Advanced Spectroscopy 20142015 Introduction to the course Advanced









- Slides: 70

Advanced Spectroscopy 2014/2015 Introduction to the course Advanced Spectroscopy Professor Dan Stærk Natural Products Research Department of Drug Design & Pharmacology Faculty of Health and Medical Sciences University of Copenhagen Open door policy –> you are always welcome to come and ask questions (or send me an e-mail) Dias 1

Advanced Spectroscopy This course will provide you with the necessary skills and knowledge to use NMR- and MS-data to determine the structure of unknown organic small-molecules. Aim of the course: ü To introduce participants to the practical application of modern spectroscopic methods for structure elucidation of small to medium-sized organic molecules. ü This involves elucidation of molecular formula, connectivity of all atoms, and determination of relative and absolute stereo-chemistry. ü Emphasis is on mass spectrometry and nuclear magnetic resonance spectroscopy, but chiroptical methods will also be introduced ü Use of modern NMR software for data processing and acquisition is an integrated part of the course. Dias 2 Advanced Spectroscopy 2014/2015

Practicalities Block structure, schedule, places ü Short Tuesdays -> lectures and class exercises ü Long Thursdays -> lectures, class exercises, computer exercises ü Increasing complexity (and in-depth knowledge) in teaching modules: Lectures -> class sessions -> computer exercises-> case exercises -> exam project. ü Lectures and class exercises in U 11. Computer exercises in D 1. ü 20 licenses to Bruker Topspin NMR program & ACD-labs installed Language ü English (both in writing and speaking). Literature ü Lambert, Gronert, Shurvell, Lightner, Organic structural Spectroscopy, 2 nd Edition 2012 ü Articles, lecture notes Absalon ü Schedule, lecture slides, discussion forum, tests, data, … Black book ü Comments (constructive, negative as well as positive) Dias 3 Advanced Spectroscopy 2014/2015

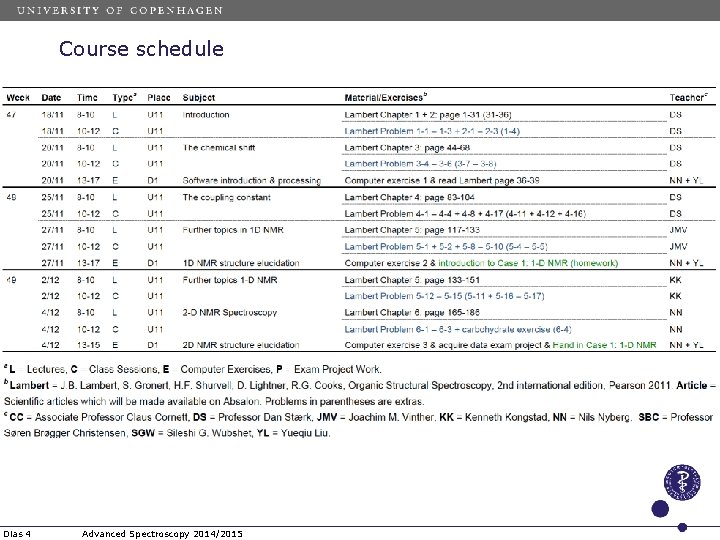
Course schedule Dias 4 Advanced Spectroscopy 2014/2015

Teachers Dan Stærk, ds@sund. ku. dk ü Professor - Natural Products Research Postdoc Nils Nyberg, nn@sund. ku. dk ü NMR manager - Natural Products Research Kenneth T. Kongstad, kjj@sund. ku. dk ü Postdoc - Natural Products Research Dias 5 Advanced Spectroscopy 2014/2015

Teachers Sileshi Wubshet, sgw@sund. ku. dk ü Postdoc - Natural Products Research Claus Cornett, clco@sund. ku. dk ü Associate professor - Department of Pharmacy Joachim M. Vinther, joachim. vinther@sund. ku. dk ü Postdoc – Natural Products Research Dias 6 Advanced Spectroscopy 2014/2015

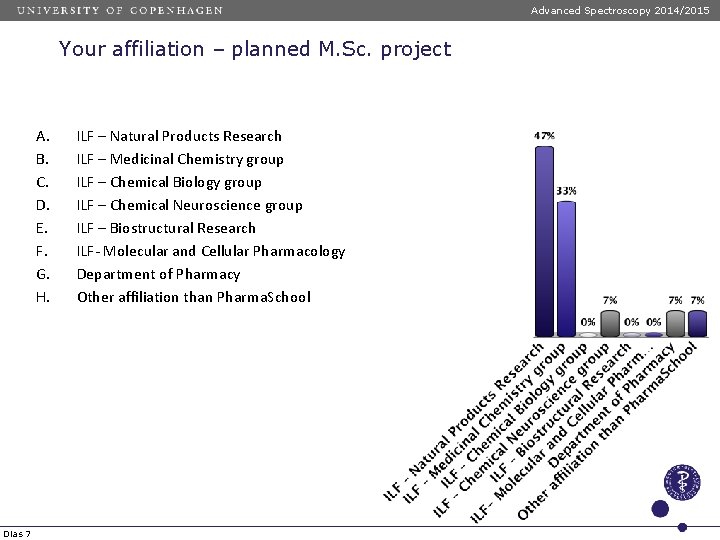
Advanced Spectroscopy 2014/2015 Your affiliation – planned M. Sc. project A. B. C. D. E. F. G. H. Dias 7 ILF – Natural Products Research ILF – Medicinal Chemistry group ILF – Chemical Biology group ILF – Chemical Neuroscience group ILF – Biostructural Research ILF- Molecular and Cellular Pharmacology Department of Pharmacy Other affiliation than Pharma. School

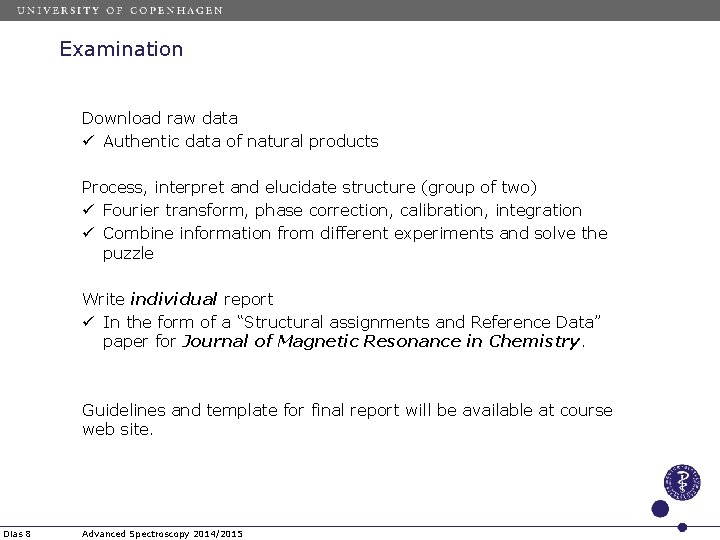
Examination Download raw data ü Authentic data of natural products Process, interpret and elucidate structure (group of two) ü Fourier transform, phase correction, calibration, integration ü Combine information from different experiments and solve the puzzle Write individual report ü In the form of a “Structural assignments and Reference Data” paper for Journal of Magnetic Resonance in Chemistry. Guidelines and template for final report will be available at course web site. Dias 8 Advanced Spectroscopy 2014/2015

