Advanced Theories of Chemical Bonding Chapter 9 Atomic
Advanced Theories of Chemical Bonding Chapter 9 Atomic Orbitals © 2009 Brooks/Cole - Cengage Molecules 1
Two Theories of Bonding • MOLECULAR ORBITAL THEORY — Robert Mullikan (18961986) • valence electrons are delocalized • valence electrons are in orbitals (called molecular orbitals) spread over entire molecule. © 2009 Brooks/Cole - Cengage 2

Two Theories of Bonding • VALENCE BOND THEORY — Linus Pauling • valence electrons are localized between atoms (or are lone pairs). • half-filled atomic orbitals overlap to form bonds. Linus Pauling, 1901 -1994 © 2009 Brooks/Cole - Cengage 3
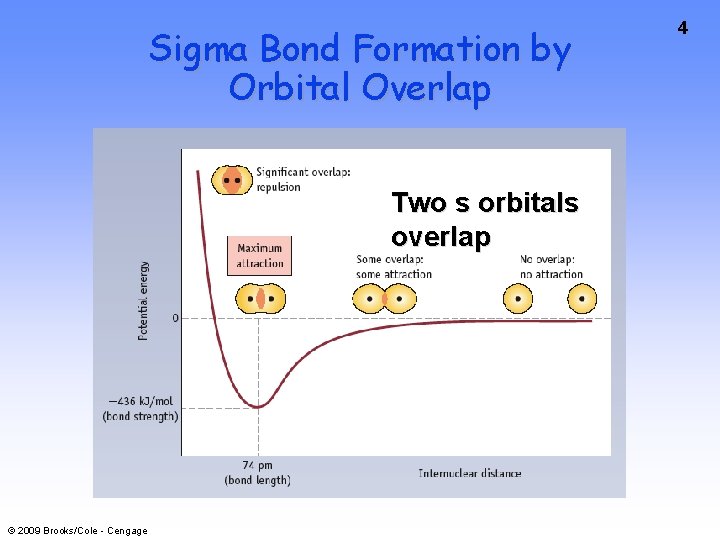
Sigma Bond Formation by Orbital Overlap Two s orbitals overlap © 2009 Brooks/Cole - Cengage 4

Sigma Bond Formation Two s orbitals overlap Two p orbitals overlap © 2009 Brooks/Cole - Cengage 5
