Advancing Care Across the Spectrum of Pancreatic Cancer









- Slides: 29

Advancing Care Across the Spectrum of Pancreatic Cancer Moderator Miguel Hidalgo Medina, MD, Ph. D Professor of Medicine, Harvard Medical School Chief, Division of Hematology/Oncology Beth Israel Deaconess Medical Center Deputy Associate Director Dana Farber Cancer Center Boston, Massachusetts

Panelists Johanna Bendell, MD Chief Development Officer Director Drug Development Unit Sarah Cannon Research Institute Tennessee Oncology Nashville, Tennessee Michael Pishvaian, MD Co-Director Pancreatic Cancer Program Director Phase I Clinical Research Program Georgetown Lombardi Comprehensive Cancer Center Washington, DC

This program will include a discussion of off-label treatment and investigational agents not approved by the FDA for use in the United States and data that were presented in abstract form. These data should be considered preliminary until published in a peer-reviewed journal.

Pancreatic Cancer • Pancreatic cancer is an increasingly common malignancy with a high mortality rate – 55, 440 new cases expected in 2018 in the United States – 44, 330 expected deaths • By 2030, pancreatic cancer will be the second most common cause of cancer death • Most patients are diagnosed in advanced stages and > 80% are inoperable at time of diagnosis ACS. Cancer facts and figures 2018.

Current Treatment for Pancreatic Cancer Is Insufficient • Surgery, chemotherapy, and radiation are the primary treatment options for pancreatic cancer • Treatments are noncurative, and survival remains low – 5 -year survival: 8% • Targeted therapies have been largely unsuccessful, despite high mutation rates • Disappointing results with immunotherapy, likely because of the immunosuppressive tumor microenvironment ACS. Cancer facts and figures 2018.

First-Line Treatment of Metastatic Pancreatic Cancer

First-Line Gemcitabine • For years, gemcitabine was the standard of care for first-line treatment of advanced pancreatic cancer, according to improved survival and clinical benefit compared with 5 -FU Figure No Longer Available m. OS: 5. 7 vs 4. 4 mo (P =. 0025) 1 -y OS: 18% vs 2% Clinical benefit (pain + KPS + weight): 23. 8% vs 4. 8% (P =. 0022) Burris HA, et al. J Clin Oncol. 1997; 15: 2403 -2413. Reprinted with permission. © 1997. American Society of Clinical Oncology. All rights reserved. Burris HA, et al. J Clin Oncol. 1997; 15: 2403 -2413.

FOLFIRINOX for First-Line Treatment • Phase 3 trial comparing FOLFIRINOX (n = 171) to gemcitabine (n = 171) in patients with previously untreated metastatic pancreatic cancer Figure No Longer Available Conroy T, et al. N Engl J Med. 2011; 364: 1817 -1825. Figure No Longer Available

Phase 3 IMPACT Trial Gemcitabine + nab-Paclitaxel 861 patients with previously untreated metastatic pancreatic cancer OS R Gemcitabine + nab-paclitaxel (n = 431) Gemcitabine (n = 430) PFS Figure No Longer Available From N Engl J Med, Von Hoff DD, et al. , Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine, 369: 1691 -1703 Copyright © 2013 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society Von Hoff DD, et al. N Engl J Med. 2013; 369: 1691 -1703.

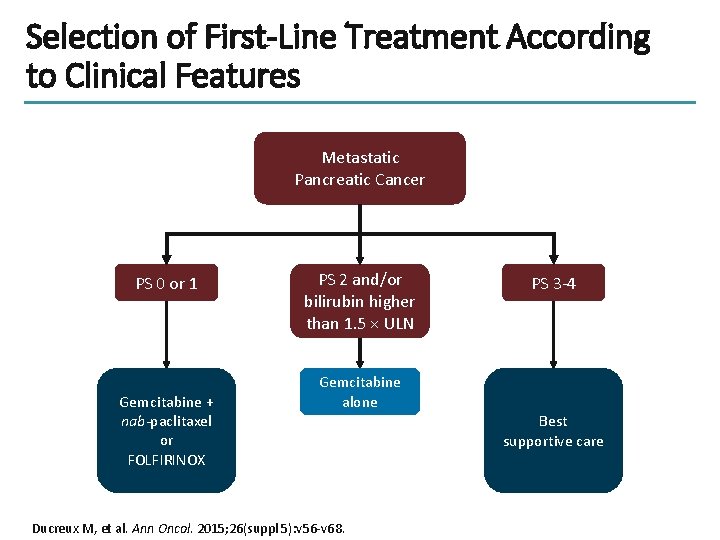
Selection of First-Line Treatment According to Clinical Features Metastatic Pancreatic Cancer PS 0 or 1 Gemcitabine + nab-paclitaxel or FOLFIRINOX PS 2 and/or bilirubin higher than 1. 5 × ULN PS 3 -4 Gemcitabine alone Ducreux M, et al. Ann Oncol. 2015; 26(suppl 5): v 56 -v 68. Best supportive care