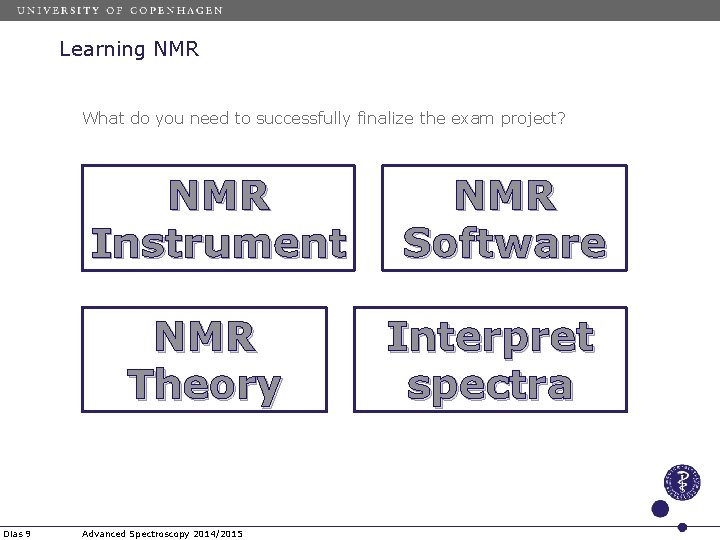
Learning NMR What do you need to successfully finalize the exam project? NMR Instrument NMR Theory Dias 9 Advanced Spectroscopy 2014/2015 NMR Software Interpret spectra

NMR software Topspin ü Software from Bruker Bio. Spin ü Expensive ü Restricted teaching licenses (20 floating) ACD/NMR-lab • Free for academic use • http: //acdlabs. com/resources/freeware/nmr_proc/ • First computer exercise session on Thursday in D 1 • Download and install program - and have a look at tutorials and demonstrations before computer exercises • Import, process and interpret spectra (supervised, 13. 00 -17. 00) Dias 10 Advanced Spectroscopy 2014/2015

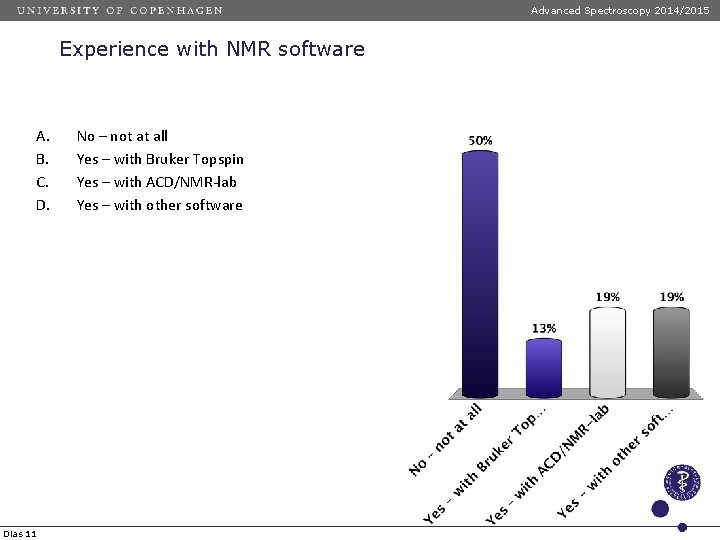
Advanced Spectroscopy 2014/2015 Experience with NMR software A. B. C. D. Dias 11 No – not at all Yes – with Bruker Topspin Yes – with ACD/NMR-lab Yes – with other software

Why spectroscopy? Spectroscopic techniques… are our “eyes” that allow us to “see” molecules – their detailed structures, i. e. , how individual atoms are connected. Knowledge of molecular structures of compounds is fundamental in all branches of chemistry. History Morphine was isolated from opium in 1805. Structure determined in 1924 (119 years later!). Today A skilled person (you) having a few mg of morphine and access to modern spectroscopic equipment would determine structure of morphine in 1 -2 days. Dias 12 Advanced Spectroscopy 2014/2015

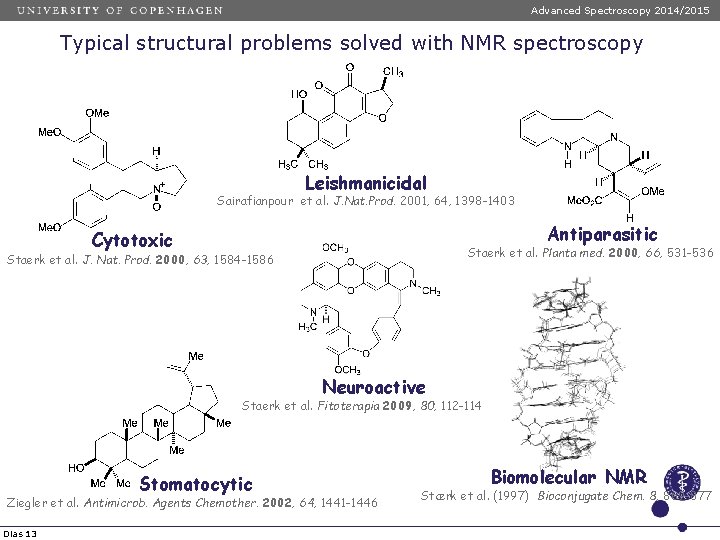
Advanced Spectroscopy 2014/2015 Typical structural problems solved with NMR spectroscopy Leishmanicidal Sairafianpour et al. J. Nat. Prod. 2001, 64, 1398 -1403 Antiparasitic Cytotoxic Staerk et al. Planta med. 2000, 66, 531 -536 Staerk et al. J. Nat. Prod. 2000, 63, 1584 -1586 Neuroactive Staerk et al. Fitoterapia 2009, 80, 112 -114 Stomatocytic Ziegler et al. Antimicrob. Agents Chemother. 2002, 64, 1441 -1446 Dias 13 Biomolecular NMR Stærk et al. (1997) Bioconjugate Chem. 8, 869 -877