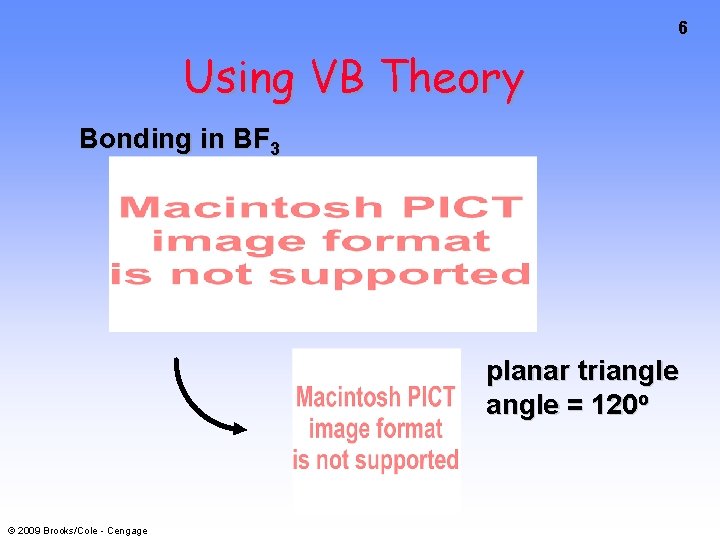
6 Using VB Theory Bonding in BF 3 planar triangle = 120 o © 2009 Brooks/Cole - Cengage
7 Bonding in BF 3 • How to account for 3 bonds 120 o apart using a spherical s orbital and p orbitals that are 90 o apart? • Pauling said to modify VB approach with ORBITAL HYBRIDIZATION • — mix available orbitals to form a new set of orbitals — HYBRID ORBITALS — that will give the maximum overlap in the correct geometry. © 2009 Brooks/Cole - Cengage
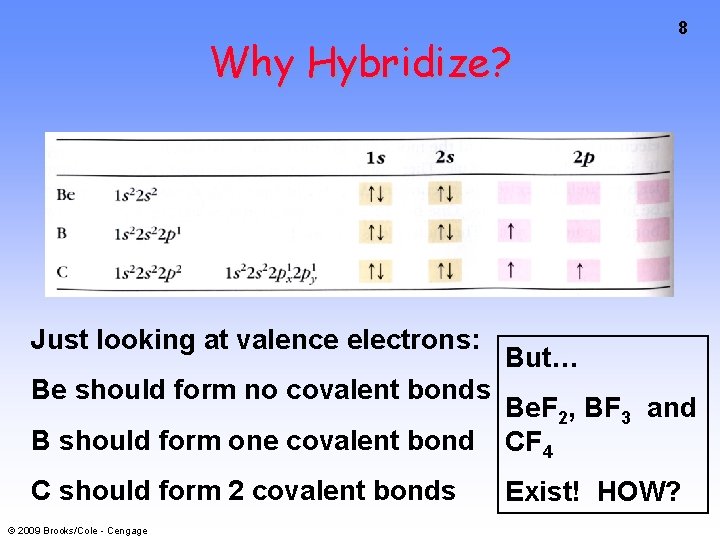
Why Hybridize? Just looking at valence electrons: Be should form no covalent bonds 8 But… B should form one covalent bond Be. F 2, BF 3 and CF 4 C should form 2 covalent bonds Exist! HOW? © 2009 Brooks/Cole - Cengage