Second-Line Treatment of Metastatic Pancreatic Cancer

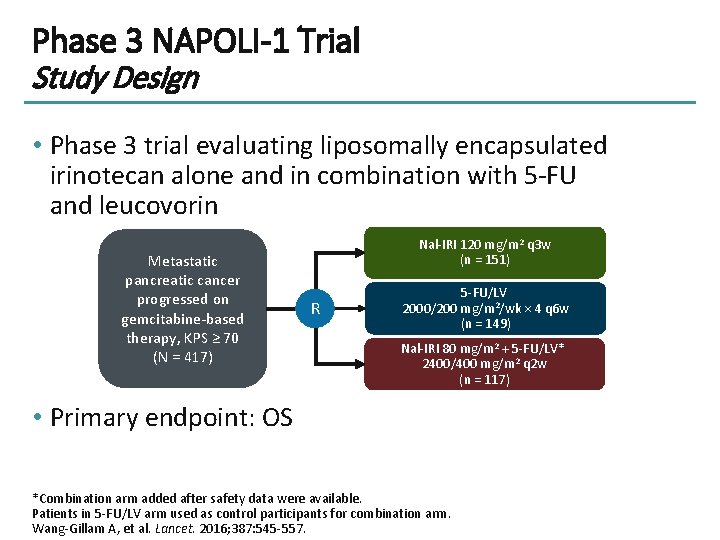
Phase 3 NAPOLI-1 Trial Study Design • Phase 3 trial evaluating liposomally encapsulated irinotecan alone and in combination with 5 -FU and leucovorin Metastatic pancreatic cancer progressed on gemcitabine-based therapy, KPS ≥ 70 (N = 417) Nal-IRI 120 mg/m 2 q 3 w (n = 151) R 5 -FU/LV 2000/200 mg/m 2/wk × 4 q 6 w (n = 149) Nal-IRI 80 mg/m 2 + 5 -FU/LV* 2400/400 mg/m 2 q 2 w (n = 117) • Primary endpoint: OS *Combination arm added after safety data were available. Patients in 5 -FU/LV arm used as control participants for combination arm. Wang-Gillam A, et al. Lancet. 2016; 387: 545 -557.

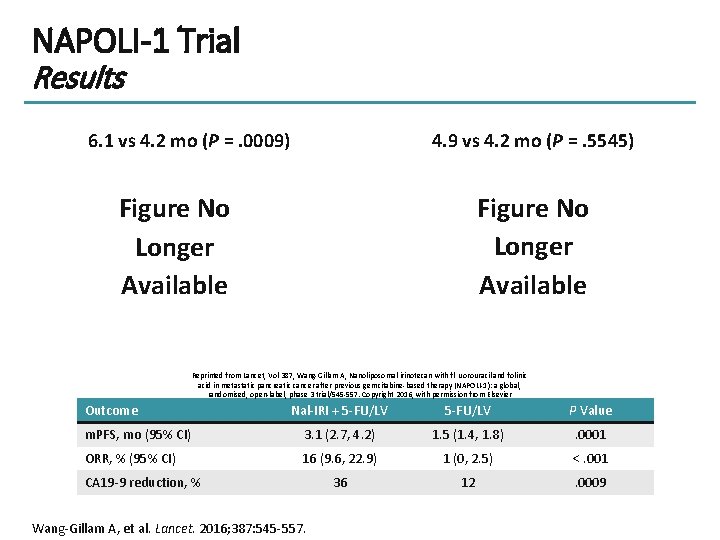
NAPOLI-1 Trial Results 6. 1 vs 4. 2 mo (P =. 0009) 4. 9 vs 4. 2 mo (P =. 5545) Figure No Longer Available Reprinted from Lancet, Vol 387, Wang-Gillam A, Nanoliposomal irinotecan with fl uorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial/545 -557. Copyright 2016, with permission from Elsevier Outcome Nal-IRI + 5 -FU/LV P Value m. PFS, mo (95% CI) 3. 1 (2. 7, 4. 2) 1. 5 (1. 4, 1. 8) . 0001 ORR, % (95% CI) 16 (9. 6, 22. 9) 1 (0, 2. 5) <. 001 36 12 . 0009 CA 19 -9 reduction, % Wang-Gillam A, et al. Lancet. 2016; 387: 545 -557.

