ADVANCES IN INFLAMMATORY BOWEL DISEASE HOPE FOR THE

ADVANCES IN INFLAMMATORY BOWEL DISEASE HOPE FOR THE CURE – WHEN VISION FOSTERS PROGRESS Seymour Katz, M. D. Clinical Professor of Medicine Albert Einstein College of Medicine
CHOICES IN INFLAMMATORY BOWEL DISEASE Binders Folders Restrict Access Open Opportunities Choose “Folders”
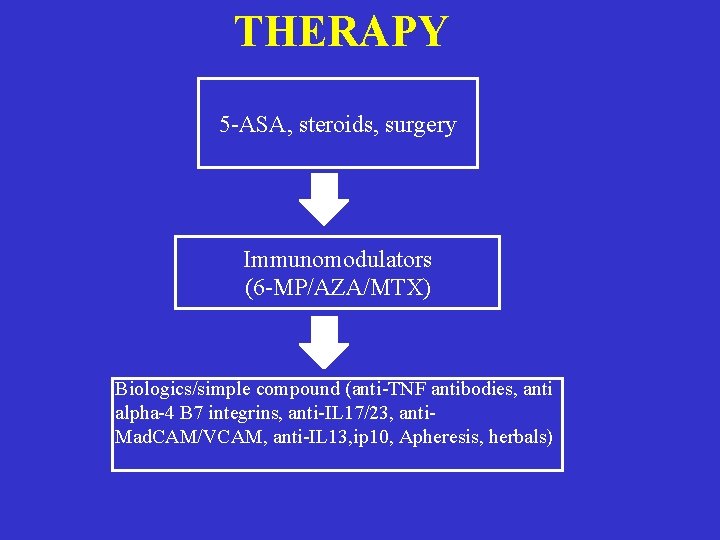
THERAPY 5 -ASA, steroids, surgery Immunomodulators (6 -MP/AZA/MTX) Biologics/simple compound (anti-TNF antibodies, anti alpha-4 B 7 integrins, anti-IL 17/23, anti. Mad. CAM/VCAM, anti-IL 13, ip 10, Apheresis, herbals)
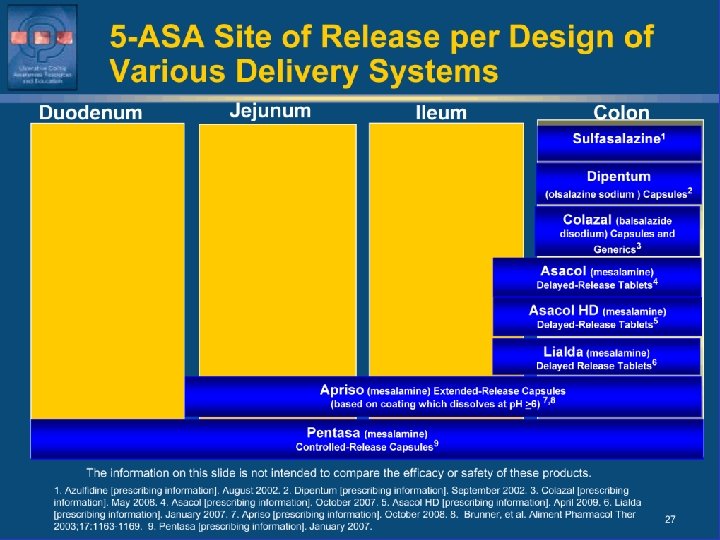
5 -ASA Site of Release per Design of Various Delivery Systems
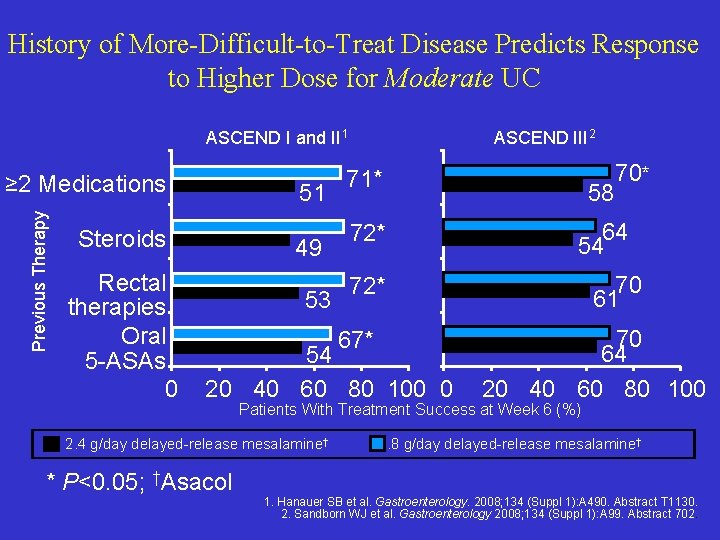
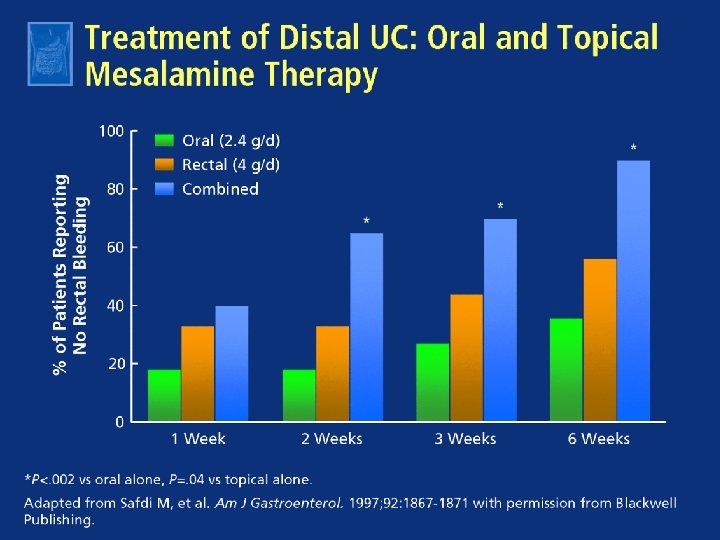
History of More-Difficult-to-Treat Disease Predicts Response to Higher Dose for Moderate UC ASCEND I and II 1 Previous Therapy ≥ 2 Medications 51 Steroids Rectal therapies Oral 5 -ASAs 0 49 53 ASCEND III 2 71* 72* 64 54 70 61 72* 67* 54 20 40 60 80 100 0 70 64 20 40 60 80 100 Patients With Treatment Success at Week 6 (%) 2. 4 g/day delayed-release mesalamine† * P<0. 05; †Asacol 58 70* 4. 8 g/day delayed-release mesalamine† 1. Hanauer SB et al. Gastroenterology. 2008; 134 (Suppl 1): A 490. Abstract T 1130. 2. Sandborn WJ et al. Gastroenterology 2008; 134 (Suppl 1): A 99. Abstract 702.

