Adverse Event Is it Serious Record in CRF
- Slides: 2

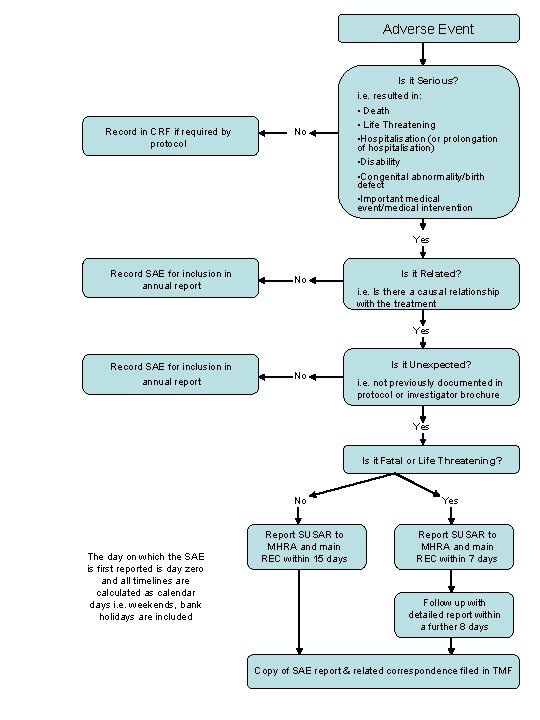
Adverse Event Is it Serious? Record in CRF if required by protocol No i. e. resulted in: • Death • Life Threatening • Hospitalisation (or prolongation of hospitalisation) • Disability • Congenital abnormality/birth defect • Important medical event/medical intervention Yes Record SAE for inclusion in annual report No Is it Related? i. e. Is there a causal relationship with the treatment Yes Record SAE for inclusion in annual report Is it Unexpected? No i. e. not previously documented in protocol or investigator brochure Yes Is it Fatal or Life Threatening? No The day on which the SAE is first reported is day zero and all timelines are calculated as calendar days i. e. weekends, bank holidays are included Report SUSAR to MHRA and main REC within 15 days Yes Report SUSAR to MHRA and main REC within 7 days Follow up with detailed report within a further 8 days Copy of SAE report & related correspondence filed in TMF

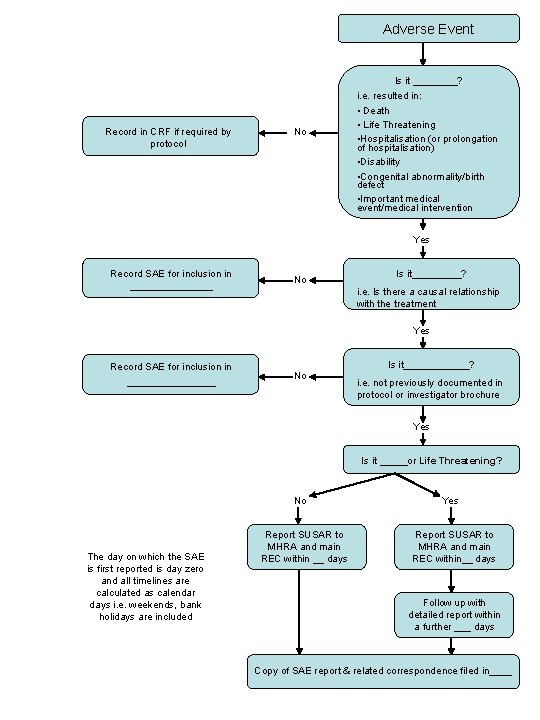
Adverse Event Is it ____? Record in CRF if required by protocol No i. e. resulted in: • Death • Life Threatening • Hospitalisation (or prolongation of hospitalisation) • Disability • Congenital abnormality/birth defect • Important medical event/medical intervention Yes Record SAE for inclusion in ________ No Is it_____? i. e. Is there a causal relationship with the treatment Yes Record SAE for inclusion in ________ Is it______? No i. e. not previously documented in protocol or investigator brochure Yes Is it _____or Life Threatening? No The day on which the SAE is first reported is day zero and all timelines are calculated as calendar days i. e. weekends, bank holidays are included Report SUSAR to MHRA and main REC within __ days Yes Report SUSAR to MHRA and main REC within__ days Follow up with detailed report within a further ___ days Copy of SAE report & related correspondence filed in____