Advanced Stage Disease Management of Disease Progression and











- Slides: 27

Advanced Stage Disease: Management of Disease Progression and Emerging Drug Protocols Howard I. Scher, MD Chief, Genitourinary Oncology Service Memorial Sloan Kettering Cancer Center October 6, 2009

Advanced Stage Disease 1. Clinical States: A framework for drug development. 2. Dissecting the lethal phenotype. 3. Targeting AR signaling: MDV 3100. 4. CTC as a biomarker.

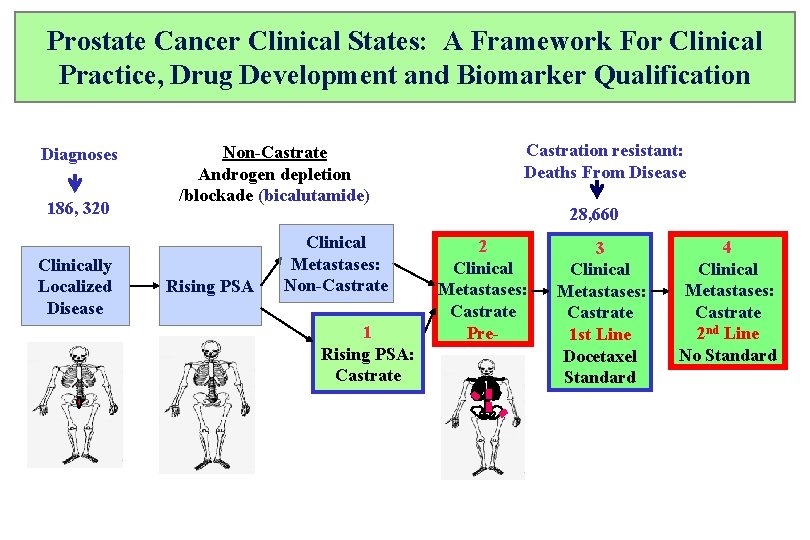
Prostate Cancer Clinical States: A Framework For Clinical Practice, Drug Development and Biomarker Qualification Diagnoses 186, 320 Clinically Localized Disease Non-Castrate Androgen depletion /blockade (bicalutamide) Rising PSA Clinical Metastases: Non-Castrate 1 Rising PSA: Castrate Castration resistant: Deaths From Disease 28, 660 2 Clinical Metastases: Castrate Pre- 3 Clinical Metastases: Castrate 1 st Line Docetaxel Standard 4 Clinical Metastases: Castrate 2 nd Line No Standard

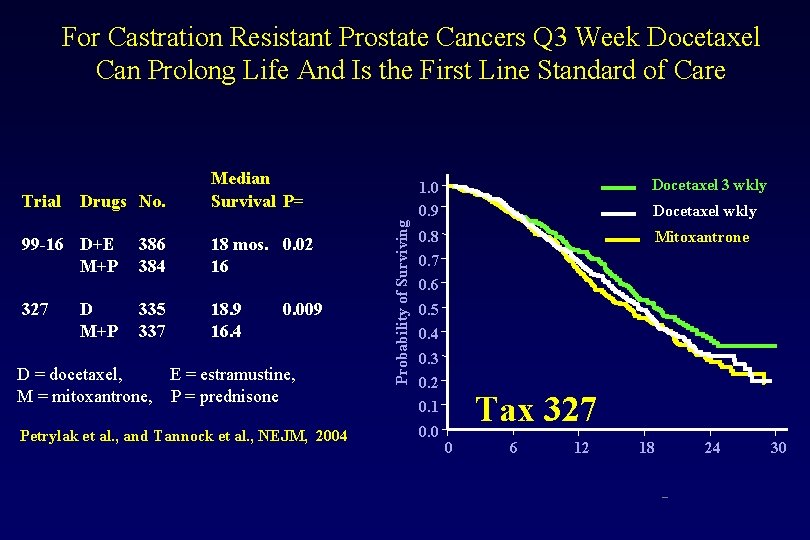
For Castration Resistant Prostate Cancers Q 3 Week Docetaxel Can Prolong Life And Is the First Line Standard of Care Drugs No. 99 -16 D+E M+P 327 D M+P 386 384 335 337 18 mos. 0. 02 16 18. 9 16. 4 0. 009 D = docetaxel, E = estramustine, M = mitoxantrone, P = prednisone Petrylak et al. , and Tannock et al. , NEJM, 2004 Probability of Surviving Trial Median Survival P= 1. 0 0. 9 Docetaxel 3 wkly 0. 8 Mitoxantrone Docetaxel wkly 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 Tax 327 0. 1 0. 0 0 6 12 18 24 – 30

Clinical Trials Are Experiments Conducted With Therapeutic Intent 1. Objective: Goals and therapeutic aim. 2. Patient population: Entry criteria: minimize heterogeneity, or enrich for specific characteristics. 3. Intervention: Mechanism: cidal, static, targeted. Dose and schedule: safety. 1. Outcomes: Endpoints: (aka response criteria). Phase 2: ? Signal, if so, how strong? Statistical design. 5. Conclusions: Was the question answered? Is continued development justified?