Safety Considerations of 5 -ASA Agents • Nephrotoxicity – – Nephrotoxicity rate 0. 26% per patient-year 1 Most often reported within the first 12 months of therapy 1 May be idiosyncratic rather than dose-related 1 Caution recommended when used in patients with known (or history of) renal disease 2, 3 – FDA recommends monitoring BUN/serum creatinine – Timely recognition of renal impairment and prompt discontinuation of 5 -ASA in affected patients is important 1 • Pregnancy/breastfeeding 4 – Safety in pregnancy demonstrated in several trials – Milk: serum ratios considered acceptably low – Considered safe for use in pregnancy and breastfeeding when indicated 1. Gisbert JP et al. Inflamm Bowel Dis. 2007; 13: 629. 2. Asacol [prescribing information] Procter & Gamble Pharmaceuticals; Sep 2006. 3. Lialda (delayed release mesalamine) [package insert]. Wayne, PA: Shire USA; Jan 2007. 4. Kane S. Gastroenterol Clin N Am. 2003; 32: 323.
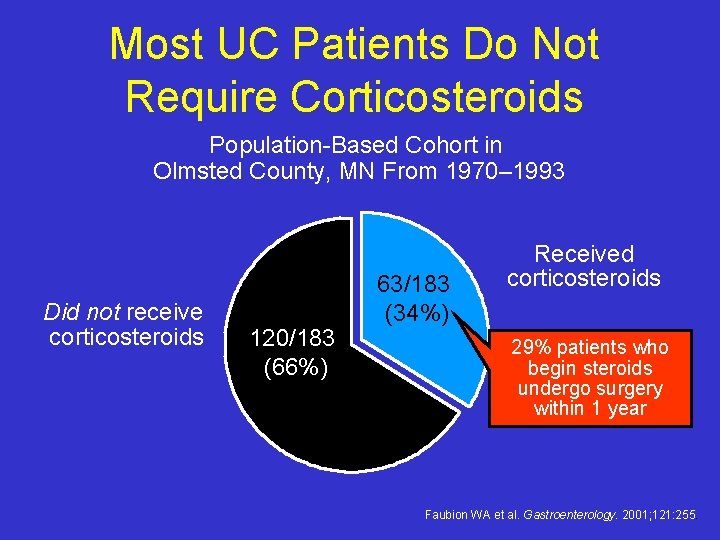
Most UC Patients Do Not Require Corticosteroids Population-Based Cohort in Olmsted County, MN From 1970– 1993 Did not receive corticosteroids 120/183 (66%) 63/183 (34%) Received corticosteroids 29% patients who begin steroids undergo surgery within 1 year Faubion WA et al. Gastroenterology. 2001; 121: 255.
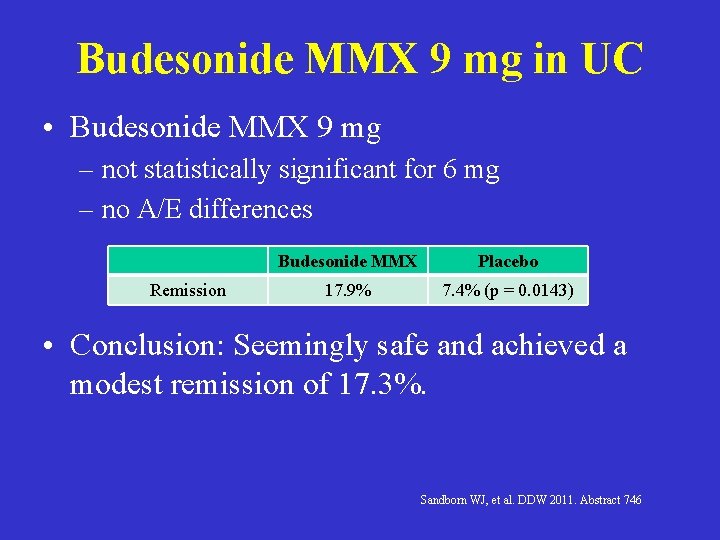
Budesonide MMX 9 mg in UC • Budesonide MMX 9 mg – not statistically significant for 6 mg – no A/E differences Remission Budesonide MMX Placebo 17. 9% 7. 4% (p = 0. 0143) • Conclusion: Seemingly safe and achieved a modest remission of 17. 3%. Sandborn WJ, et al. DDW 2011. Abstract 746
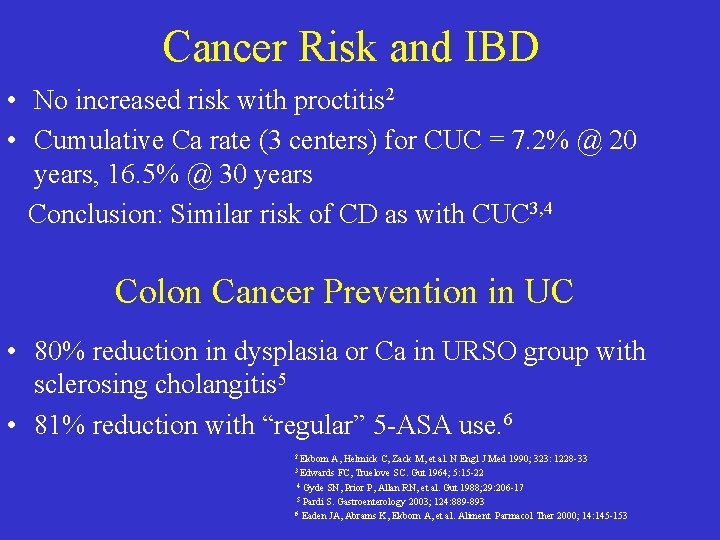
Cancer Risk and IBD • No increased risk with proctitis 2 • Cumulative Ca rate (3 centers) for CUC = 7. 2% @ 20 years, 16. 5% @ 30 years Conclusion: Similar risk of CD as with CUC 3, 4 Colon Cancer Prevention in UC • 80% reduction in dysplasia or Ca in URSO group with sclerosing cholangitis 5 • 81% reduction with “regular” 5 -ASA use. 6 2 Ekbom A, Helmick C, Zack M, et al. N Engl J Med 1990; 323: 1228 -33 FC, Truelove SC. Gut 1964; 5: 15 -22 4 Gyde SN, Prior P, Allan RN, et al. Gut 1988; 29: 206 -17 5 Pardi S. Gastroenterology 2003; 124: 889 -893 6 Eaden JA, Abrams K, Ekborn A, et al. Aliment Parmacol Ther 2000; 14: 145 -153 3 Edwards