Hybrid Orbitals: Why? • To explain the bonding in molecules like Be. F 2, BF 3 and CF 4, Linus Pauling proposed that orbitals become ‘hybridized’ – Hybrid orbitals are orbitals created by mixing the s, p or d orbitals of an atom. © 2009 Brooks/Cole - Cengage 9
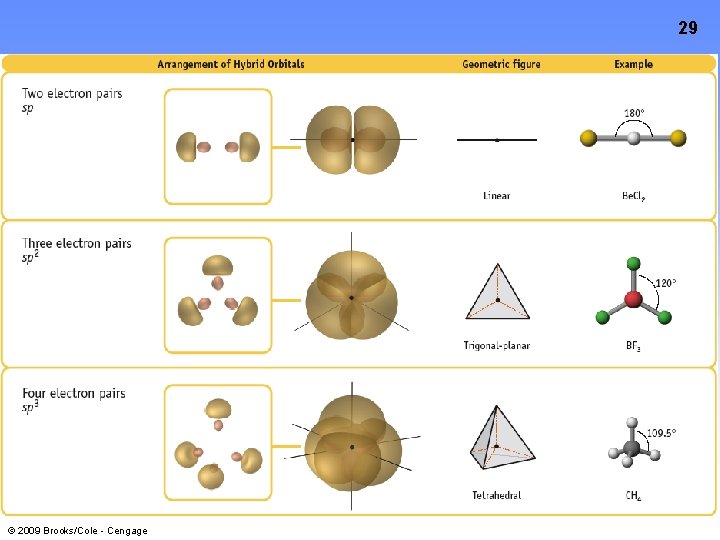
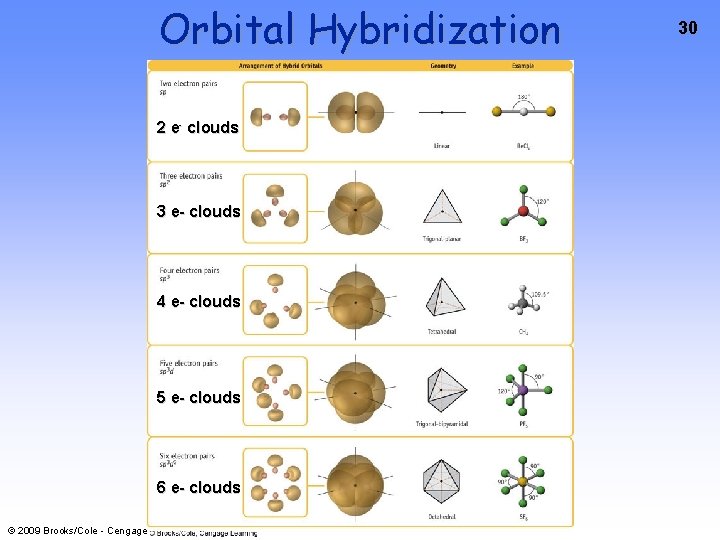
10 Hybrid Orbitals: The Rules 1. The number or hybrid orbitals is ALWAYS equal to the number of atomic orbitals that are combined to make the hybrid set 2. Hybrid orbital sets are always built by combining an s orbital with as many p or d orbitals necessary to accommodate the bonding and lone pairs on the central atom (Remember Electron Pair Geometry? ) 3. The Hybrid Orbitals are directed TOWARDS the terminal atoms • © 2009 Brooks/Cole - Cengage This results in a better orbital overlap AND stronger bonds between the central and terminal atoms
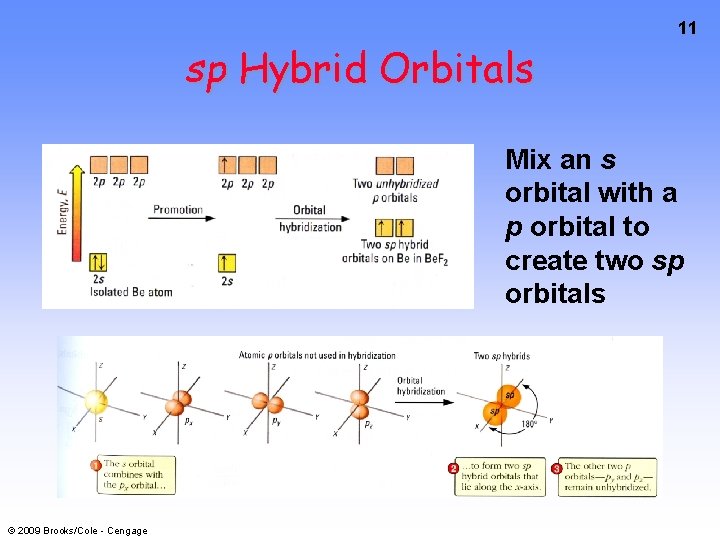