Outcome Measures Are Biomarkers To be Validated Analytically and Qualified Clinically 1. 2. 3. 4. Insure a drug is no longer working before stopping therapy. Report PSA changes using waterfall plots. Confirm bone scan findings with a second scan. Eliminate overall response as an outcome: focus on time to event.

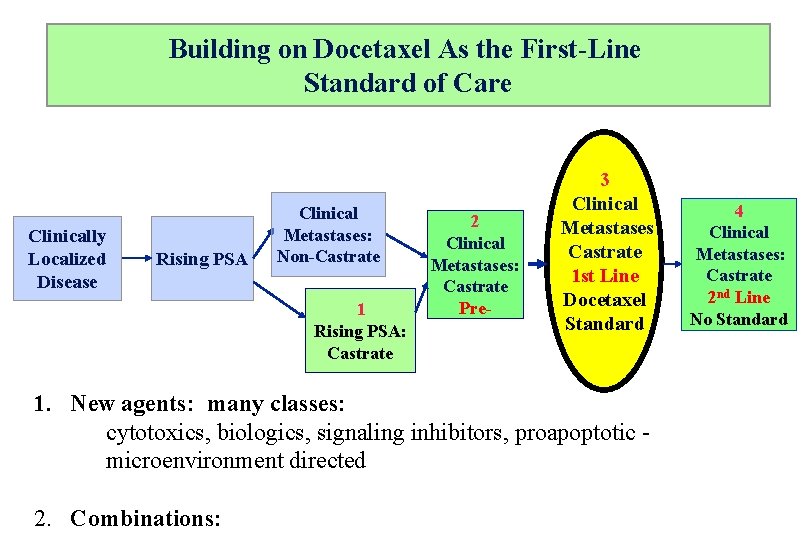
Building on Docetaxel As the First-Line Standard of Care Clinically Localized Disease Rising PSA Clinical Metastases: Non-Castrate 1 Rising PSA: Castrate 2 Clinical Metastases: Castrate Pre- 3 Clinical Metastases Castrate 1 st Line Docetaxel Standard 1. New agents: many classes: cytotoxics, biologics, signaling inhibitors, proapoptotic microenvironment directed 2. Combinations: 4 Clinical Metastases: Castrate 2 nd Line No Standard

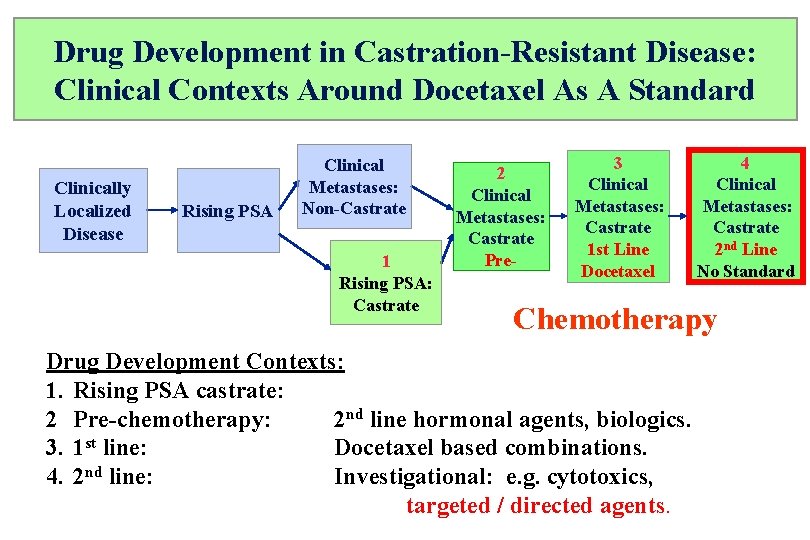
Drug Development in Castration-Resistant Disease: Clinical Contexts Around Docetaxel As A Standard Clinically Localized Disease Rising PSA Clinical Metastases: Non-Castrate 1 Rising PSA: Castrate 2 Clinical Metastases: Castrate Pre- 3 Clinical Metastases: Castrate 1 st Line Docetaxel 4 Clinical Metastases: Castrate 2 nd Line No Standard Chemotherapy Drug Development Contexts: 1. Rising PSA castrate: 2 Pre-chemotherapy: 2 nd line hormonal agents, biologics. 3. 1 st line: Docetaxel based combinations. 4. 2 nd line: Investigational: e. g. cytotoxics, targeted / directed agents.