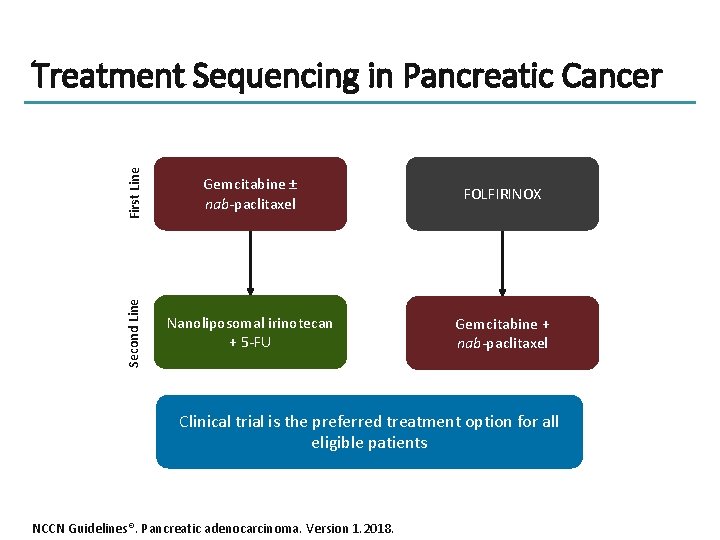
First Line Gemcitabine ± nab-paclitaxel FOLFIRINOX Second Line Treatment Sequencing in Pancreatic Cancer Nanoliposomal irinotecan + 5 -FU Gemcitabine + nab-paclitaxel Clinical trial is the preferred treatment option for all eligible patients NCCN Guidelines®. Pancreatic adenocarcinoma. Version 1. 2018.

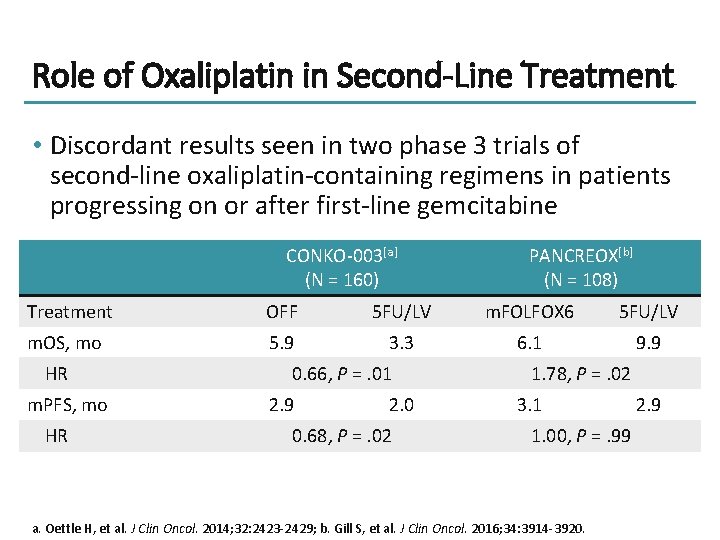
Role of Oxaliplatin in Second-Line Treatment • Discordant results seen in two phase 3 trials of second-line oxaliplatin-containing regimens in patients progressing on or after first-line gemcitabine CONKO-003[a] (N = 160) PANCREOX[b] (N = 108) Treatment OFF 5 FU/LV m. FOLFOX 6 5 FU/LV m. OS, mo 5. 9 3. 3 6. 1 9. 9 HR m. PFS, mo HR 0. 66, P =. 01 2. 9 2. 0 0. 68, P =. 02 1. 78, P =. 02 3. 1 1. 00, P =. 99 a. Oettle H, et al. J Clin Oncol. 2014; 32: 2423 -2429; b. Gill S, et al. J Clin Oncol. 2016; 34: 3914 -3920. 2. 9