sp Hybrid Orbitals 11 Mix an s orbital with a p orbital to create two sp orbitals © 2009 Brooks/Cole - Cengage
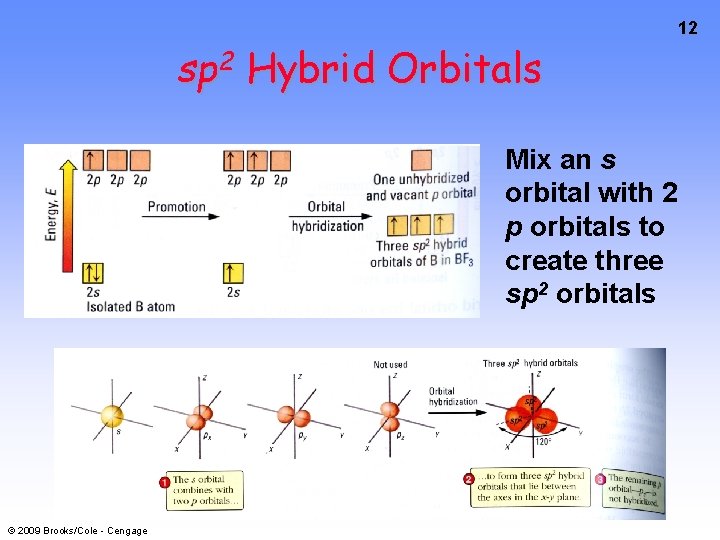
sp 2 Hybrid Orbitals Mix an s orbital with 2 p orbitals to create three sp 2 orbitals © 2009 Brooks/Cole - Cengage 12
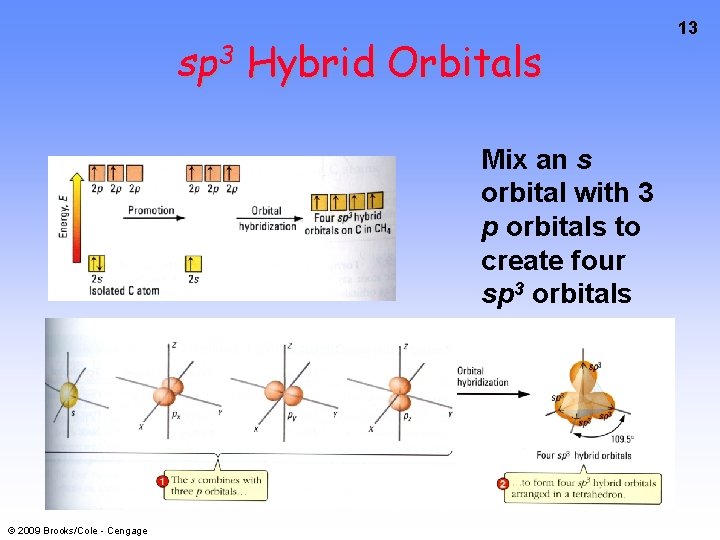
sp 3 Hybrid Orbitals Mix an s orbital with 3 p orbitals to create four sp 3 orbitals © 2009 Brooks/Cole - Cengage 13
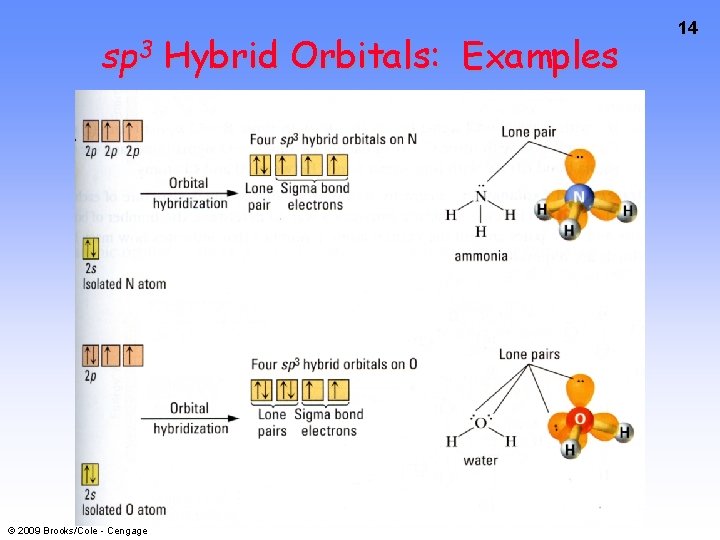
sp 3 Hybrid Orbitals: Examples © 2009 Brooks/Cole - Cengage 14
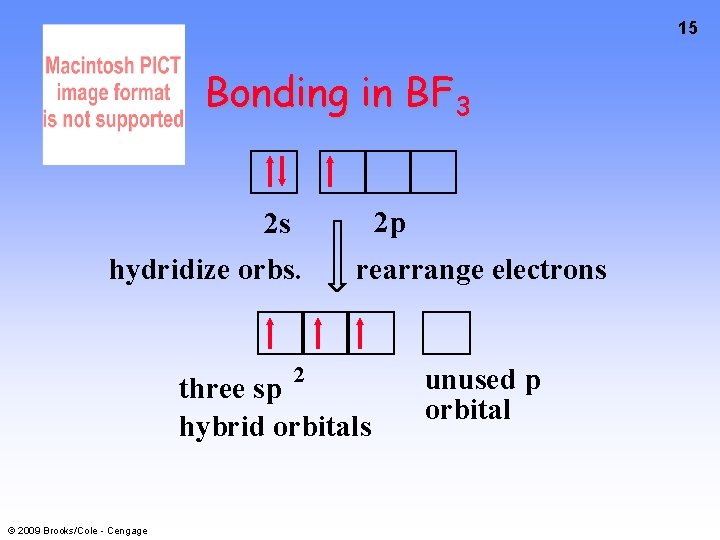
15 Bonding in BF 3 2 p 2 s hydridize orbs. 2 rearrange electrons three sp hybrid orbitals © 2009 Brooks/Cole - Cengage unused p orbital
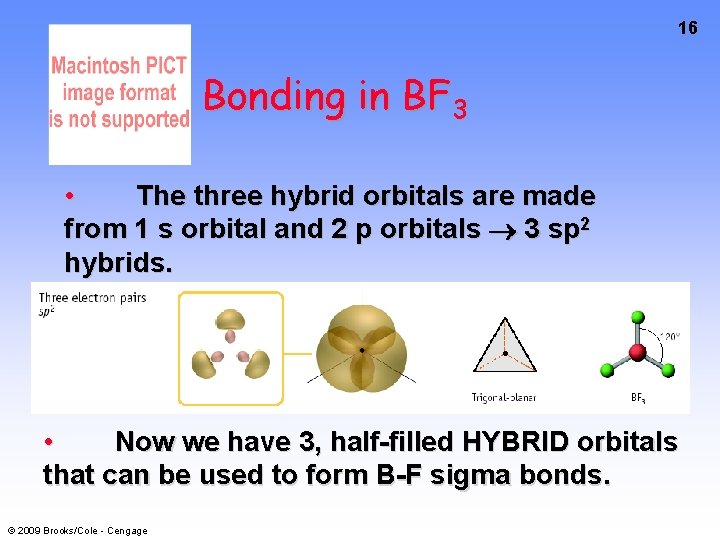
16 Bonding in BF 3 • The three hybrid orbitals are made from 1 s orbital and 2 p orbitals 3 sp 2 hybrids. • Now we have 3, half-filled HYBRID orbitals that can be used to form B-F sigma bonds. © 2009 Brooks/Cole - Cengage