A Partial List of Taxotere Combinations Under Evaluation As First-Line Therapy Phase 3 1. + Avastin (anti-VEGF Ab) Genentech (CALGB) 2. + Atrasentan Abbott (SWOG) 3. + ZD 4054 Astra-Zeneca (ENTHUSE) 4. + VEGF-trap Sanofi-Aventis 5. + dasatinib BMS 6. + Gossypol (BCL-2) Ascenta 7. + clusterin antisense Oncogenix With caveat the PSA changes are misleading! - accrued

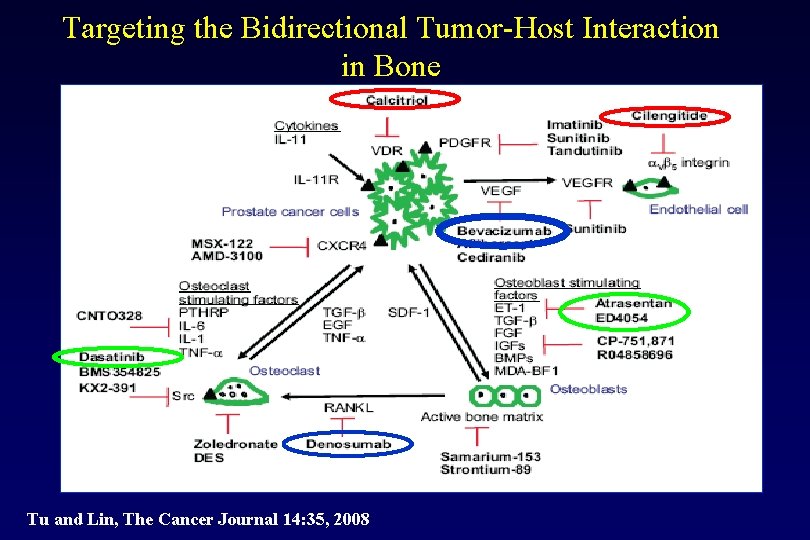
Targeting the Bidirectional Tumor-Host Interaction in Bone Tu and Lin, The Cancer Journal 14: 35, 2008

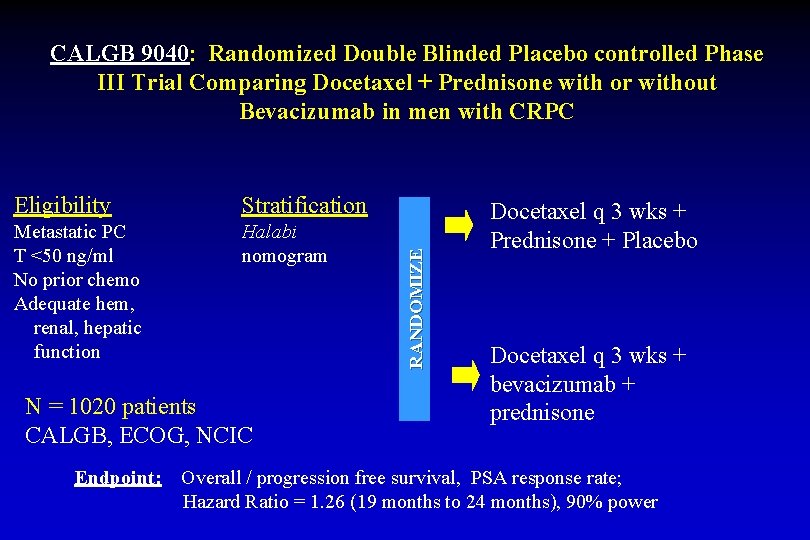
Eligibility Stratification Metastatic PC T <50 ng/ml No prior chemo Adequate hem, renal, hepatic function Halabi nomogram N = 1020 patients CALGB, ECOG, NCIC Endpoint: RANDOMIZE CALGB 9040: Randomized Double Blinded Placebo controlled Phase III Trial Comparing Docetaxel + Prednisone with or without Bevacizumab in men with CRPC Docetaxel q 3 wks + Prednisone + Placebo Docetaxel q 3 wks + bevacizumab + prednisone Overall / progression free survival, PSA response rate; Hazard Ratio = 1. 26 (19 months to 24 months), 90% power

Advanced Stage Disease 1. Clinical States: A framework for drug development. 2. Dissecting the lethal phenotype. 3. Targeting AR signaling: MDV 3100. 4. CTC as a biomarker.