Emerging Therapies for Advanced Pancreatic Cancer

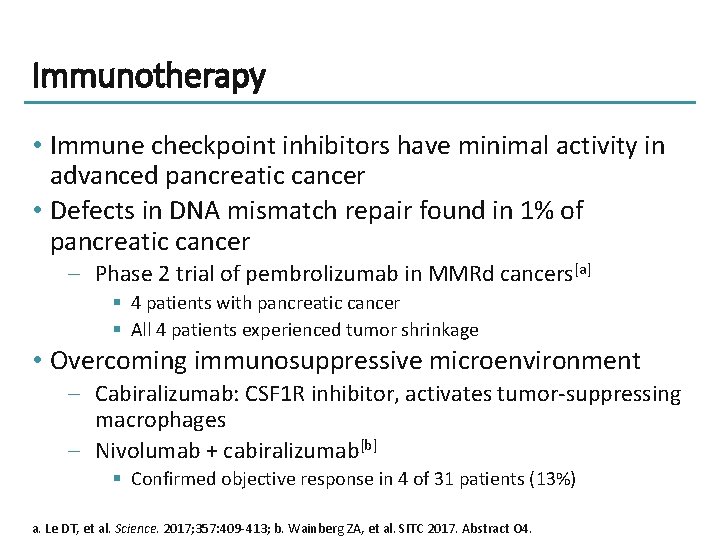
Immunotherapy • Immune checkpoint inhibitors have minimal activity in advanced pancreatic cancer • Defects in DNA mismatch repair found in 1% of pancreatic cancer – Phase 2 trial of pembrolizumab in MMRd cancers[a] § 4 patients with pancreatic cancer § All 4 patients experienced tumor shrinkage • Overcoming immunosuppressive microenvironment – Cabiralizumab: CSF 1 R inhibitor, activates tumor-suppressing macrophages – Nivolumab + cabiralizumab[b] § Confirmed objective response in 4 of 31 patients (13%) a. Le DT, et al. Science. 2017; 357: 409 -413; b. Wainberg ZA, et al. SITC 2017. Abstract O 4.

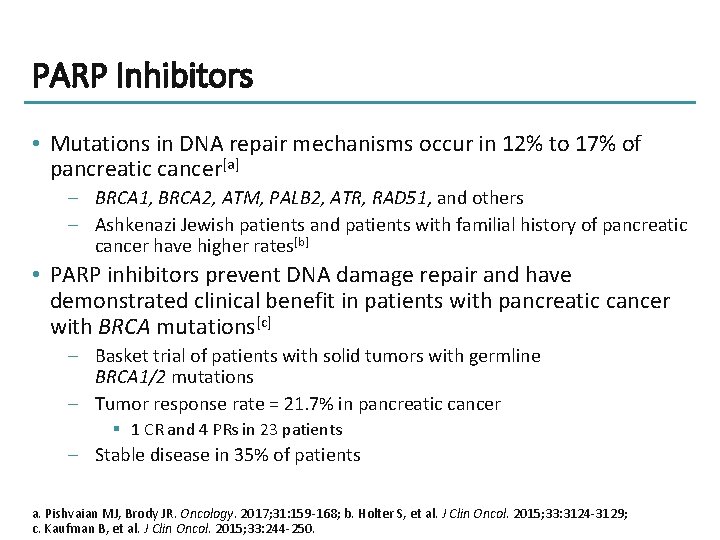
PARP Inhibitors • Mutations in DNA repair mechanisms occur in 12% to 17% of pancreatic cancer[a] – BRCA 1, BRCA 2, ATM, PALB 2, ATR, RAD 51, and others – Ashkenazi Jewish patients and patients with familial history of pancreatic cancer have higher rates[b] • PARP inhibitors prevent DNA damage repair and have demonstrated clinical benefit in patients with pancreatic cancer with BRCA mutations[c] – Basket trial of patients with solid tumors with germline BRCA 1/2 mutations – Tumor response rate = 21. 7% in pancreatic cancer § 1 CR and 4 PRs in 23 patients – Stable disease in 35% of patients a. Pishvaian MJ, Brody JR. Oncology. 2017; 31: 159 -168; b. Holter S, et al. J Clin Oncol. 2015; 33: 3124 -3129; c. Kaufman B, et al. J Clin Oncol. 2015; 33: 244 -250.