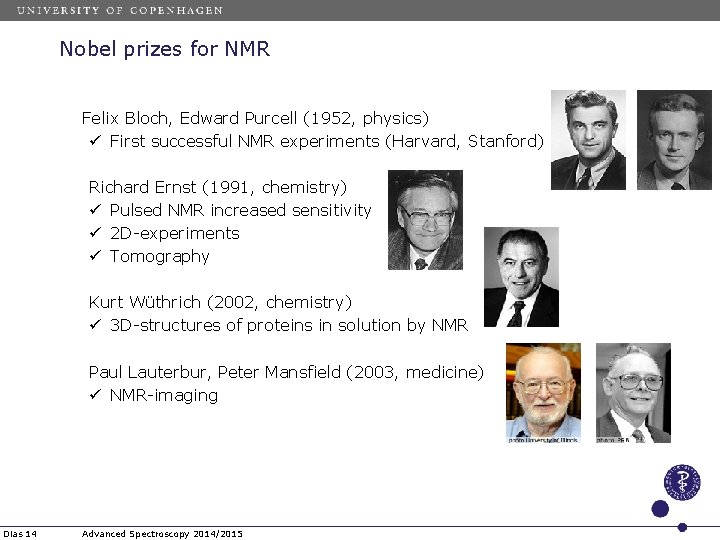
Nobel prizes for NMR Felix Bloch, Edward Purcell (1952, physics) ü First successful NMR experiments (Harvard, Stanford) Richard Ernst (1991, chemistry) ü Pulsed NMR increased sensitivity ü 2 D-experiments ü Tomography Kurt Wüthrich (2002, chemistry) ü 3 D-structures of proteins in solution by NMR Paul Lauterbur, Peter Mansfield (2003, medicine) ü NMR-imaging Dias 14 Advanced Spectroscopy 2014/2015

Spectroscopic methods Dias 15 Advanced Spectroscopy 2014/2015

Spectroscopic methods - solving the puzzle Ultraviolet/visible spectroscopy (UV/Vis) ü Chromophores, conjugated double bonds Infrared spectroscopy (IR) ü Functional groups Mass spectrometry (MS) ü Molecular mass, fragmentation patterns Nuclear magnetic resonance spectroscopy (NMR) ü Detailed structural information: functional groups, connectivity, relative stereochemistry ü Dynamic information, conformational information Chiroptical methods (CD, ORD) ü Absolute configuration of molecules X-ray, neutron, electron diffraction ü Gives complete structural information (but limited applicability) Dias 16 Advanced Spectroscopy 2014/2015

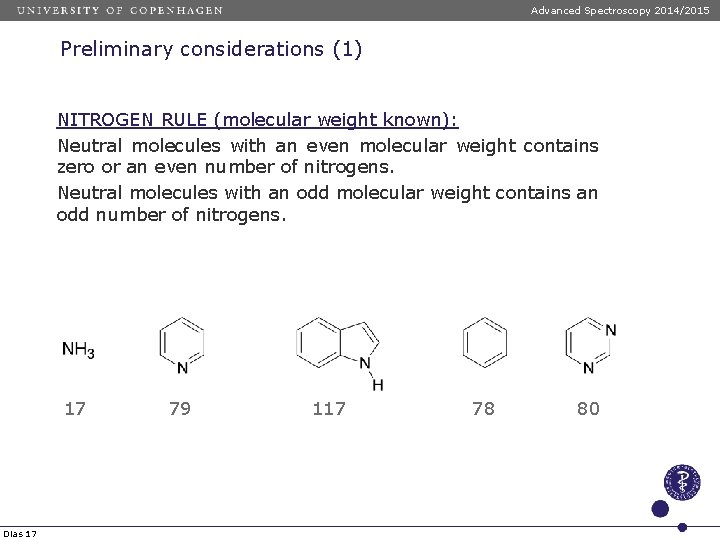
Advanced Spectroscopy 2014/2015 Preliminary considerations (1) NITROGEN RULE (molecular weight known): Neutral molecules with an even molecular weight contains zero or an even number of nitrogens. Neutral molecules with an odd molecular weight contains an odd number of nitrogens. 17 Dias 17 79 117 78 80

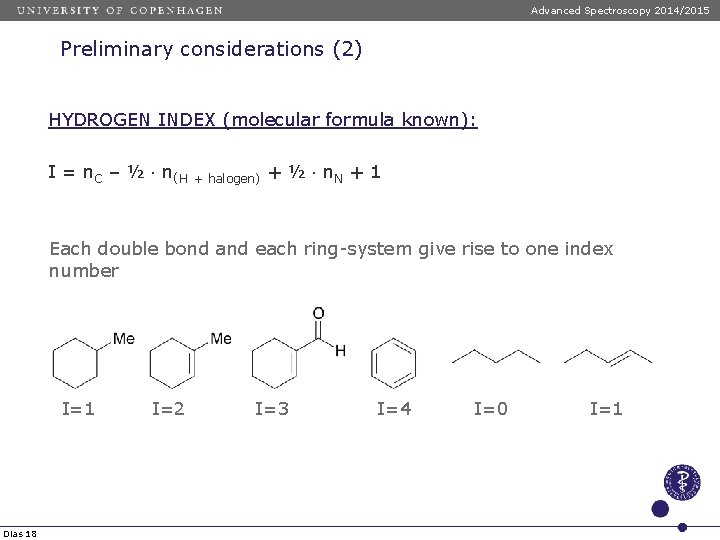
Advanced Spectroscopy 2014/2015 Preliminary considerations (2) HYDROGEN INDEX (molecular formula known): I = n. C – ½ n(H + halogen) + ½ n. N + 1 Each double bond and each ring-system give rise to one index number I=1 Dias 18 I=2 I=3 I=4 I=0 I=1

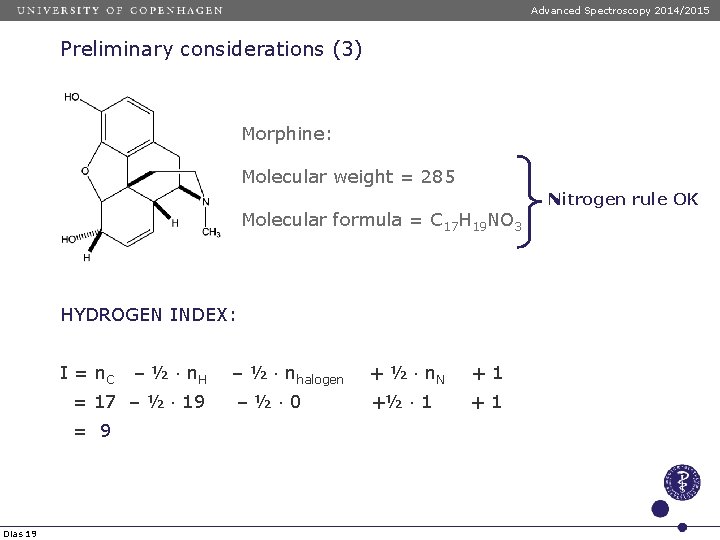
Advanced Spectroscopy 2014/2015 Preliminary considerations (3) Morphine: Molecular weight = 285 Molecular formula = C 17 H 19 NO 3 HYDROGEN INDEX: I = n. C – ½ n. H = 17 – ½ 19 = 9 Dias 19 – ½ nhalogen + ½ n. N +1 –½ 0 +½ 1 +1 Nitrogen rule OK