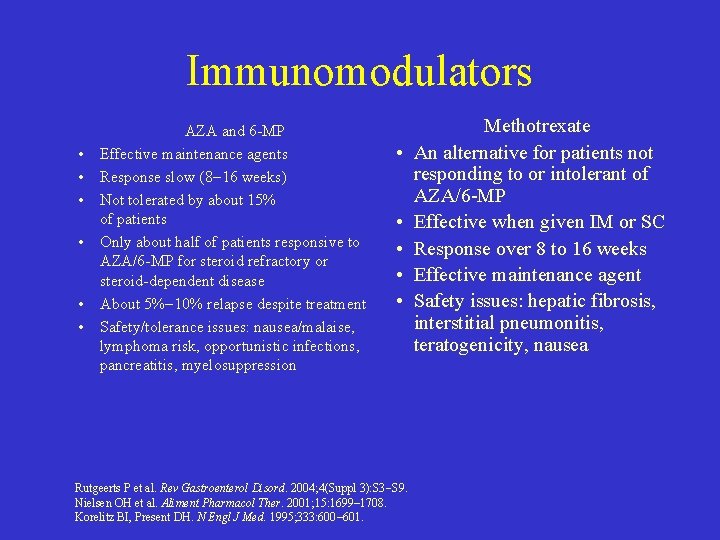
Immunomodulators • • • AZA and 6 -MP Effective maintenance agents Response slow (8 16 weeks) Not tolerated by about 15% of patients Only about half of patients responsive to AZA/6 -MP for steroid refractory or steroid-dependent disease About 5% 10% relapse despite treatment Safety/tolerance issues: nausea/malaise, lymphoma risk, opportunistic infections, pancreatitis, myelosuppression • • • Rutgeerts P et al. Rev Gastroenterol Disord. 2004; 4(Suppl 3): S 3 S 9. Nielsen OH et al. Aliment Pharmacol Ther. 2001; 15: 1699– 1708. Korelitz BI, Present DH. N Engl J Med. 1995; 333: 600– 601. Methotrexate An alternative for patients not responding to or intolerant of AZA/6 -MP Effective when given IM or SC Response over 8 to 16 weeks Effective maintenance agent Safety issues: hepatic fibrosis, interstitial pneumonitis, teratogenicity, nausea
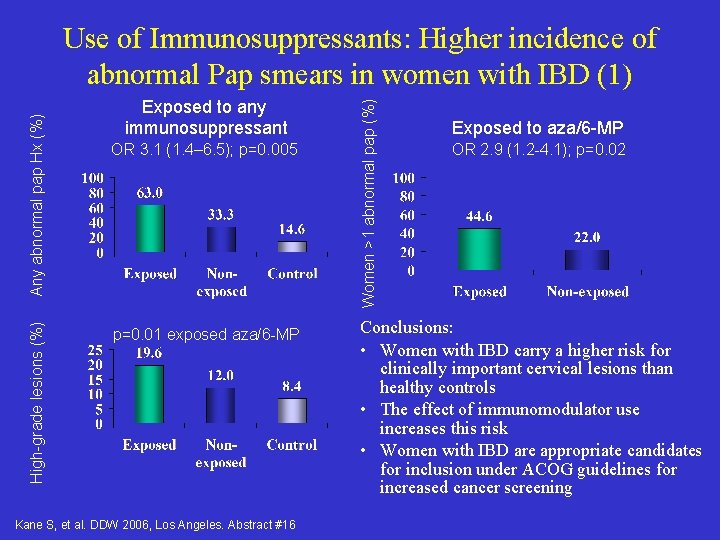
High-grade lesions (%) Exposed to any immunosuppressant OR 3. 1 (1. 4– 6. 5); p=0. 005 p=0. 01 exposed aza/6 -MP Kane S, et al. DDW 2006, Los Angeles. Abstract #16 Women >1 abnormal pap (%) Any abnormal pap Hx (%) Use of Immunosuppressants: Higher incidence of abnormal Pap smears in women with IBD (1) Exposed to aza/6 -MP OR 2. 9 (1. 2 -4. 1); p=0. 02 Conclusions: • Women with IBD carry a higher risk for clinically important cervical lesions than healthy controls • The effect of immunomodulator use increases this risk • Women with IBD are appropriate candidates for inclusion under ACOG guidelines for increased cancer screening
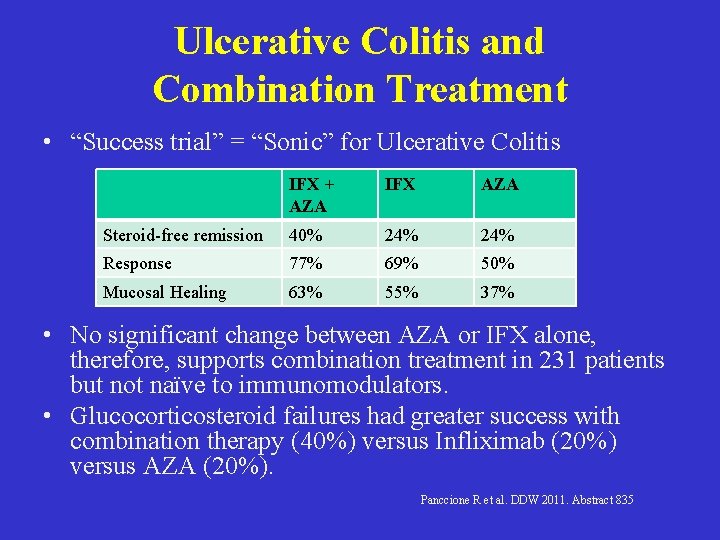
Ulcerative Colitis and Combination Treatment • “Success trial” = “Sonic” for Ulcerative Colitis IFX + AZA IFX AZA Steroid-free remission 40% 24% Response 77% 69% 50% Mucosal Healing 63% 55% 37% • No significant change between AZA or IFX alone, therefore, supports combination treatment in 231 patients but not naïve to immunomodulators. • Glucocorticosteroid failures had greater success with combination therapy (40%) versus Infliximab (20%) versus AZA (20%). Panccione R et al. DDW 2011. Abstract 835