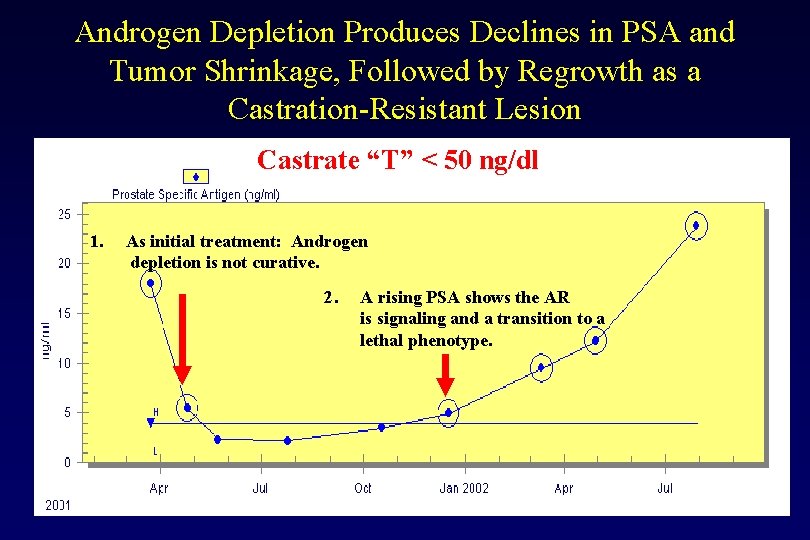
Androgen Depletion Produces Declines in PSA and Tumor Shrinkage, Followed by Regrowth as a Castration-Resistant Lesion Castrate “T” < 50 ng/dl 1. As initial treatment: Androgen depletion is not curative. 2. A rising PSA shows the AR is signaling and a transition to a lethal phenotype.

Clinical Insights into Castration-Resistant Progression Guiding Drug Development 1. Rising PSA levels are consistent with continued AR signaling. 2. Clinical significance of AR targeting is reinforced by the response to secondary hormone therapies, as well as the “withdrawal”/ “discontinuation” of anti-androgens. 3. This suggests antagonists later functions as an agonist as the disease progresses. 4. The AR ligand binding domain is clinically relevant and contributes to progression.

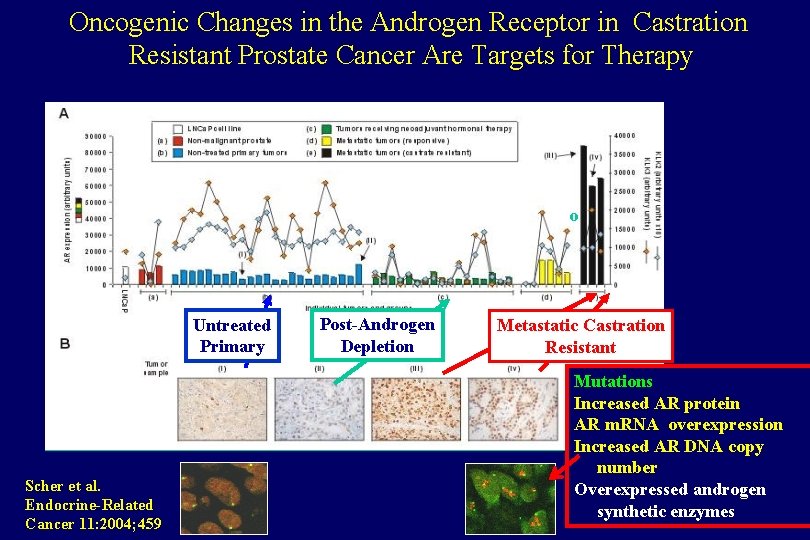
Oncogenic Changes in the Androgen Receptor in Castration Resistant Prostate Cancer Are Targets for Therapy o Untreated Primary Scher et al. Endocrine-Related Cancer 11: 2004; 459 Post-Androgen Depletion Metastatic Castration Resistant Mutations Increased AR protein AR m. RNA overexpression Increased AR DNA copy number Overexpressed androgen synthetic enzymes

Advanced Stage Disease 1. Clinical States: A framework for drug development. 2. Dissecting the lethal phenotype. 3. Targeting AR signaling: MDV 3100. 4. CTC as a biomarker.