Advanced Spectroscopy 2014/2015 Exercise 1 - preliminary considerations How many rings and/or double bonds does a molecule with the molecular formula C 8 H 10 O 4 possess? A. B. C. D. E. F. G. Dias 20 1 2 3 4 5 6 7

Advanced Spectroscopy 2014/2015 Exercise 1 – preliminary considerations How many rings and/or double bonds does a molecule with the molecular formula C 8 H 10 O 4 possess? HYDROGEN INDEX: I = n. C Dias 21 – ½ n. H – ½ nhalogen + ½ n. N +1

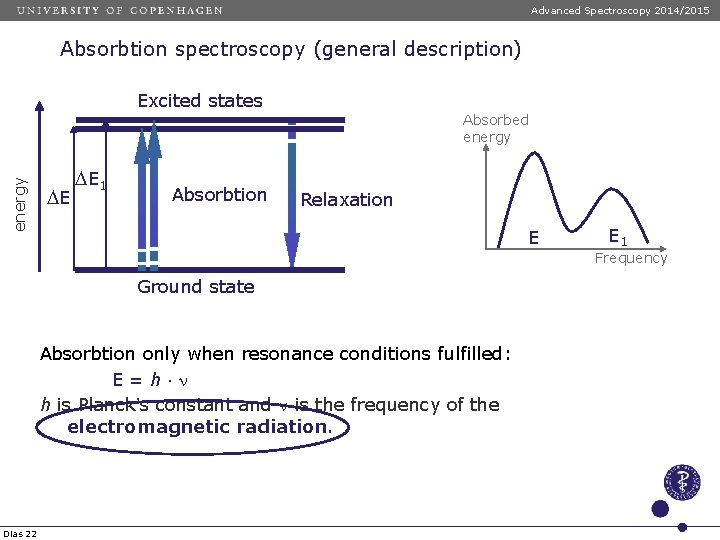
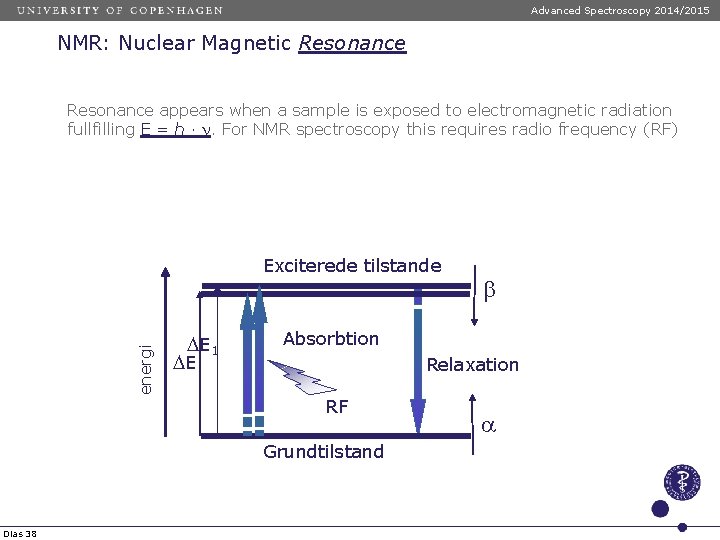
Advanced Spectroscopy 2014/2015 Absorbtion spectroscopy (general description) Excited states energy Absorbed energy DE D E 1 Absorbtion Relaxation E E 1 Frequency Ground state Absorbtion only when resonance conditions fulfilled: E=h n h is Planck’s constant and n is the frequency of the electromagnetic radiation. Dias 22

The electromagnetic spectrum NMR! Dias 23 Advanced Spectroscopy 2014/2015

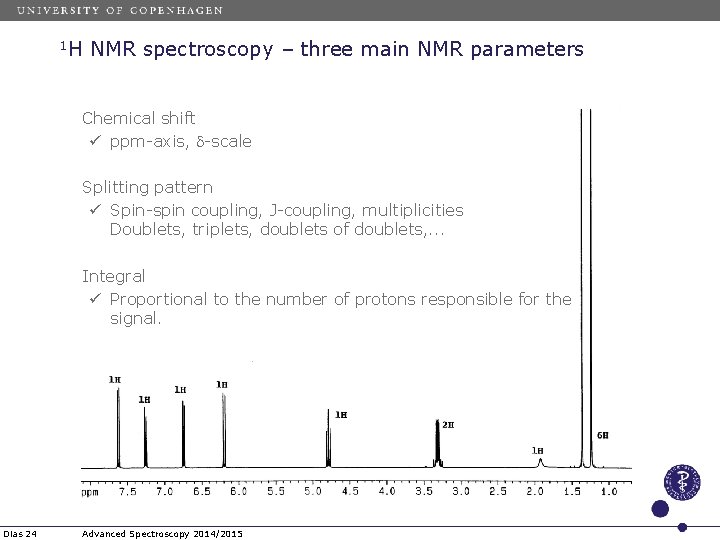
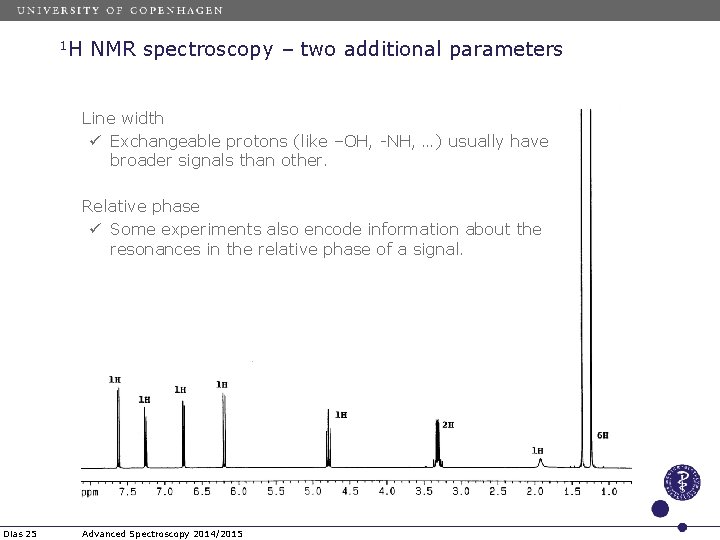
1 H NMR spectroscopy – three main NMR parameters Chemical shift ü ppm-axis, d-scale Splitting pattern ü Spin-spin coupling, J-coupling, multiplicities Doublets, triplets, doublets of doublets, . . . Integral ü Proportional to the number of protons responsible for the signal. Dias 24 Advanced Spectroscopy 2014/2015

