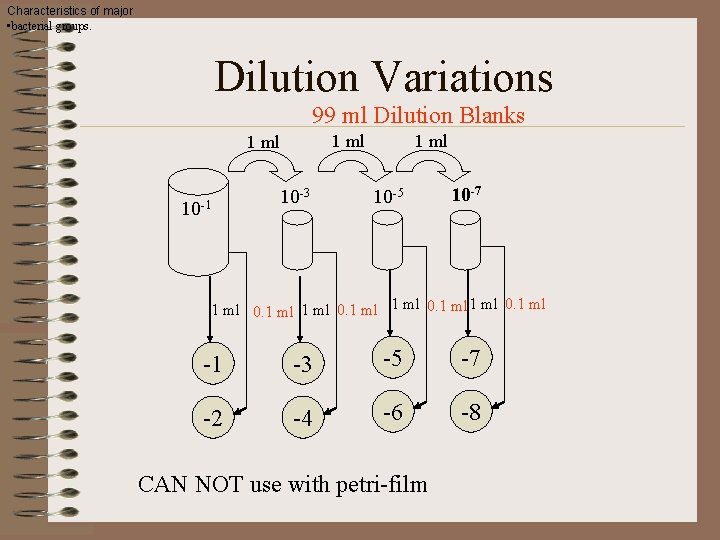
Characteristics of major • bacterial groups. Dilution Variations 99 ml Dilution Blanks 1 ml 10 -1 10 -3 1 ml 10 -5 10 -7 1 ml 0. 1 ml -1 -3 -5 -7 -2 -4 -6 -8 CAN NOT use with petri-film
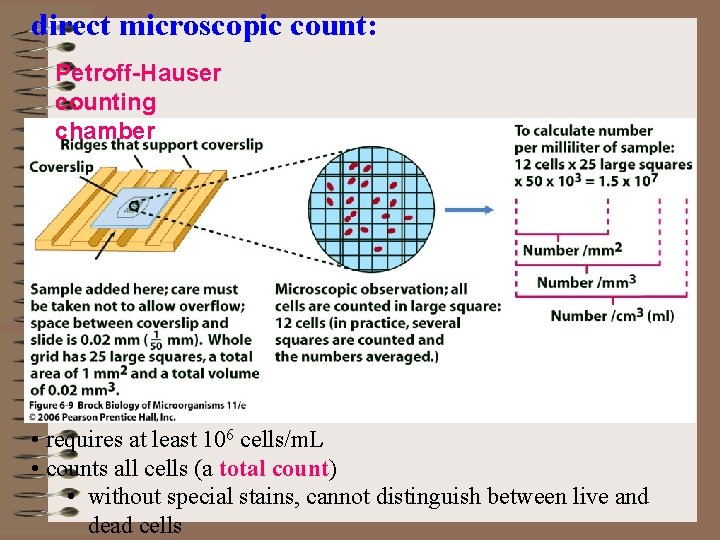
direct microscopic count: Petroff-Hauser counting chamber • requires at least 106 cells/m. L • counts all cells (a total count) • without special stains, cannot distinguish between live and dead cells

viable count: • only those cells that are viable and able to grow and form colonies under the provided conditions are counted • results reported as colony forming units/m. L (CFU/m. L)
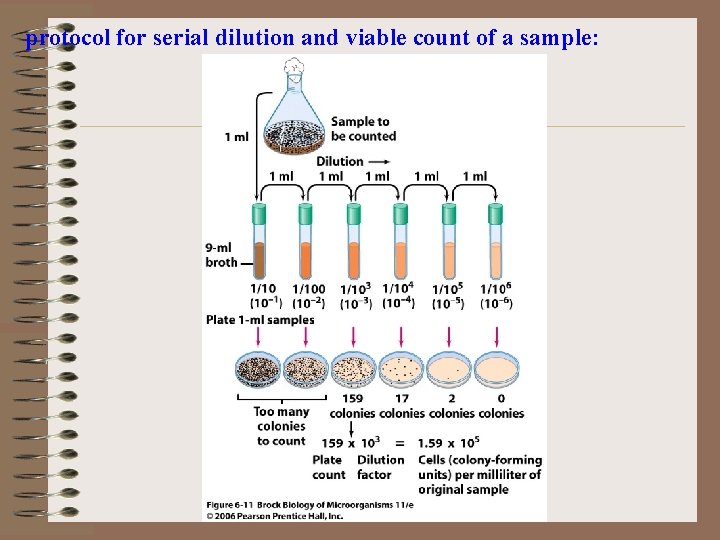
protocol for serial dilution and viable count of a sample:
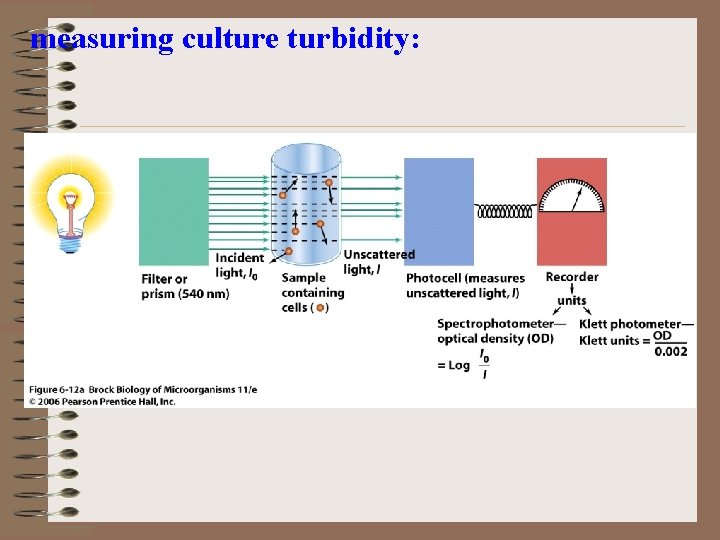
measuring culture turbidity:
- Slides: 25