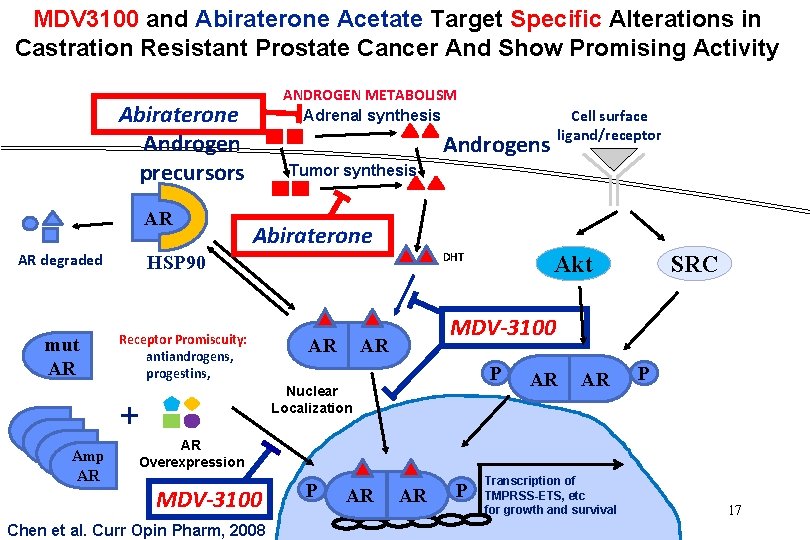
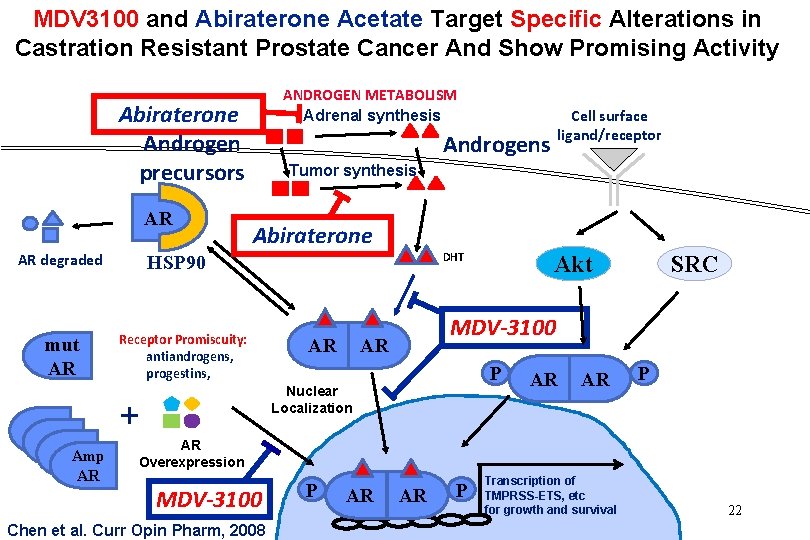
MDV 3100 and Abiraterone Acetate Target Specific Alterations in Castration Resistant Prostate Cancer And Show Promising Activity ANDROGEN METABOLISM Adrenal synthesis Abiraterone Androgen precursors AR AR degraded mut AR AR Amp AR Cell surface ligand/receptor Androgens Tumor synthesis Abiraterone DHT HSP 90 Receptor Promiscuity: antiandrogens, progestins, AR SRC MDV-3100 AR P Nuclear Localization + Akt AR AR P AR Overexpression MDV-3100 Chen et al. Curr Opin Pharm, 2008 P AR AR P Transcription of TMPRSS-ETS, etc for growth and survival 17

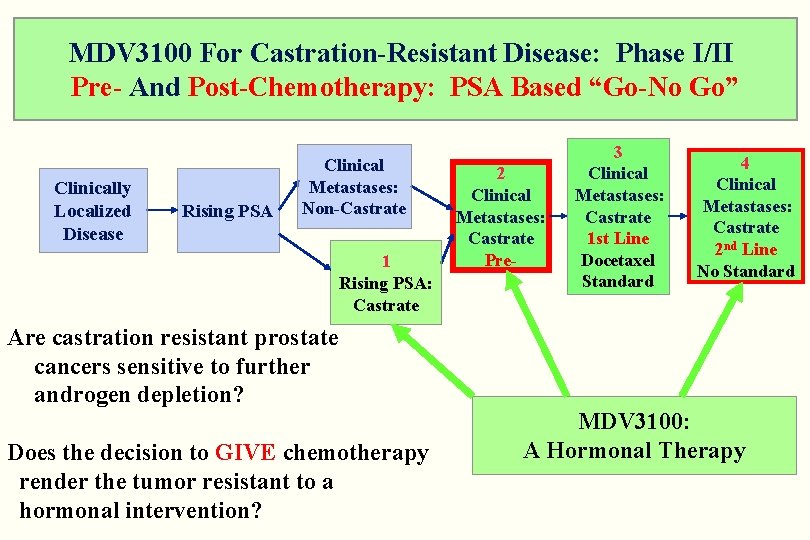
MDV 3100 For Castration-Resistant Disease: Phase I/II Pre- And Post-Chemotherapy: PSA Based “Go-No Go” Clinically Localized Disease Rising PSA Clinical Metastases: Non-Castrate 1 Rising PSA: Castrate 2 Clinical Metastases: Castrate Pre- 3 Clinical Metastases: Castrate 1 st Line Docetaxel Standard 4 Clinical Metastases: Castrate 2 nd Line No Standard Are castration resistant prostate cancers sensitive to further androgen depletion? Does the decision to GIVE chemotherapy render the tumor resistant to a hormonal intervention? MDV 3100: A Hormonal Therapy