1 H NMR spectroscopy – two additional parameters Line width ü Exchangeable protons (like –OH, -NH, …) usually have broader signals than other. Relative phase ü Some experiments also encode information about the resonances in the relative phase of a signal. Dias 25 Advanced Spectroscopy 2014/2015

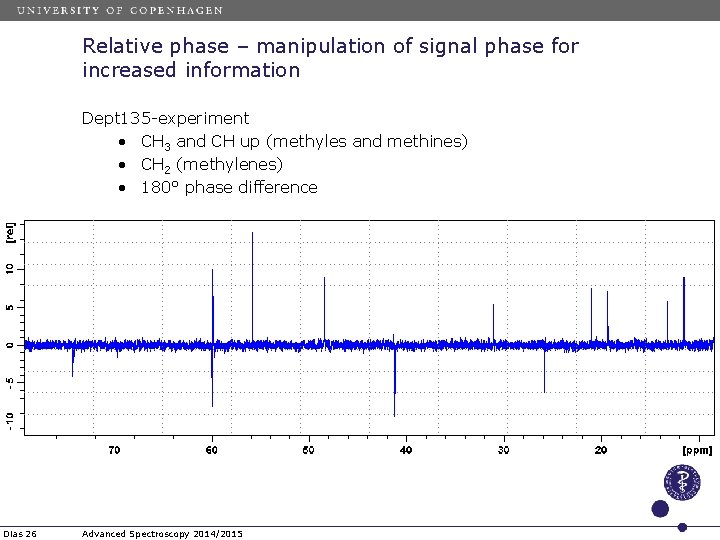
Relative phase – manipulation of signal phase for increased information Dept 135 -experiment • CH 3 and CH up (methyles and methines) • CH 2 (methylenes) • 180° phase difference Dias 26 Advanced Spectroscopy 2014/2015

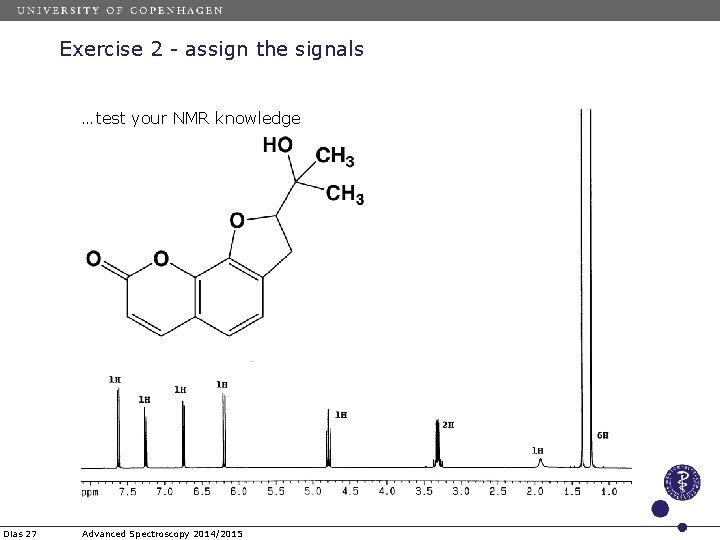
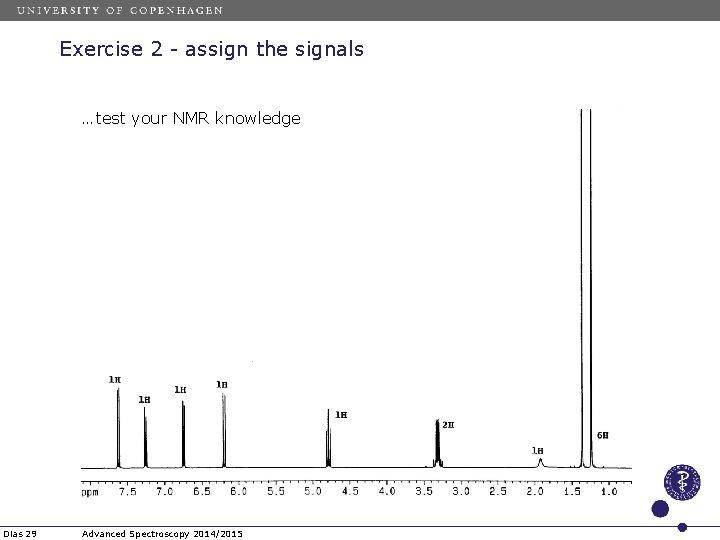
Exercise 2 - assign the signals …test your NMR knowledge Dias 27 Advanced Spectroscopy 2014/2015

Exercise 2 - assign the signals …test your NMR knowledge Dias 28 Advanced Spectroscopy 2014/2015

Exercise 2 - assign the signals …test your NMR knowledge Dias 29 Advanced Spectroscopy 2014/2015

Basic principles of NMR Nuclear: concerns the nuclei of atoms. Magnetic: uses the magnetic properties of the nuclei. Resonance: physics term describing oscillations. Dias 30 Advanced Spectroscopy 2014/2015

NMR: Nuclear Magnetic Resonance Nuclei = protons + neutrons Odd number of either protons OR neutrons the atom has magnetic moment. Atoms with magnetic moment can be studied with NMR. Number of protons + number of neutrons Number of protons ( = Atomic number ) Dias 31 Advanced Spectroscopy 2014/2015