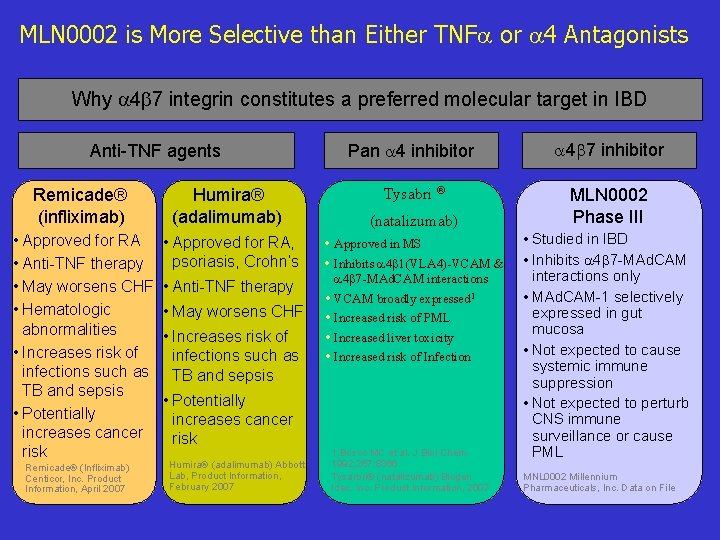
MLN 0002 is More Selective than Either TNF or 4 Antagonists Why 4 7 integrin constitutes a preferred molecular target in IBD Anti-TNF agents Pan 4 inhibitor 4 7 inhibitor Tysabri ® MLN 0002 Phase III Remicade® (infliximab) Humira® (adalimumab) (natalizumab) • Approved for RA • Anti-TNF therapy • May worsens CHF • Hematologic abnormalities • Increases risk of infections such as TB and sepsis • Potentially increases cancer risk • Approved for RA, psoriasis, Crohn’s • Approved in MS • Inhibits 4 1(VLA 4)-VCAM & Remicade® (Infliximab) Centicor, Inc. Product Information, April 2007 • Anti-TNF therapy • May worsens CHF • Increases risk of infections such as TB and sepsis • Potentially increases cancer risk Humira® (adalimumab) Abbott Lab, Product Information, February 2007 4 7 -MAd. CAM interactions • VCAM broadly expressed 1 • Increased risk of PML • Increased liver toxicity • Increased risk of Infection 1. Bosco MC et al. J Biol Chem 1992; 267; 8366 Tysarbri® (natalizumab) Biogen Idec, Inc. Product Information, 2007 • Studied in IBD • Inhibits 4 7 -MAd. CAM interactions only • MAd. CAM-1 selectively expressed in gut mucosa • Not expected to cause systemic immune suppression • Not expected to perturb CNS immune surveillance or cause PML MNL 0002 Millennium Pharmaceuticals, Inc. Data on File
Oral Janus Kinase Inhibitor • Inhibiting JAK 1 and 3 (which signals IL-2, -4, -7, -9, -15, and – 21) in 139 patients showed NO value in Crohns although response in CRP, and fecal calprotectin. 1 • A dose dependent response did occur in ulcerative colitis patients. 2 1. Sandborn WJ, et al. DDW 2011. Abstract 745 2. Sandborn WJ, et al. DDW 2011. Abstract 594
Rhu. Mab 7 in UC • Rhu. MAb 7 is a humanized monoclonal antibody to 7 of the integrins 4 7 and E 7 given as a single dose or placebo side in a 4: 1 ratio Rhu. Mab 7 Placebo Response 10/15 2/3 Remission 3/15 0/3 – 65% of 48 patients had prior Infliximab exposure. Rutgeerts P, et al. DDW 2011. Abstract 748