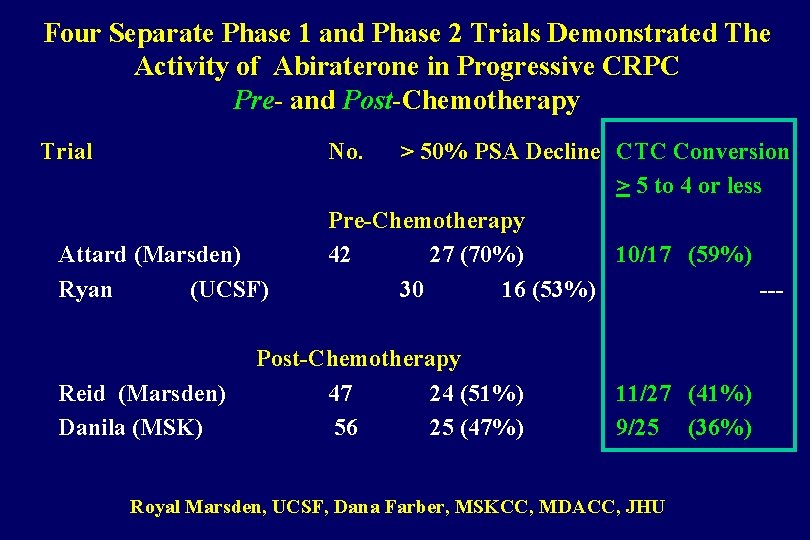
Four Separate Phase 1 and Phase 2 Trials Demonstrated The Activity of Abiraterone in Progressive CRPC Pre- and Post-Chemotherapy Trial No. Attard (Marsden) Ryan (UCSF) Reid (Marsden) Danila (MSK) > 50% PSA Decline CTC Conversion > 5 to 4 or less Pre-Chemotherapy 42 27 (70%) 10/17 (59%) 30 16 (53%) --- Post-Chemotherapy 47 24 (51%) 56 25 (47%) 11/27 (41%) 9/25 (36%) Royal Marsden, UCSF, Dana Farber, MSKCC, MDACC, JHU

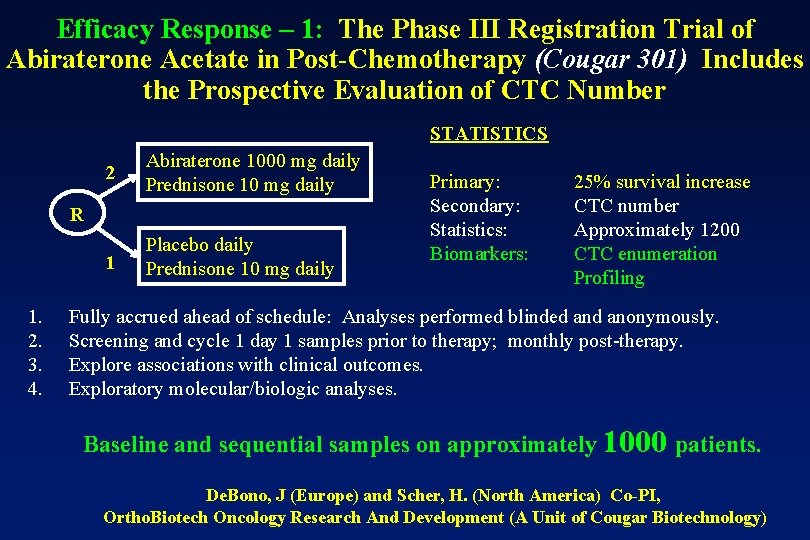
Efficacy Response – 1: The Phase III Registration Trial of Abiraterone Acetate in Post-Chemotherapy (Cougar 301) Includes the Prospective Evaluation of CTC Number STATISTICS 2 Abiraterone 1000 mg daily Prednisone 10 mg daily 1 Placebo daily Prednisone 10 mg daily R 1. 2. 3. 4. Primary: Secondary: Statistics: Biomarkers: 25% survival increase CTC number Approximately 1200 CTC enumeration Profiling Fully accrued ahead of schedule: Analyses performed blinded anonymously. Screening and cycle 1 day 1 samples prior to therapy; monthly post-therapy. Explore associations with clinical outcomes. Exploratory molecular/biologic analyses. Baseline and sequential samples on approximately 1000 patients. De. Bono, J (Europe) and Scher, H. (North America) Co-PI, Ortho. Biotech Oncology Research And Development (A Unit of Cougar Biotechnology)