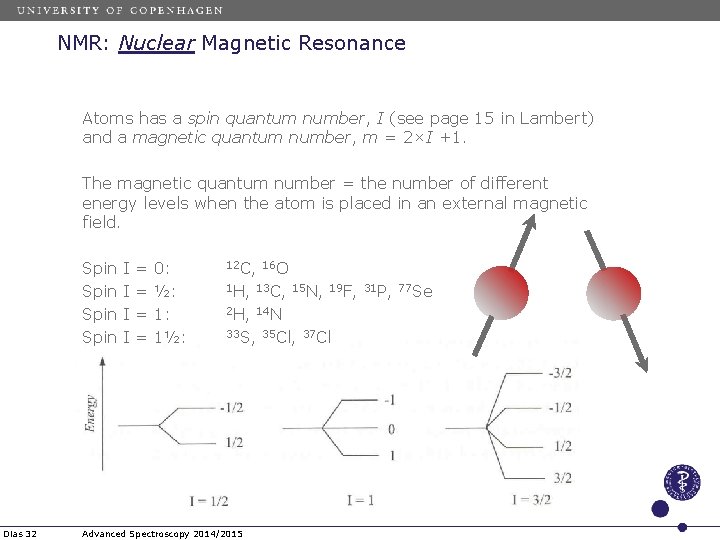
NMR: Nuclear Magnetic Resonance Atoms has a spin quantum number, I (see page 15 in Lambert) and a magnetic quantum number, m = 2×I +1. The magnetic quantum number = the number of different energy levels when the atom is placed in an external magnetic field. Spin Dias 32 I I = = 0: ½: 1: 1½: 12 C, 16 O 1 H, 13 C, 15 N, 19 F, 31 P, 77 Se 2 H, 14 N 33 S, 35 Cl, 37 Cl Advanced Spectroscopy 2014/2015

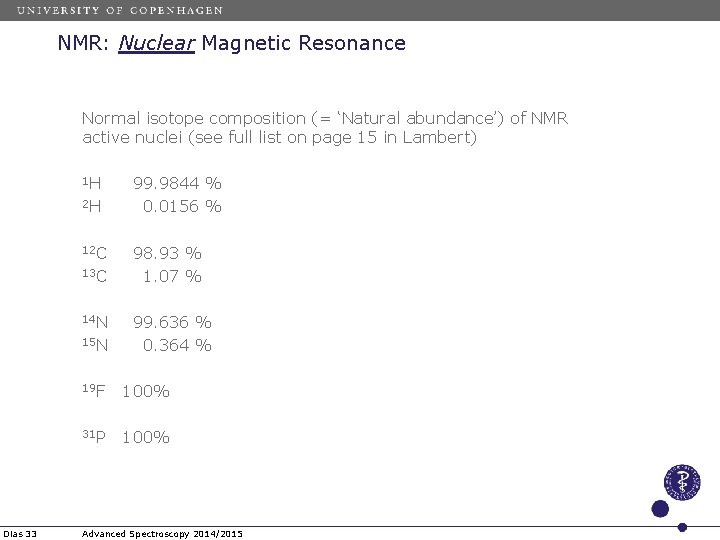
NMR: Nuclear Magnetic Resonance Normal isotope composition (= ‘Natural abundance’) of NMR active nuclei (see full list on page 15 in Lambert) 1 H 2 H 12 C 13 C 14 N 15 N Dias 33 99. 9844 % 0. 0156 % 98. 93 % 1. 07 % 99. 636 % 0. 364 % 19 F 100% 31 P 100% Advanced Spectroscopy 2014/2015

Advanced Spectroscopy 2014/2015 Is 23 Na a NMR active nucleus and what is the natural abundance A. B. C. D. Dias 34 Yes – 100% No – 100% Yes – 75. 43% No – 75. 43%

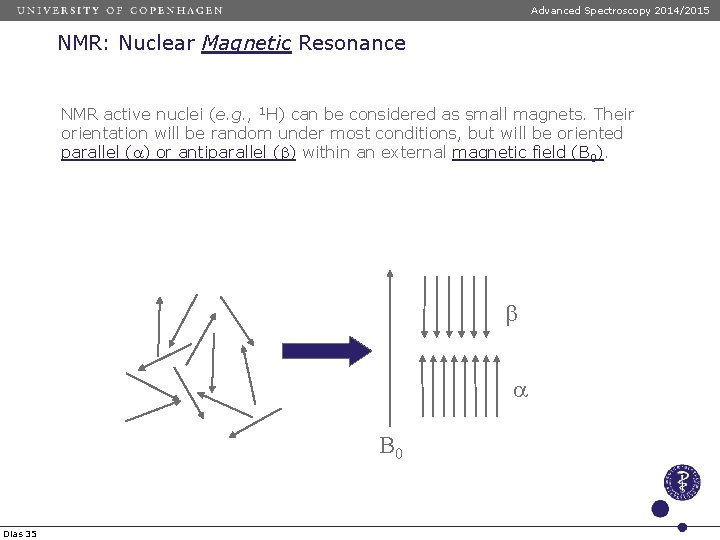
Advanced Spectroscopy 2014/2015 NMR: Nuclear Magnetic Resonance NMR active nuclei (e. g. , 1 H) can be considered as small magnets. Their orientation will be random under most conditions, but will be oriented parallel (a) or antiparallel (b) within an external magnetic field (B 0). b a B 0 Dias 35

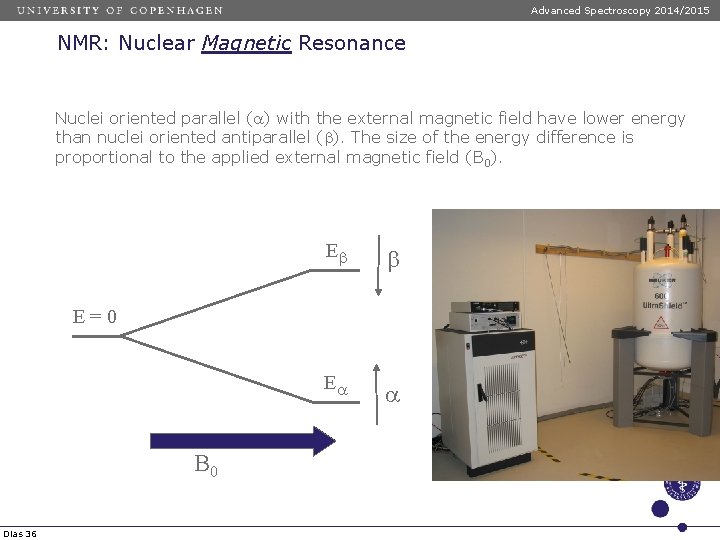
Advanced Spectroscopy 2014/2015 NMR: Nuclear Magnetic Resonance Nuclei oriented parallel (a) with the external magnetic field have lower energy than nuclei oriented antiparallel (b). The size of the energy difference is proportional to the applied external magnetic field (B 0). Eb b Ea a E=0 B 0 Dias 36

NMR: Nuclear Magnetic Resonance A system prefers some frequencies over others… A small energy input at the right frequency will give large oscillations… Dias 37 Advanced Spectroscopy 2014/2015