Bonding in BF 3 An orbital from each F overlaps one of the sp 2 hybrids to form a B-F bond. © 2009 Brooks/Cole - Cengage 17
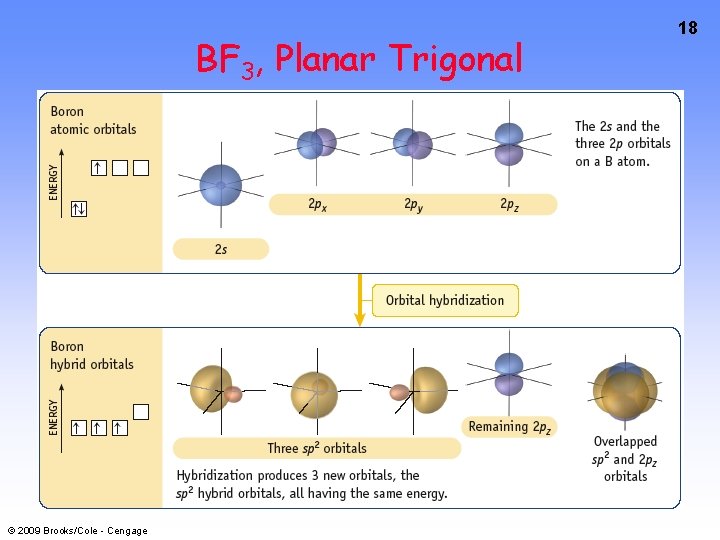
BF 3, Planar Trigonal © 2009 Brooks/Cole - Cengage 18
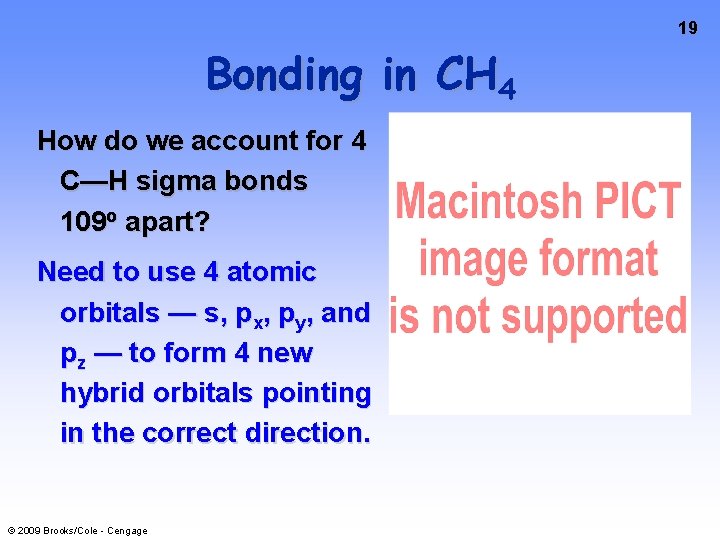
19 Bonding in CH 4 How do we account for 4 C—H sigma bonds 109 o apart? Need to use 4 atomic orbitals — s, px, py, and pz — to form 4 new hybrid orbitals pointing in the correct direction. © 2009 Brooks/Cole - Cengage
Bonding in a Tetrahedron Formation of Hybrid Atomic Orbitals 4 C atom orbitals hybridize to form four equivalent sp 3 hybrid atomic orbitals. © 2009 Brooks/Cole - Cengage 20
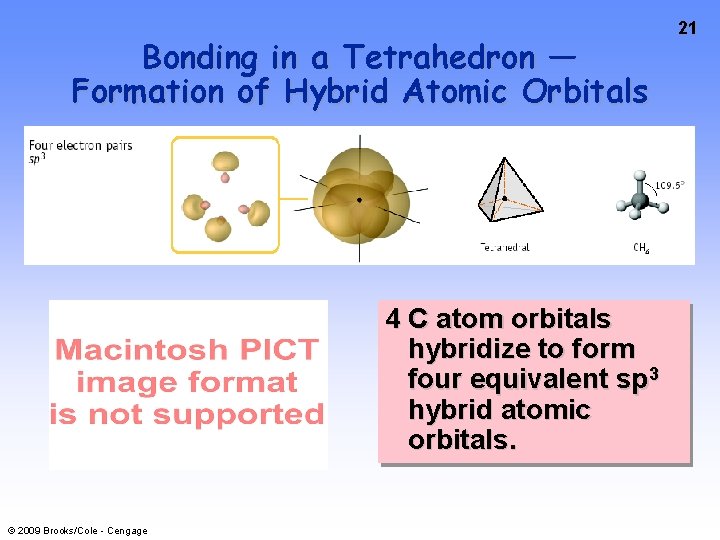