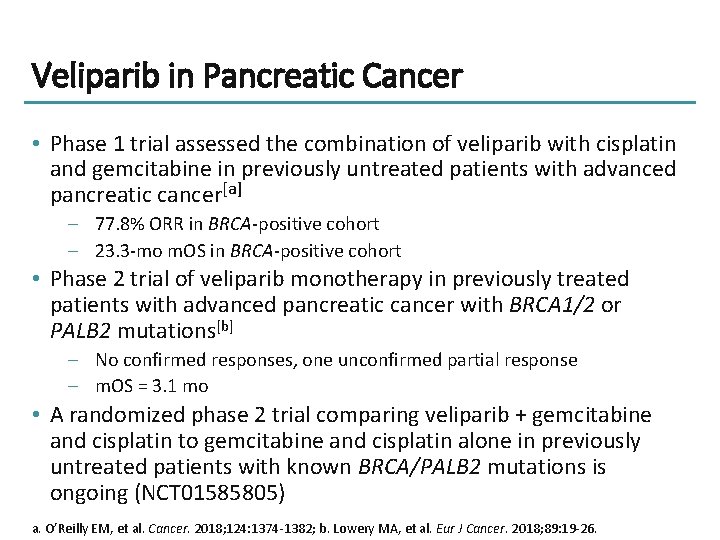
Veliparib in Pancreatic Cancer • Phase 1 trial assessed the combination of veliparib with cisplatin and gemcitabine in previously untreated patients with advanced pancreatic cancer[a] – 77. 8% ORR in BRCA-positive cohort – 23. 3 -mo m. OS in BRCA-positive cohort • Phase 2 trial of veliparib monotherapy in previously treated patients with advanced pancreatic cancer with BRCA 1/2 or PALB 2 mutations[b] – No confirmed responses, one unconfirmed partial response – m. OS = 3. 1 mo • A randomized phase 2 trial comparing veliparib + gemcitabine and cisplatin to gemcitabine and cisplatin alone in previously untreated patients with known BRCA/PALB 2 mutations is ongoing (NCT 01585805) a. O’Reilly EM, et al. Cancer. 2018; 124: 1374 -1382; b. Lowery MA, et al. Eur J Cancer. 2018; 89: 19 -26.

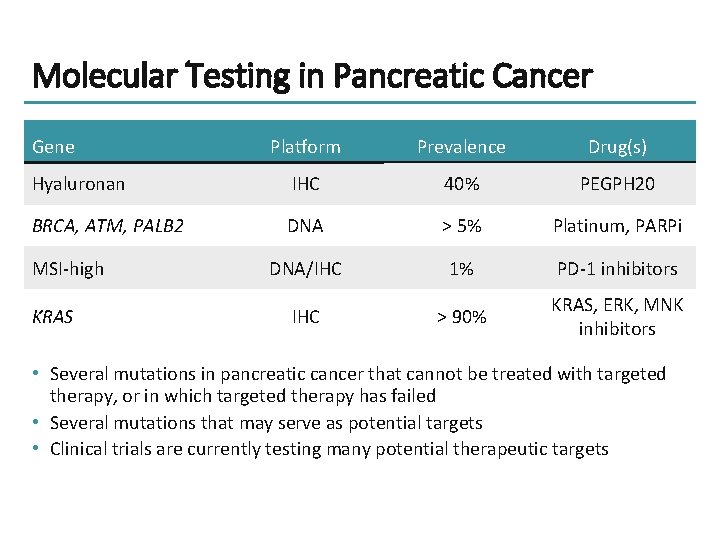
Molecular Testing in Pancreatic Cancer Gene Platform Prevalence Drug(s) Hyaluronan IHC 40% PEGPH 20 BRCA, ATM, PALB 2 DNA > 5% Platinum, PARPi DNA/IHC 1% PD-1 inhibitors IHC > 90% KRAS, ERK, MNK inhibitors MSI-high KRAS • Several mutations in pancreatic cancer that cannot be treated with targeted therapy, or in which targeted therapy has failed • Several mutations that may serve as potential targets • Clinical trials are currently testing many potential therapeutic targets