Advanced Stage Disease 1. Clinical States: A framework for drug development. 2. Dissecting the lethal phenotype. 3. Targeting AR signaling: MDV 3100. 4. CTC as a biomarker.

MDV 3100 and Abiraterone Acetate Target Specific Alterations in Castration Resistant Prostate Cancer And Show Promising Activity ANDROGEN METABOLISM Adrenal synthesis Abiraterone Androgen precursors AR AR degraded mut AR AR Amp AR Cell surface ligand/receptor Androgens Tumor synthesis Abiraterone DHT HSP 90 Receptor Promiscuity: antiandrogens, progestins, AR SRC MDV-3100 AR P Nuclear Localization + Akt AR AR P AR Overexpression MDV-3100 Chen et al. Curr Opin Pharm, 2008 P AR AR P Transcription of TMPRSS-ETS, etc for growth and survival 22

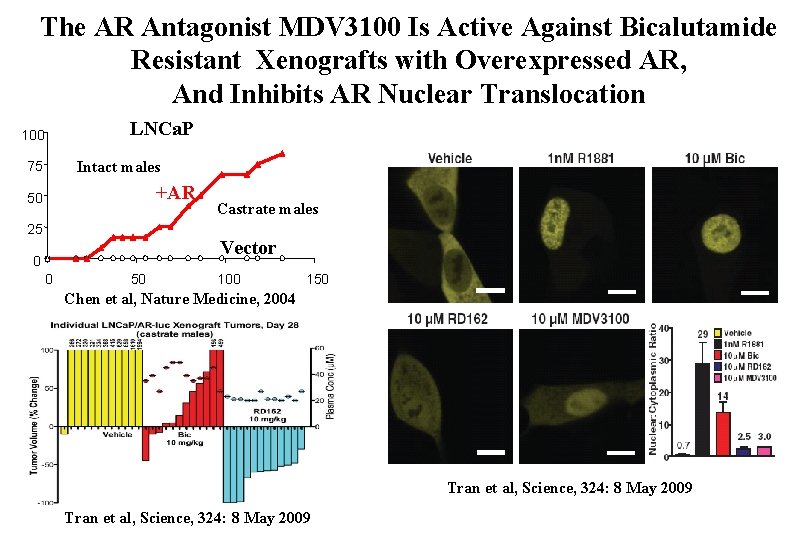
The AR Antagonist MDV 3100 Is Active Against Bicalutamide Resistant Xenografts with Overexpressed AR, And Inhibits AR Nuclear Translocation LNCa. P 100 Intact males 75 +AR 50 25 Castrate males Vector 0 0 50 100 150 Chen et al, Nature Medicine, 2004 Tran et al, Science, 324: 8 May 2009