Bonding in a Tetrahedron — Formation of Hybrid Atomic Orbitals 4 C atom orbitals hybridize to form four equivalent sp 3 hybrid atomic orbitals. © 2009 Brooks/Cole - Cengage 21
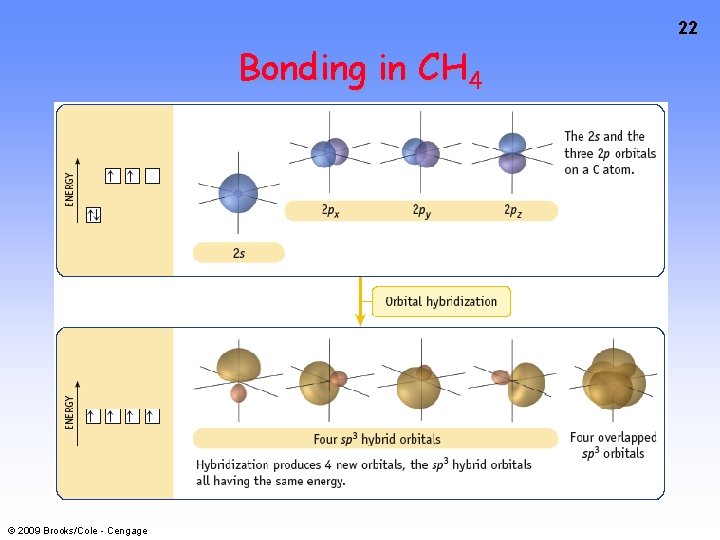
22 Bonding in CH 4 © 2009 Brooks/Cole - Cengage
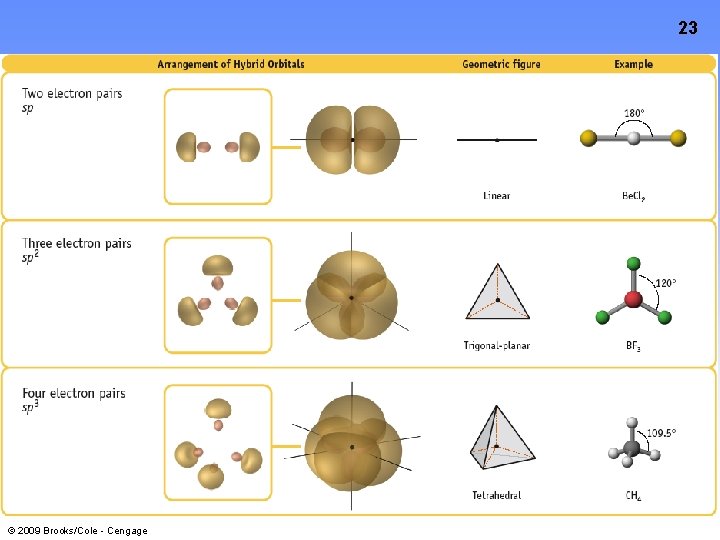
23 © 2009 Brooks/Cole - Cengage
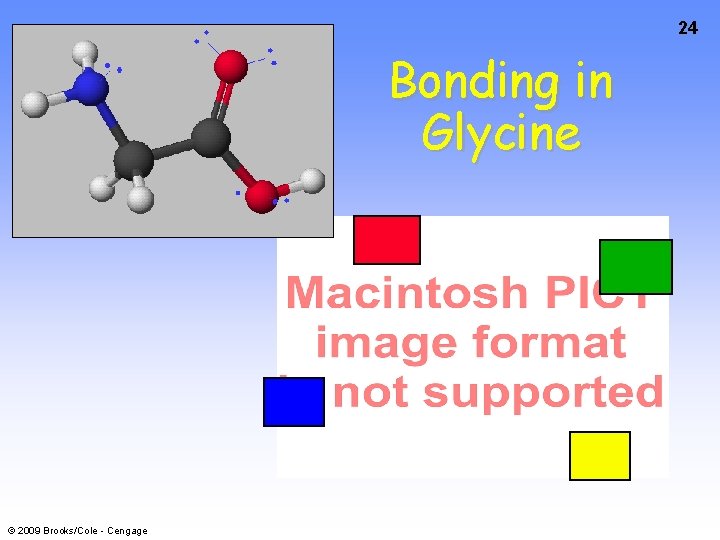
24 Bonding in Glycine © 2009 Brooks/Cole - Cengage
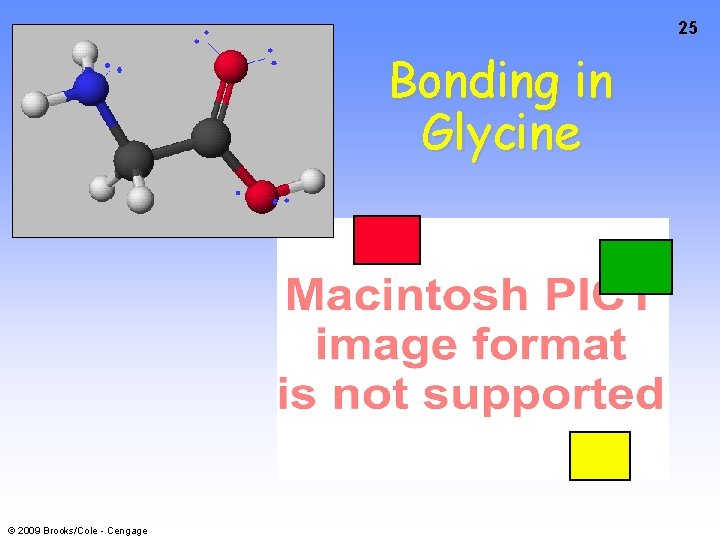
25 Bonding in Glycine © 2009 Brooks/Cole - Cengage
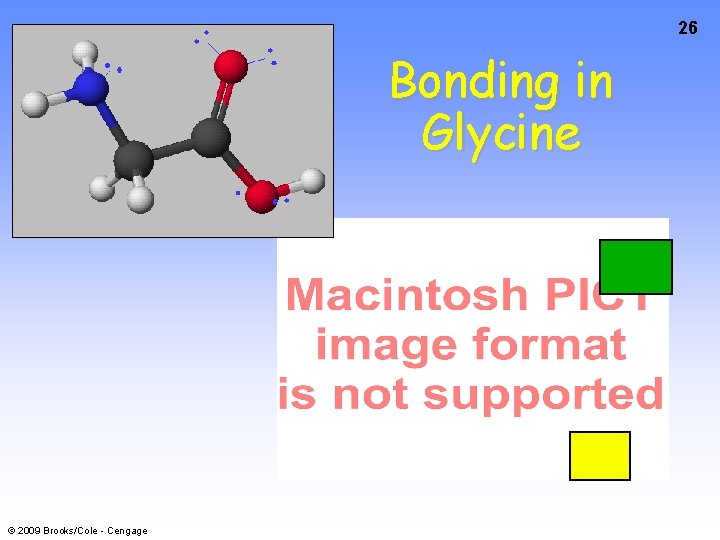
26 Bonding in Glycine © 2009 Brooks/Cole - Cengage
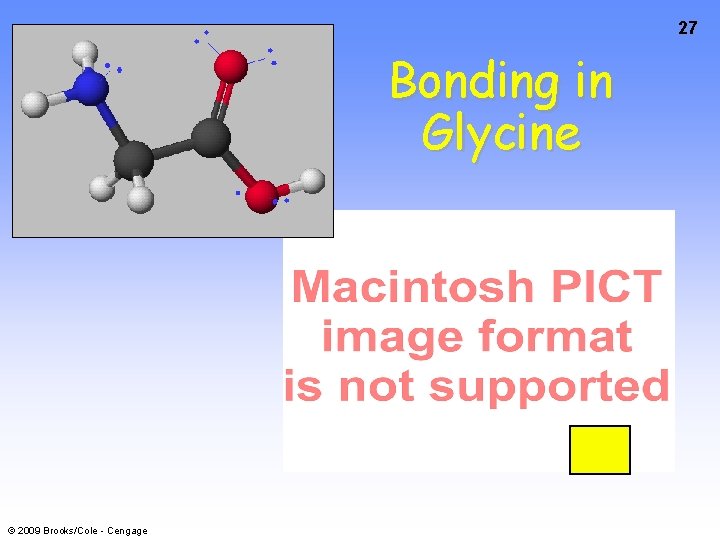
27 Bonding in Glycine © 2009 Brooks/Cole - Cengage
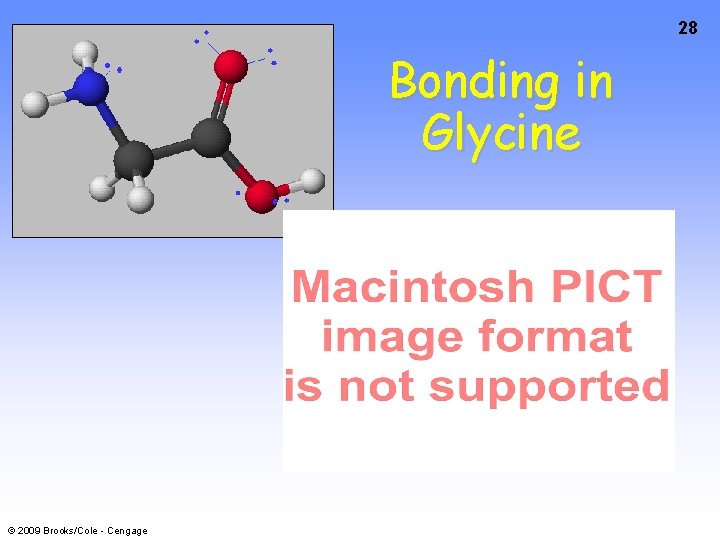
28 Bonding in Glycine © 2009 Brooks/Cole - Cengage
29 © 2009 Brooks/Cole - Cengage
Orbital Hybridization 2 e- clouds 3 e- clouds 4 e- clouds 5 e- clouds 6 e- clouds © 2009 Brooks/Cole - Cengage 30