Advances in Early-Stage Pancreatic Cancer

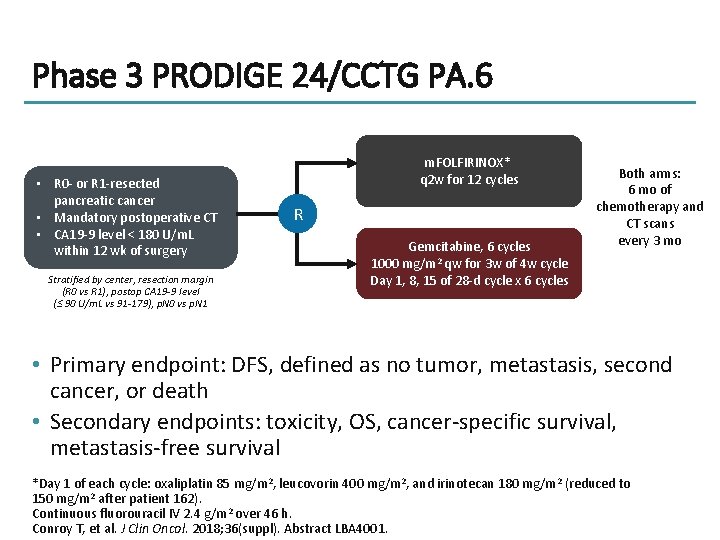
Phase 3 PRODIGE 24/CCTG PA. 6 • R 0 - or R 1 -resected pancreatic cancer • Mandatory postoperative CT • CA 19 -9 level < 180 U/m. L within 12 wk of surgery Stratified by center, resection margin (R 0 vs R 1), postop CA 19 -9 level (≤ 90 U/m. L vs 91 -179), p. N 0 vs p. N 1 m. FOLFIRINOX* q 2 w for 12 cycles R Gemcitabine, 6 cycles 1000 mg/m 2 qw for 3 w of 4 w cycle Day 1, 8, 15 of 28 -d cycle x 6 cycles Both arms: 6 mo of chemotherapy and CT scans every 3 mo • Primary endpoint: DFS, defined as no tumor, metastasis, second cancer, or death • Secondary endpoints: toxicity, OS, cancer-specific survival, metastasis-free survival *Day 1 of each cycle: oxaliplatin 85 mg/m 2, leucovorin 400 mg/m 2, and irinotecan 180 mg/m 2 (reduced to 150 mg/m 2 after patient 162). Continuous fluorouracil IV 2. 4 g/m 2 over 46 h. Conroy T, et al. J Clin Oncol. 2018; 36(suppl). Abstract LBA 4001.