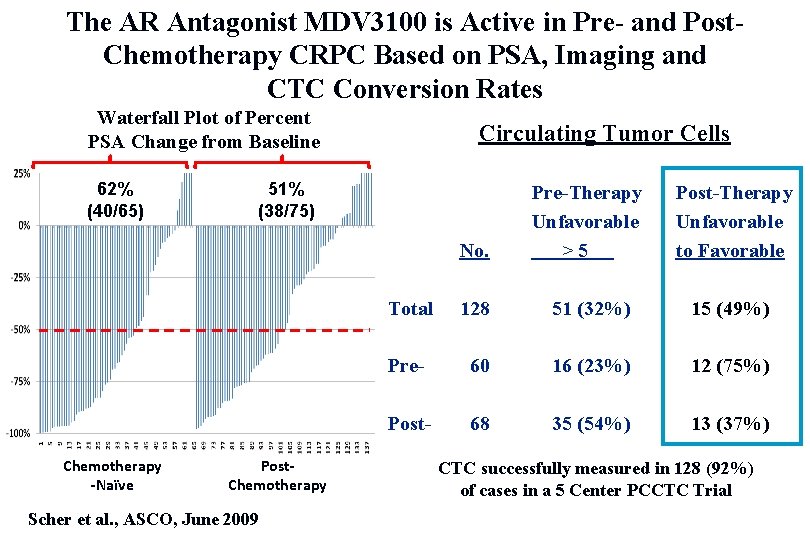
The AR Antagonist MDV 3100 is Active in Pre- and Post. Chemotherapy CRPC Based on PSA, Imaging and CTC Conversion Rates Waterfall Plot of Percent PSA Change from Baseline 62% (40/65) Chemotherapy -Naïve Circulating Tumor Cells 51% (38/75) Post. Chemotherapy Scher et al. , ASCO, June 2009 No. Pre-Therapy Unfavorable >5 Post-Therapy Unfavorable to Favorable Total 128 51 (32%) 15 (49%) Pre- 60 16 (23%) 12 (75%) Post- 68 35 (54%) 13 (37%) CTC successfully measured in 128 (92%) of cases in a 5 Center PCCTC Trial

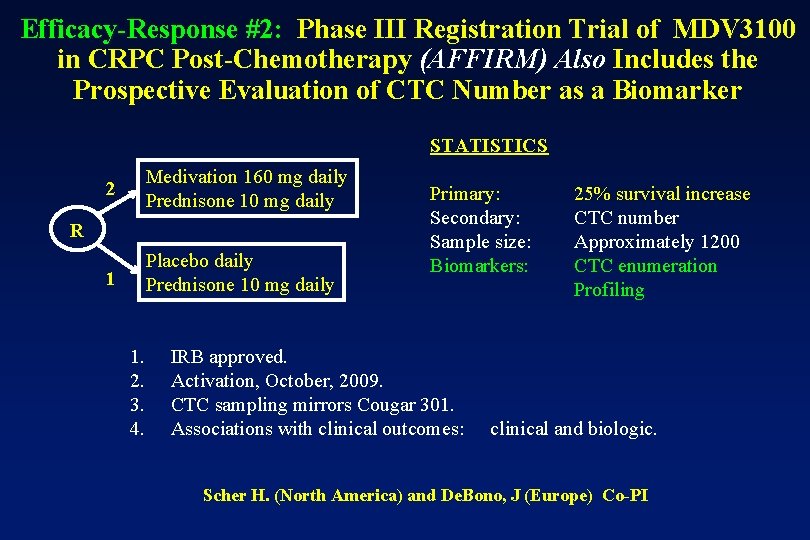
Efficacy-Response #2: Phase III Registration Trial of MDV 3100 in CRPC Post-Chemotherapy (AFFIRM) Also Includes the Prospective Evaluation of CTC Number as a Biomarker STATISTICS 2 Medivation 160 mg daily Prednisone 10 mg daily 1 Placebo daily Prednisone 10 mg daily R 1. 2. 3. 4. Primary: Secondary: Sample size: Biomarkers: IRB approved. Activation, October, 2009. CTC sampling mirrors Cougar 301. Associations with clinical outcomes: 25% survival increase CTC number Approximately 1200 CTC enumeration Profiling clinical and biologic. Scher H. (North America) and De. Bono, J (Europe) Co-PI

Therapy Development: A Multidisciplinary Team Daniel Danila David Solit Dana Rathkopf Michael Morris Nicholas Mitsiades Charles Sawyers Yu Chen Nicola Clegg Royal Marsden: Johann de Bono Gerhart Attard Neal Rosen U. Miami: Richard Cote Martin Fleisher Hans Lilja Rita Espinosa. Gonzalez Aseem Anand Adriana Heguy Margaret Leversha Jan Hendrix Oscar Lin OHSU: Tom Beer CSHL: U Washington: Celestia Higano Bruce Montgomery Glenn Heller Larry Schwartz Hedvig Steven Solomon Chris Sander Nikki Schultz †William Gerald Anu Gopalan Victor Reuter Richard Mac. Combie MGH SU 2 C: Dan Haber FDA BQRT: Federico Goodsaid MDACC: Chris Logothetis Eleni Efstathiou DFCI: Steven Larson Peter Smith Jones Ortho Biotechnology (Cougar): Arturo Molina Chris Haqq Medivation: Lynn Seely Mohammed Hirmand Veridex: Robert Mc. Cormack Mary-Ellen Taplin U Michigan: Maha Hussain NIH SPORE; DOD PCCTC Prostate Cancer Foundation STARR Foundation , FNIH De. Witt Wallace