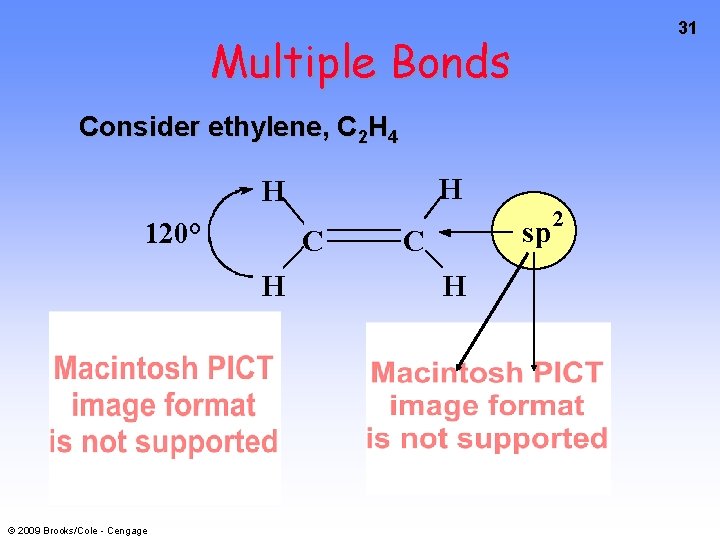
31 Multiple Bonds Consider ethylene, C 2 H 4 H H 120° C H © 2009 Brooks/Cole - Cengage sp C H 2
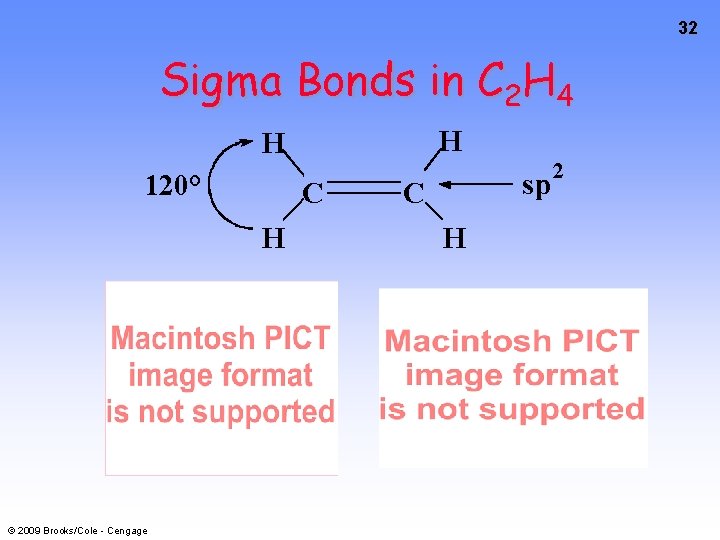
32 Sigma Bonds in C 2 H 4 H H 120° C H © 2009 Brooks/Cole - Cengage sp C H 2
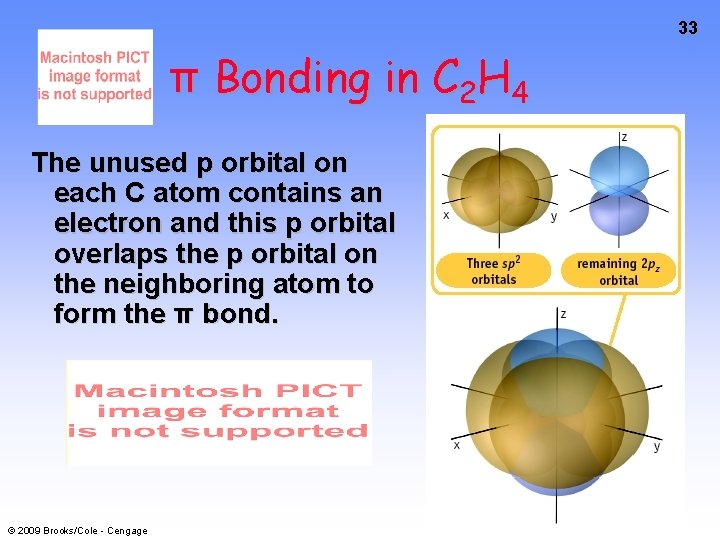
33 π Bonding in C 2 H 4 The unused p orbital on each C atom contains an electron and this p orbital overlaps the p orbital on the neighboring atom to form the π bond. © 2009 Brooks/Cole - Cengage
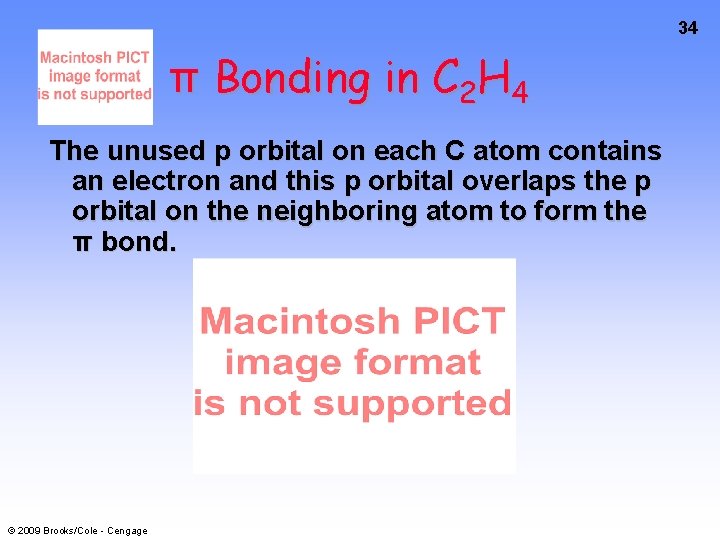
34 π Bonding in C 2 H 4 The unused p orbital on each C atom contains an electron and this p orbital overlaps the p orbital on the neighboring atom to form the π bond. © 2009 Brooks/Cole - Cengage
35 Multiple Bonding in C 2 H 4 © 2009 Brooks/Cole - Cengage
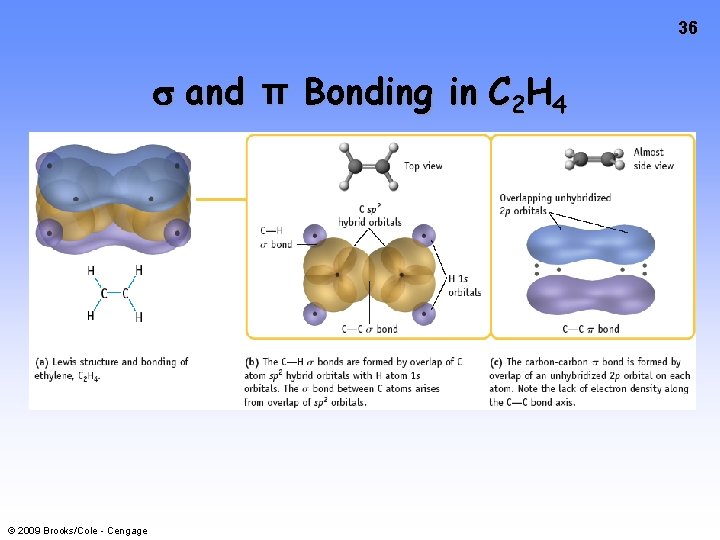