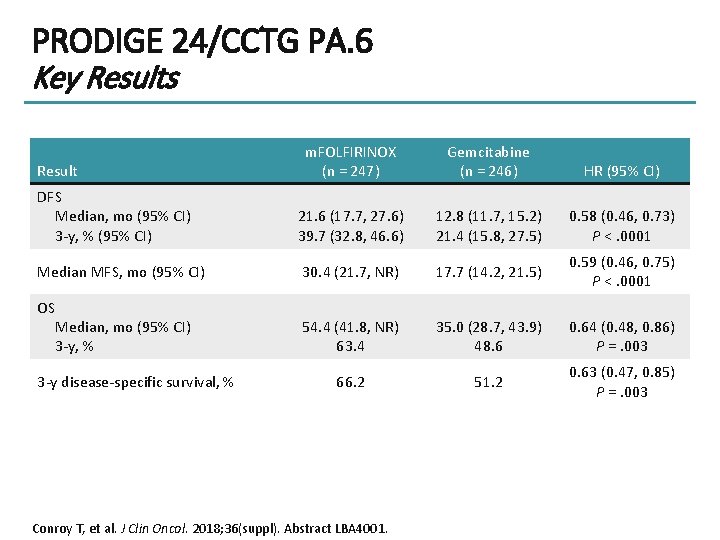
PRODIGE 24/CCTG PA. 6 Key Results m. FOLFIRINOX (n = 247) Gemcitabine (n = 246) HR (95% CI) DFS Median, mo (95% CI) 3 -y, % (95% CI) 21. 6 (17. 7, 27. 6) 39. 7 (32. 8, 46. 6) 12. 8 (11. 7, 15. 2) 21. 4 (15. 8, 27. 5) 0. 58 (0. 46, 0. 73) P <. 0001 Median MFS, mo (95% CI) 30. 4 (21. 7, NR) 17. 7 (14. 2, 21. 5) 0. 59 (0. 46, 0. 75) P <. 0001 OS Median, mo (95% CI) 3 -y, % 54. 4 (41. 8, NR) 63. 4 35. 0 (28. 7, 43. 9) 48. 6 0. 64 (0. 48, 0. 86) P =. 003 66. 2 51. 2 0. 63 (0. 47, 0. 85) P =. 003 Result 3 -y disease-specific survival, % Conroy T, et al. J Clin Oncol. 2018; 36(suppl). Abstract LBA 4001.

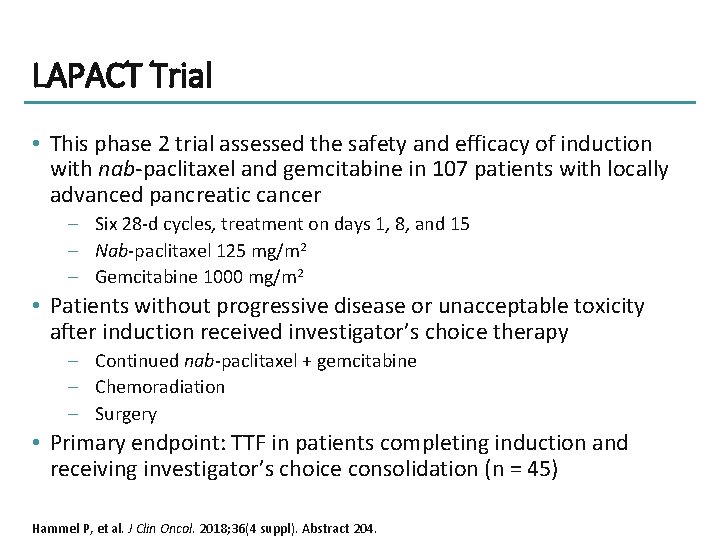
LAPACT Trial • This phase 2 trial assessed the safety and efficacy of induction with nab-paclitaxel and gemcitabine in 107 patients with locally advanced pancreatic cancer – Six 28 -d cycles, treatment on days 1, 8, and 15 – Nab-paclitaxel 125 mg/m 2 – Gemcitabine 1000 mg/m 2 • Patients without progressive disease or unacceptable toxicity after induction received investigator’s choice therapy – Continued nab-paclitaxel + gemcitabine – Chemoradiation – Surgery • Primary endpoint: TTF in patients completing induction and receiving investigator’s choice consolidation (n = 45) Hammel P, et al. J Clin Oncol. 2018; 36(4 suppl). Abstract 204.

LAPACT Trial Results • Response to induction therapy – ORR = 32. 7% – DCR = 77. 6% • Results in patients receiving consolidation – m. TTF: 8. 8 mo (historic controls: 6. 6 mo) – m. PFS: 10. 8 mo • Patients undergoing resection: 16/107 (15%) – 7 achieved R 0 resection Hammel P, et al. J Clin Oncol. 2018; 36(4 suppl). Abstract 204.