Curcumin for long-term maintenance therapy in patients with UC Background • Pharmacologically active phytochemical derived from turmeric • Suppresses NF-k. B Study design • Multicenter, double-blinded, placebo-controlled trial • Patients with quiescent UC (CAI ≤ 4 for 4 weeks) randomized to curcumin 2 g/day (n=45) or placebo (n=44) • Continued on sulfasalazine or 5 -ASA • Primary analysis: Relapse rates by 6 months Hanai H, et al. DDW 2006, #572
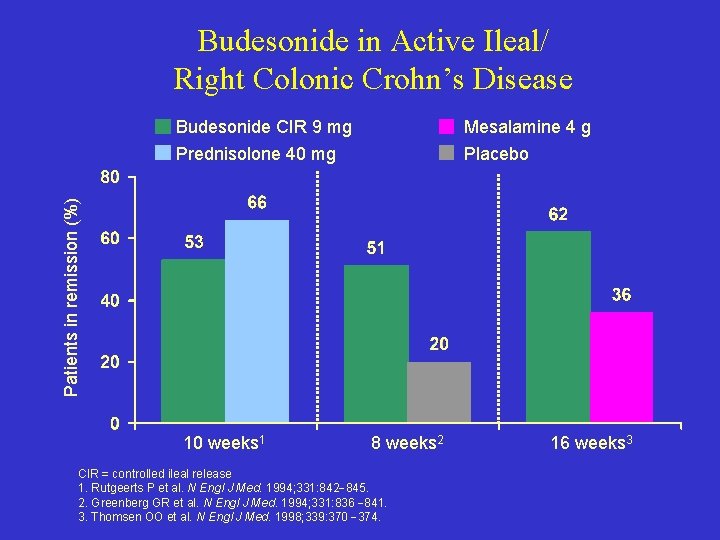
Budesonide in Active Ileal/ Right Colonic Crohn’s Disease Mesalamine 4 g Prednisolone 40 mg Placebo Patients in remission (%) Budesonide CIR 9 mg 10 weeks 1 8 weeks 2 CIR = controlled ileal release 1. Rutgeerts P et al. N Engl J Med. 1994; 331: 842 845. 2. Greenberg GR et al. N Engl J Med. 1994; 331: 836 841. 3. Thomsen OO et al. N Engl J Med. 1998; 339: 370 374. 16 weeks 3

Biologic Therapy: Mucosal Healing vs. Clinical Remission Adverse events Risks vs. Benefits Symptom relief vs. deep mucosal healing
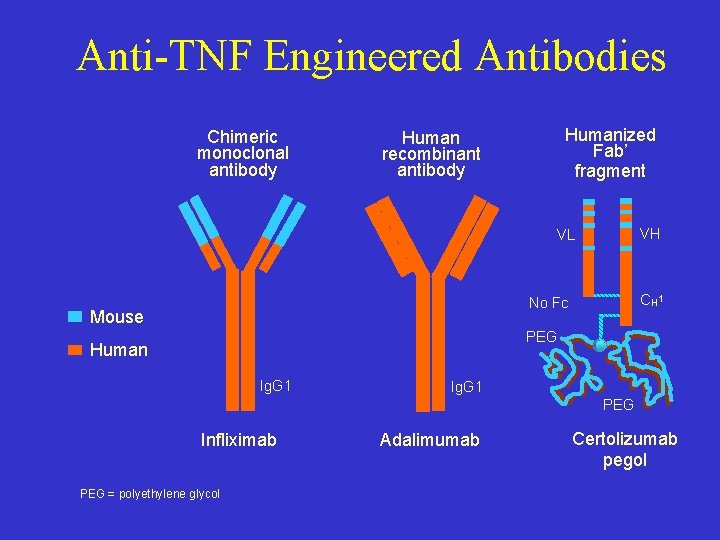
Anti-TNF Engineered Antibodies Chimeric monoclonal antibody Humanized Fab’ fragment Human recombinant antibody VH VL CH 1 No Fc Mouse PEG Human Ig. G 1 PEG Infliximab PEG = polyethylene glycol Adalimumab Certolizumab pegol
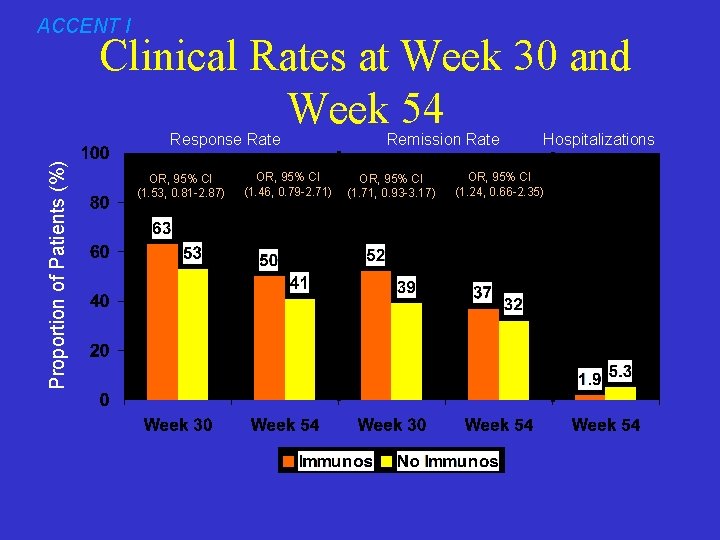
ACCENT I Clinical Rates at Week 30 and Week 54 Proportion of Patients (%) Response Rate OR, 95% CI (1. 53, 0. 81 -2. 87) OR, 95% CI (1. 46, 0. 79 -2. 71) Remission Rate OR, 95% CI (1. 71, 0. 93 -3. 17) Hospitalizations OR, 95% CI (1. 24, 0. 66 -2. 35)

Closer look at the Mayo experience with opportunistic infections Herpes zoster Candida albicans Herpes Simplex CMV EBV Histoplasmosis Blastomycosis Streptococcus E. Coli Mycobacterium marinum Mycobacterium fortuitum Cryptococcus Mycobacterium gordonae 28 26 18 12 8 2 1 1 1 1 Toruner et al. Gastro 2008; 134: 929
20 year old male receiving anti-TNF + Immunomodulator Therapy for 1 year Risk of Developing NH Lymphoma IM medication monotherapy Risk without combination therapy