36 and π Bonding in C 2 H 4 © 2009 Brooks/Cole - Cengage
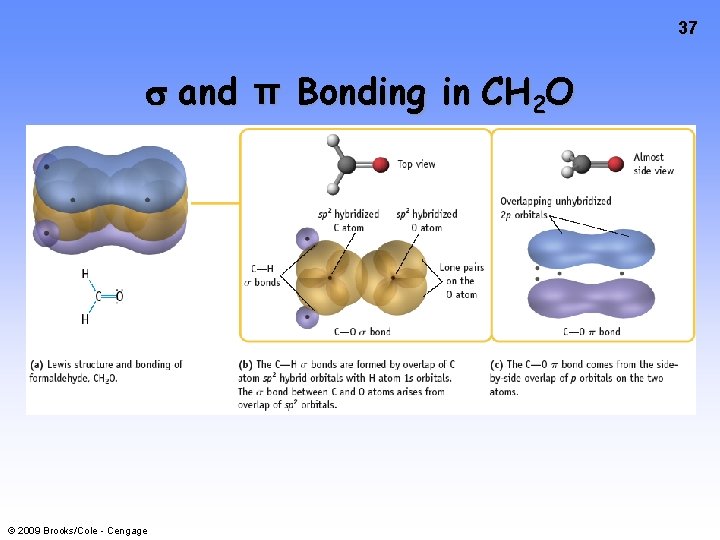
37 and π Bonding in CH 2 O © 2009 Brooks/Cole - Cengage
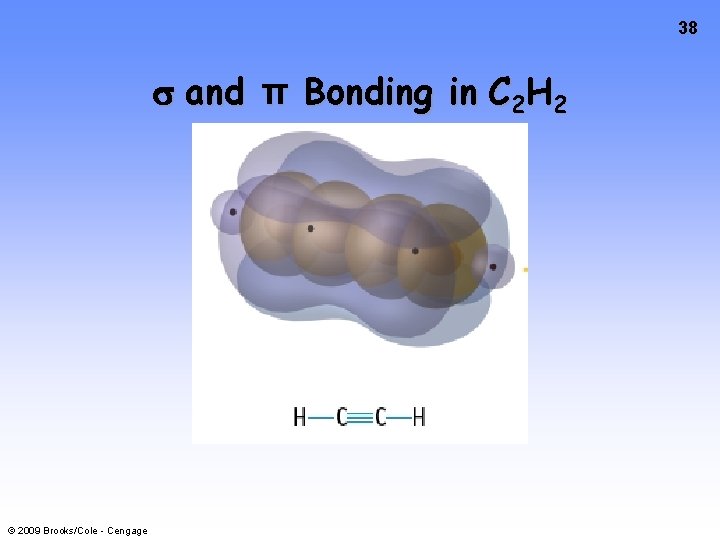
38 and π Bonding in C 2 H 2 © 2009 Brooks/Cole - Cengage
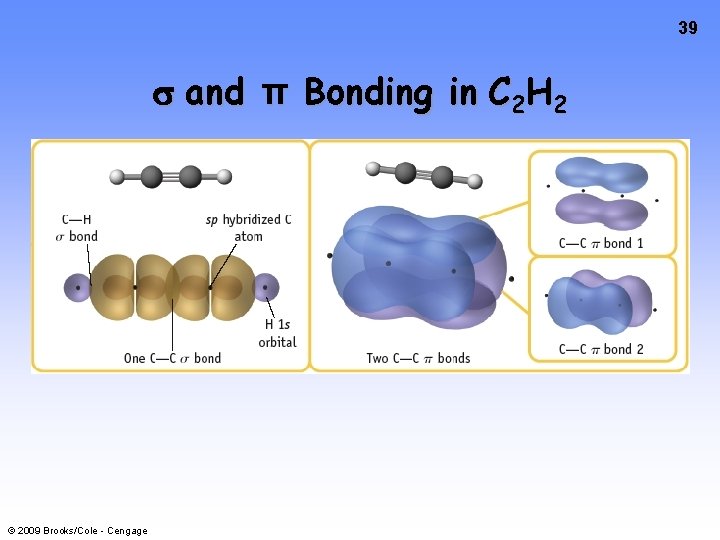
39 and π Bonding in C 2 H 2 © 2009 Brooks/Cole - Cengage
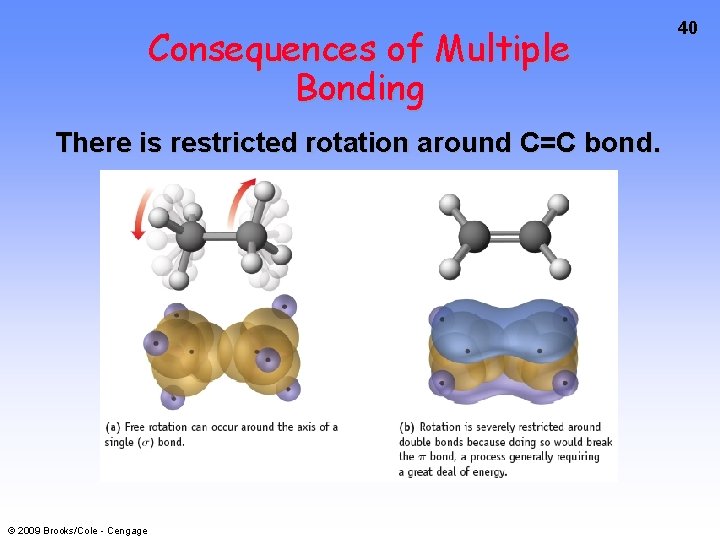
Consequences of Multiple Bonding There is restricted rotation around C=C bond. © 2009 Brooks/Cole - Cengage 40
Consequences of Multiple Bonding Restricted rotation around C=C bond. © 2009 Brooks/Cole - Cengage 41
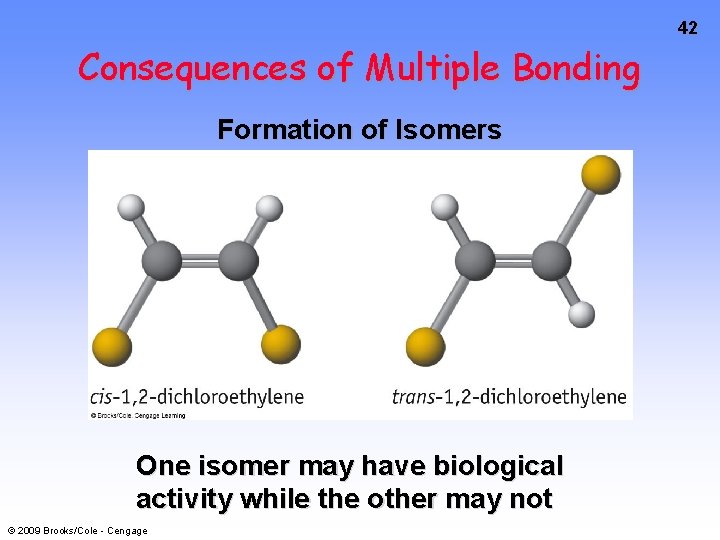
42 Consequences of Multiple Bonding Formation of Isomers One isomer may have biological activity while the other may not © 2009 Brooks/Cole - Cengage
43 Double Bonds and Vision © 2009 Brooks/Cole - Cengage
- Slides: 43