Advanced Spectroscopy 2014/2015 NMR: Nuclear Magnetic Resonance appears when a sample is exposed to electromagnetic radiation fullfilling E = h · n. For NMR spectroscopy this requires radio frequency (RF) energi Exciterede tilstande D E 1 DE Absorbtion Relaxation RF Grundtilstand Dias 38 b a

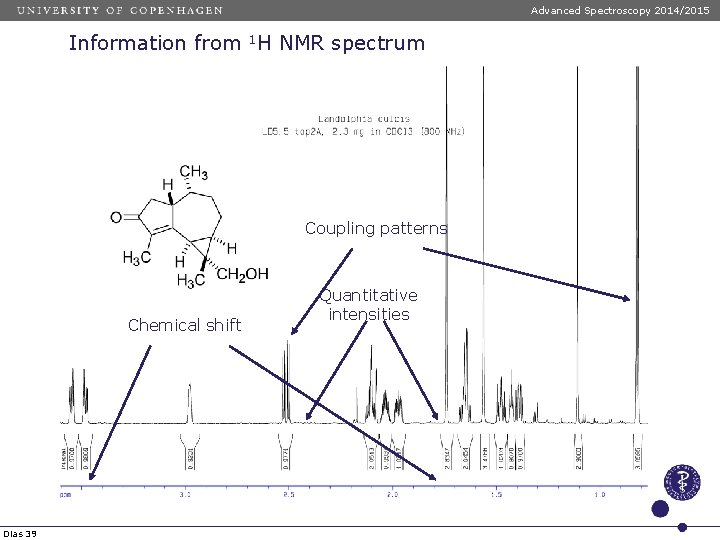
Advanced Spectroscopy 2014/2015 Information from 1 H NMR spectrum Coupling patterns Chemical shift Dias 39 Quantitative intensities

Advanced Spectroscopy 2014/2015 Basic 1 H NMR parameters Integrals (= area under curve = intensities): ü tells how many H that are giving rise to the signal (only integers) ü integral-labels (sum normalized to number of H from molecular formula) ü integral-curves (heights normalized to number of H from molecular formula) Chemical shift: ü position of resonance signals on the frequency axis ü give information about the chemical surroundings ü d always given in ppm (part per million) Coupling pattern (= multiplicity = splitting): ü give information about the number of neighboring H ü typical coupling through 2 -4 bonds ü coupling constant J = distance between the individual lines in a coupling pattern (always given in Hz) ü equivalent H’s does not give rise to splitting Dias 40

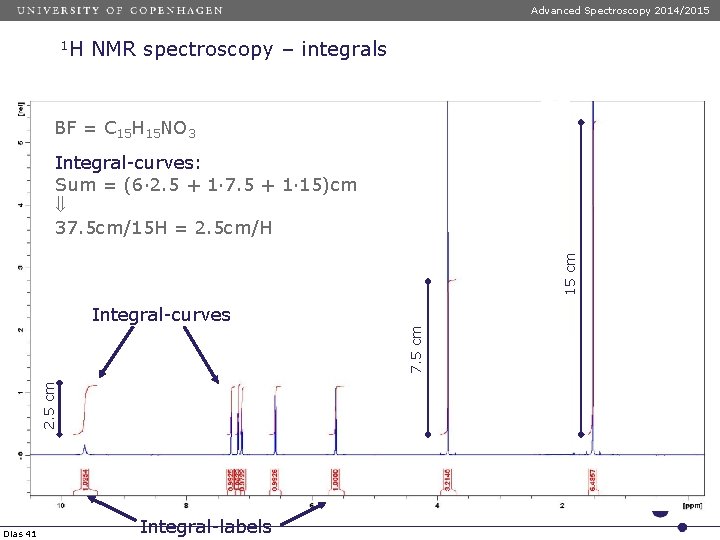
Advanced Spectroscopy 2014/2015 1 H NMR spectroscopy – integrals BF = C 15 H 15 NO 3 15 cm Integral-curves: Sum = (6· 2. 5 + 1· 7. 5 + 1· 15)cm 37. 5 cm/15 H = 2. 5 cm/H 2. 5 cm 7. 5 cm Integral-curves Dias 41 Integral-labels

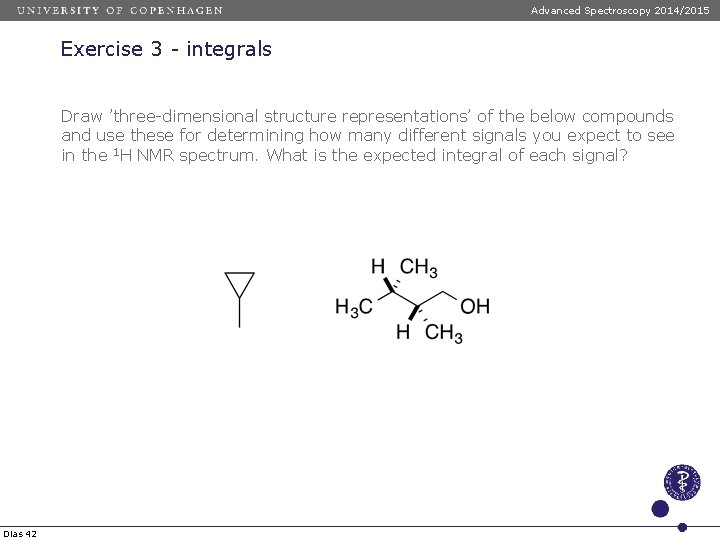
Advanced Spectroscopy 2014/2015 Exercise 3 - integrals Draw ’three-dimensional structure representations’ of the below compounds and use these for determining how many different signals you expect to see in the 1 H NMR spectrum. What is the expected integral of each signal? Dias 42

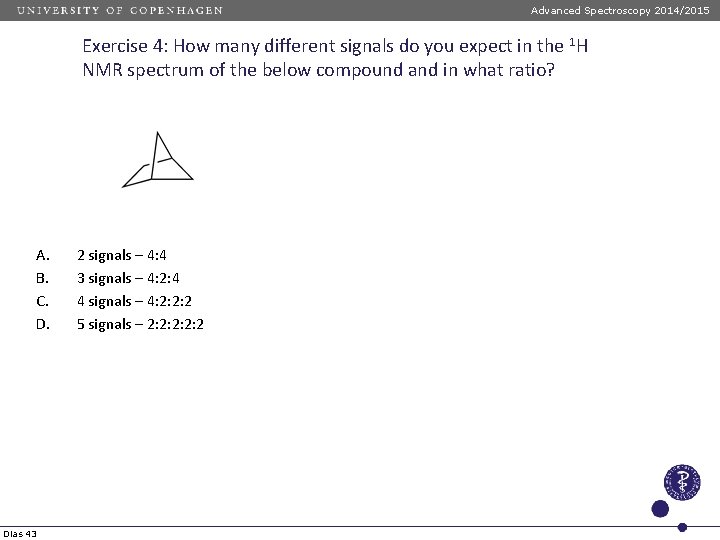
Advanced Spectroscopy 2014/2015 Exercise 4: How many different signals do you expect in the 1 H NMR spectrum of the below compound and in what ratio? A. B. C. D. Dias 43 2 signals – 4: 4 3 signals – 4: 2: 4 4 signals – 4: 2: 2: 2 5 signals – 2: 2: 2