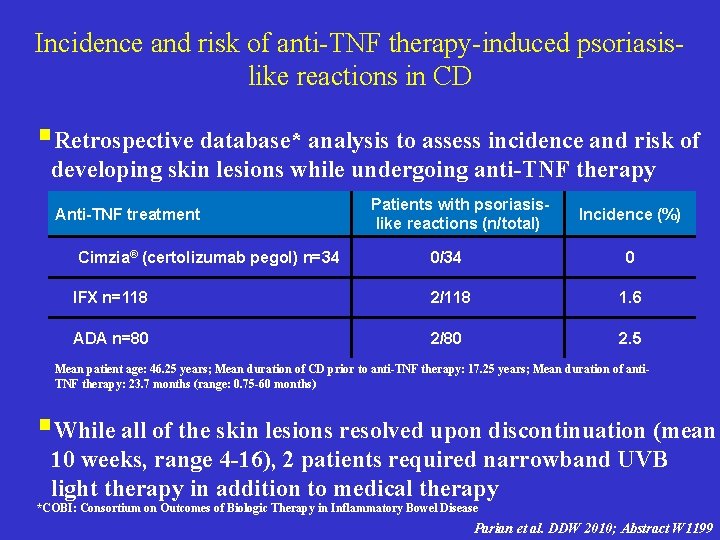
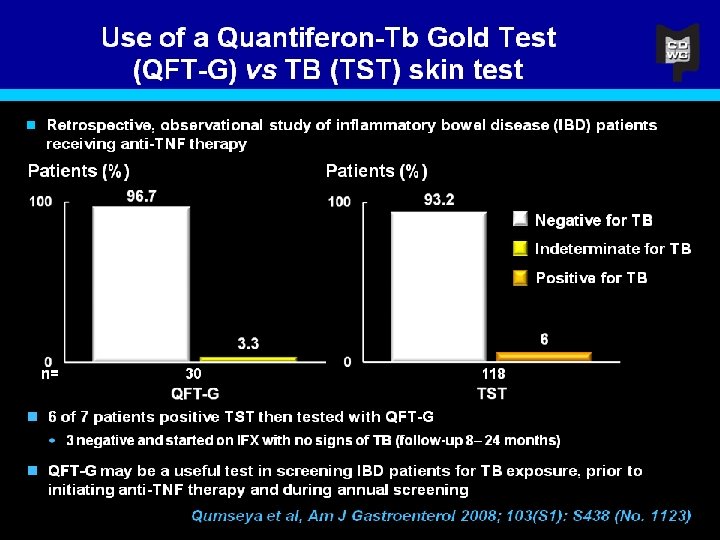
Incidence and risk of anti-TNF therapy-induced psoriasislike reactions in CD §Retrospective database* analysis to assess incidence and risk of developing skin lesions while undergoing anti-TNF therapy Anti-TNF treatment Cimzia® (certolizumab pegol) n=34 Patients with psoriasislike reactions (n/total) Incidence (%) 0/34 0 IFX n=118 2/118 1. 6 ADA n=80 2/80 2. 5 Mean patient age: 46. 25 years; Mean duration of CD prior to anti-TNF therapy: 17. 25 years; Mean duration of anti. TNF therapy: 23. 7 months (range: 0. 75 -60 months) §While all of the skin lesions resolved upon discontinuation (mean 10 weeks, range 4 -16), 2 patients required narrowband UVB light therapy in addition to medical therapy *COBI: Consortium on Outcomes of Biologic Therapy in Inflammatory Bowel Disease Parian et al. DDW 2010; Abstract W 1199
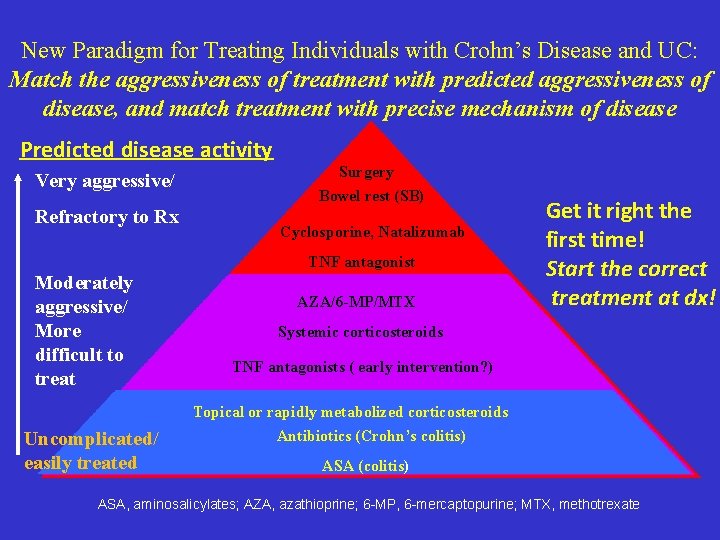
New Paradigm for Treating Individuals with Crohn’s Disease and UC: Match the aggressiveness of treatment with predicted aggressiveness of disease, and match treatment with precise mechanism of disease Predicted disease activity Very aggressive/ Refractory to Rx Surgery Bowel rest (SB) Cyclosporine, Natalizumab TNF antagonist Moderately aggressive/ More difficult to treat Uncomplicated/ easily treated AZA/6 -MP/MTX Get it right the first time! Start the correct treatment at dx! Systemic corticosteroids TNF antagonists ( early intervention? ) Topical or rapidly metabolized corticosteroids Antibiotics (Crohn’s colitis) ASA (colitis) ASA, aminosalicylates; AZA, azathioprine; 6 -MP, 6 -mercaptopurine; MTX, methotrexate
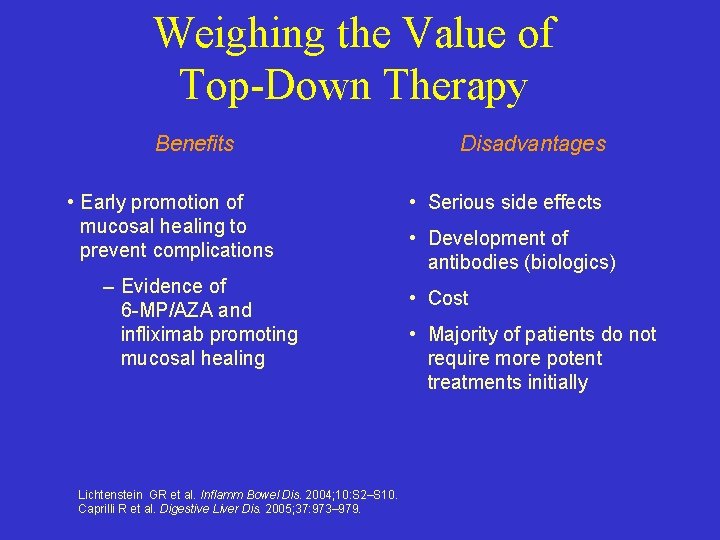
Weighing the Value of Top-Down Therapy Benefits • Early promotion of mucosal healing to prevent complications – Evidence of 6 -MP/AZA and infliximab promoting mucosal healing Lichtenstein GR et al. Inflamm Bowel Dis. 2004; 10: S 2–S 10. Caprilli R et al. Digestive Liver Dis. 2005; 37: 973– 979. Disadvantages • Serious side effects • Development of antibodies (biologics) • Cost • Majority of patients do not require more potent treatments initially