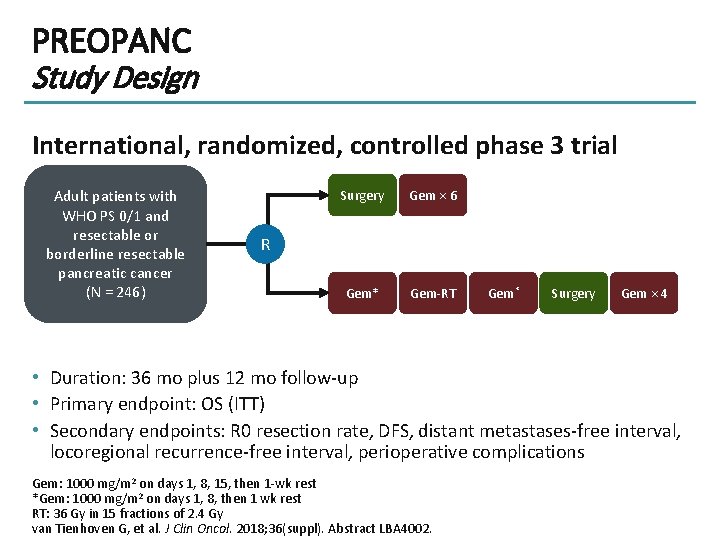
PREOPANC Study Design International, randomized, controlled phase 3 trial Adult patients with WHO PS 0/1 and resectable or borderline resectable pancreatic cancer (N = 246) Surgery Gem × 6 Gem* Gem-RT R Gem* Surgery Gem × 4 • Duration: 36 mo plus 12 mo follow-up • Primary endpoint: OS (ITT) • Secondary endpoints: R 0 resection rate, DFS, distant metastases-free interval, locoregional recurrence-free interval, perioperative complications Gem: 1000 mg/m 2 on days 1, 8, 15, then 1 -wk rest *Gem: 1000 mg/m 2 on days 1, 8, then 1 wk rest RT: 36 Gy in 15 fractions of 2. 4 Gy van Tienhoven G, et al. J Clin Oncol. 2018; 36(suppl). Abstract LBA 4002.

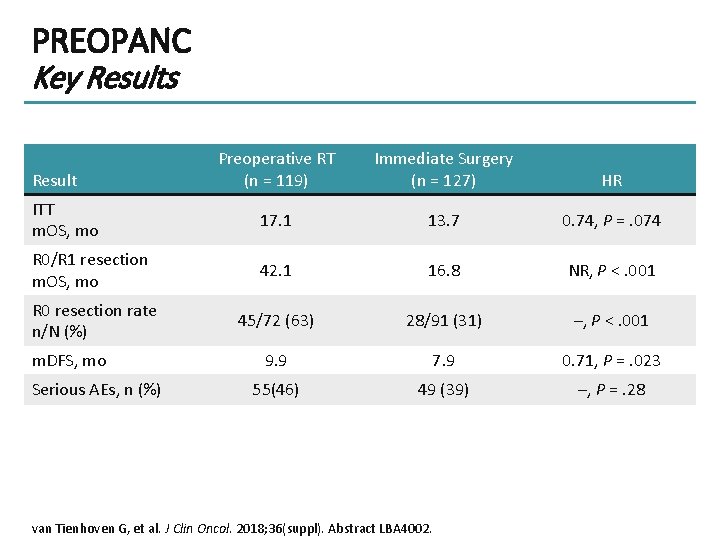
PREOPANC Key Results Preoperative RT (n = 119) Immediate Surgery (n = 127) HR ITT m. OS, mo 17. 1 13. 7 0. 74, P =. 074 R 0/R 1 resection m. OS, mo 42. 1 16. 8 NR, P <. 001 R 0 resection rate n/N (%) 45/72 (63) 28/91 (31) –, P <. 001 9. 9 7. 9 0. 71, P =. 023 55(46) 49 (39) –, P =. 28 Result m. DFS, mo Serious AEs, n (%) van Tienhoven G, et al. J Clin Oncol. 2018; 36(suppl). Abstract LBA 4002.

Take-Home Messages • The future of pancreatic cancer is bright, with new treatment options and sequencing strategies improving patient outcomes across the continuum of disease • Patients should be referred to centers of excellence for best possible treatment outcomes • Incorporating new technologies, including genomic profiling and biomarker-driven treatment selection, will help improve long-term outcomes – Use of single-agent checkpoint inhibitors should be limited to patients with high MSI • Several new treatments are on the horizon with the potential to radically improve patient outcomes

Thank you for participating in this activity. To proceed to the online CME test, click on the Earn Credit link on this page.