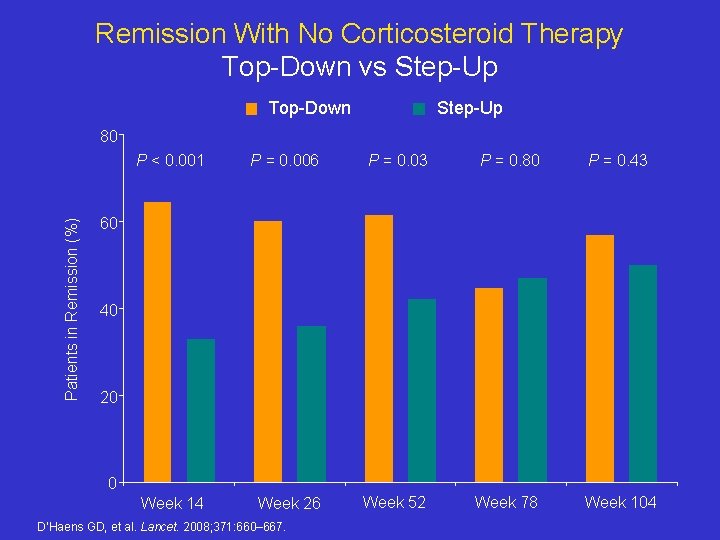
Remission With No Corticosteroid Therapy Top-Down vs Step-Up Top-Down Step-Up Patients in Remission (%) 80 P < 0. 001 P = 0. 006 P = 0. 03 P = 0. 80 P = 0. 43 Week 14 Week 26 Week 52 Week 78 Week 104 60 40 20 0 D’Haens GD, et al. Lancet. 2008; 371: 660– 667.
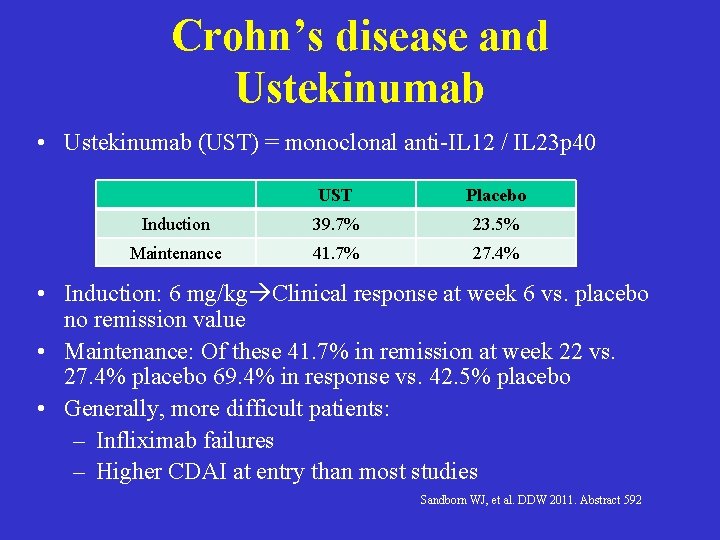
Crohn’s disease and Ustekinumab • Ustekinumab (UST) = monoclonal anti-IL 12 / IL 23 p 40 UST Placebo Induction 39. 7% 23. 5% Maintenance 41. 7% 27. 4% • Induction: 6 mg/kg Clinical response at week 6 vs. placebo no remission value • Maintenance: Of these 41. 7% in remission at week 22 vs. 27. 4% placebo 69. 4% in response vs. 42. 5% placebo • Generally, more difficult patients: – Infliximab failures – Higher CDAI at entry than most studies Sandborn WJ, et al. DDW 2011. Abstract 592
Progressive Multifoca. I Leukoencephalopathy • 3 cases of PML • 2 years of combined natalizumab/beta interferon therapy- one fatality • FDA review: voluntary suspension of the program (9/05 resubmitted BLA)
Risks vs. Benefits Risks: • Infection – vigilance regarding: C. diff. , CMV, TBC; vaccination update. • Lymphoma – true for all biologic / IMD (4 x), esp. males < 30 with HSTCL • M. S. • Lupus • Require labs: every 4 months, retest TBC annually Benefits: Q: Benefits with biologic / IMD counseling? A: Yes, better result, less A/E

Who Should Take Immunosuppressant / Biologic Therapy? Q: Does the patient’s disease severity require IMD / Biologic? A: Appropriate for moderate to severe IBD failing conventional Rx. or steroid-depending. • NOT if: 1. 2. 3. 4. 5. • Active (i. e. untreated) infection Untreated malignancies (melanoma, lymphoma, renal cell Ca, lung Ca, or presently in active chemotherapy). Uncontrolled CHF Neurologic disease (M. S. , autoimmune neurological disease) Fibrostenotic “strictures”: surgery required YES if: 1. Proven active IBD and NOT IBS, bacterial overgrowth mimicking IBD pain / diarrhea.

Discussion before Biologic / IMD • Is disease severe enough to warrant biologic / IMD? • Can patient tolerate A/E? • Is patient “psychologically” accepting of Rx. ? (i. e. “too dangerous”, non-compliant with schedule) • Will patient’s health insurance cover Rx. ? • Is MD’s staff prepared to assist with insurance obstacles? • Comment: NO “ONE SIZE FITS ALL” – Need to Individualize therapy
UNIMAGINABLE ADVANCES • Now: – 124 genetic loci in IBD • • Gene signatures: NPC 1 L 1, TAF 5 L and FOLH 1 distinguishes Ileal in CD/UC 24 gene ratios highest in IBD>IBS IRGM rs 10065172 CD – Microbiome – Biomarkers • Diagnostic – – Goal: Platform for individualizing therapy • Predict response to therapy PR 3 -ANCA only found in UC patients Anti-CBir 1, anti-Omp. C, anti-I 2 found in CD Fecal calprotectin, lactoferrin Anti-Glycan antibodies (g. ASCA, ACCA, ALCA and AMCA) bot not in African-Americans
The Vision • Define functions of selected IBD-related genes. Of the 124 genes associated with Crohn’s and UC how they increase risk is unknown. • Identify biomarkers and genes that will help predict at the time of diagnosis when child will develop severe vs. mild Crohn’s disease and an individual’s response to different therapies.
Microbiome: • Familial influence on Microbione composition: early colonization or early diet change more important than genetic background of host • Diet dramatically changes bacterial composition and gene expression • Selective manipulation of the microbial composition and/or function in individual patients could revolutionize treatment of IBD

“OLDER” IBD PATIENT THERAPY • Distinguish: “fit” from “frail” elderly – “Fit” – don’t exclude based on age – Frail” – decrease mobility, polypharmacy, comorbidity, decrease cognition • Therefore, limit goals, don’t DESTABILIZE Fit Frail Vs.
Layers of Care for the Older IBD Patient COMORBIDITY POLYPHARMACY MOBILITY COGNITION IBD 53
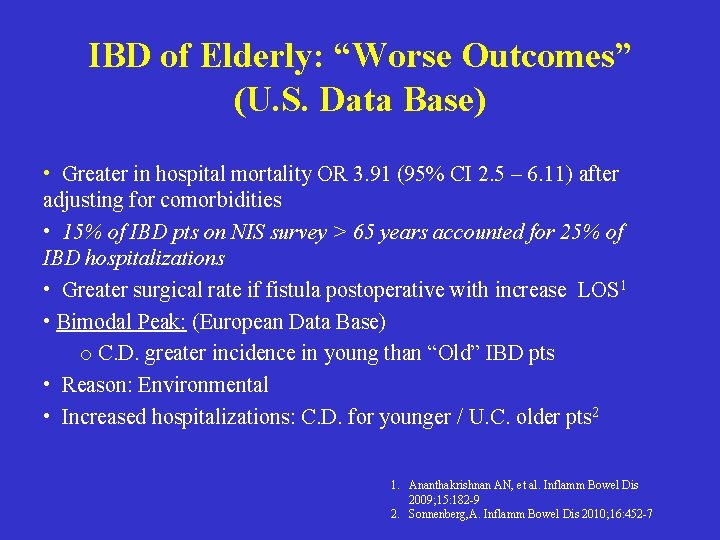
IBD of Elderly: “Worse Outcomes” (U. S. Data Base) • Greater in hospital mortality OR 3. 91 (95% CI 2. 5 – 6. 11) after adjusting for comorbidities • 15% of IBD pts on NIS survey > 65 years accounted for 25% of IBD hospitalizations • Greater surgical rate if fistula postoperative with increase LOS 1 • Bimodal Peak: (European Data Base) o C. D. greater incidence in young than “Old” IBD pts • Reason: Environmental • Increased hospitalizations: C. D. for younger / U. C. older pts 2 1. Ananthakrishnan AN, et al. Inflamm Bowel Dis 2009; 15: 182 -9 2. Sonnenberg, A. Inflamm Bowel Dis 2010; 16: 452 -7
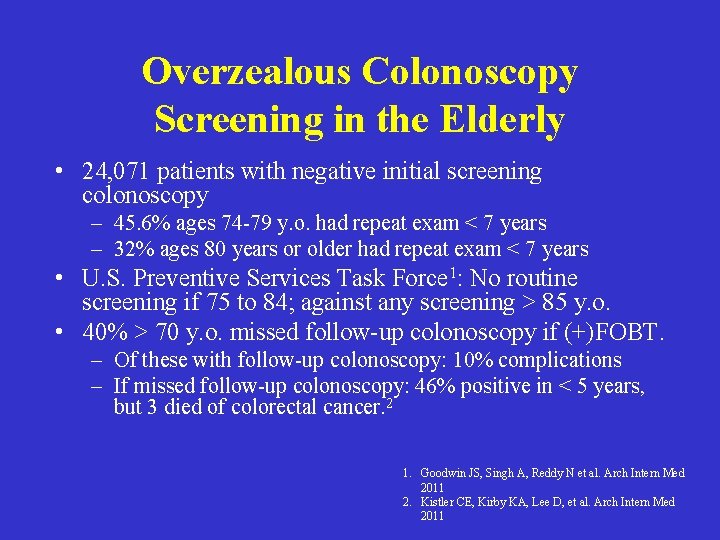
Overzealous Colonoscopy Screening in the Elderly • 24, 071 patients with negative initial screening colonoscopy – 45. 6% ages 74 -79 y. o. had repeat exam < 7 years – 32% ages 80 years or older had repeat exam < 7 years • U. S. Preventive Services Task Force 1: No routine screening if 75 to 84; against any screening > 85 y. o. • 40% > 70 y. o. missed follow-up colonoscopy if (+)FOBT. – Of these with follow-up colonoscopy: 10% complications – If missed follow-up colonoscopy: 46% positive in < 5 years, but 3 died of colorectal cancer. 2 1. Goodwin JS, Singh A, Reddy N et al. Arch Intern Med 2011 2. Kistler CE, Kirby KA, Lee D, et al. Arch Intern Med 2011

CONCLUSION • “HOPE PREVAILS”
- Slides: 43