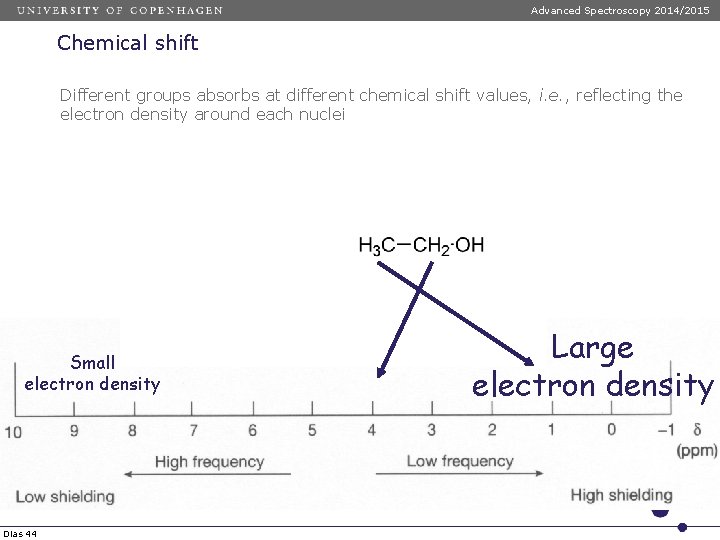
Advanced Spectroscopy 2014/2015 Chemical shift Different groups absorbs at different chemical shift values, i. e. , reflecting the electron density around each nuclei Small electron density Dias 44 Large electron density

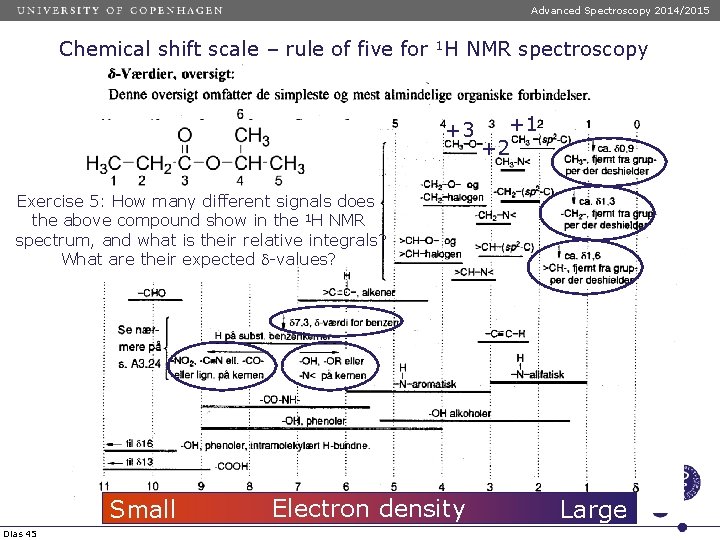
Advanced Spectroscopy 2014/2015 Chemical shift scale – rule of five for 1 H NMR spectroscopy Chemical shift tabeller +3 +1 +2 Exercise 5: How many different signals does the above compound show in the 1 H NMR spectrum, and what is their relative integrals? What are their expected d-values? Small Dias 45 Electron density Large

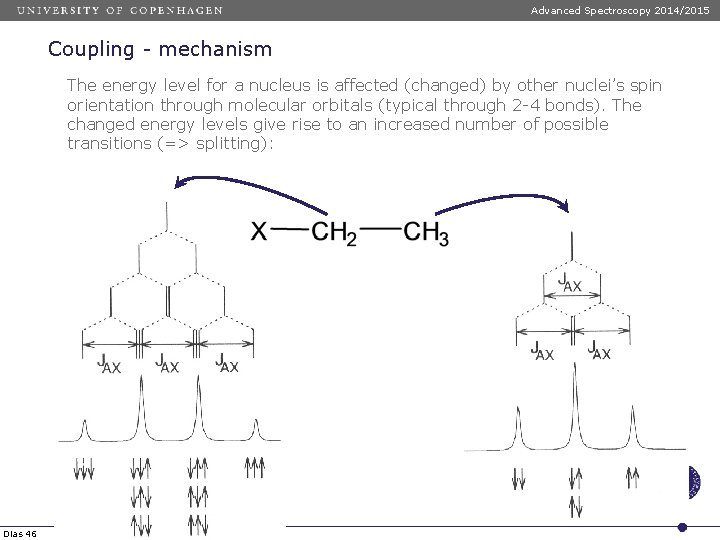
Advanced Spectroscopy 2014/2015 Coupling - mechanism The energy level for a nucleus is affected (changed) by other nuclei’s spin orientation through molecular orbitals (typical through 2 -4 bonds). The changed energy levels give rise to an increased number of possible transitions (=> splitting): Dias 46

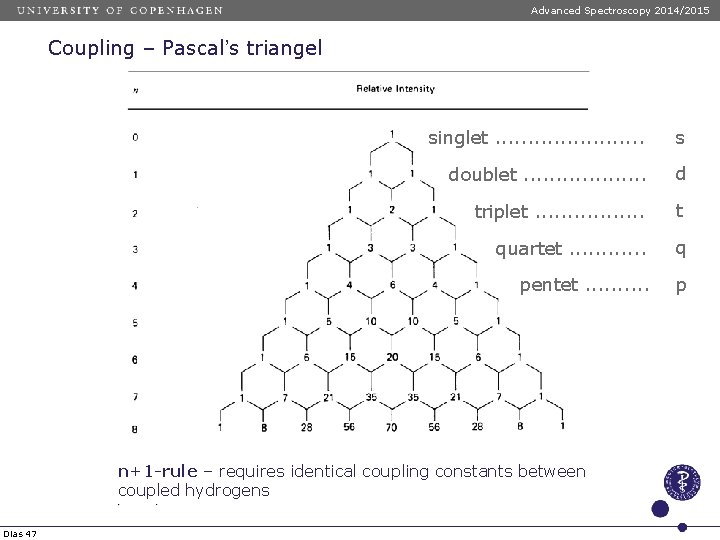
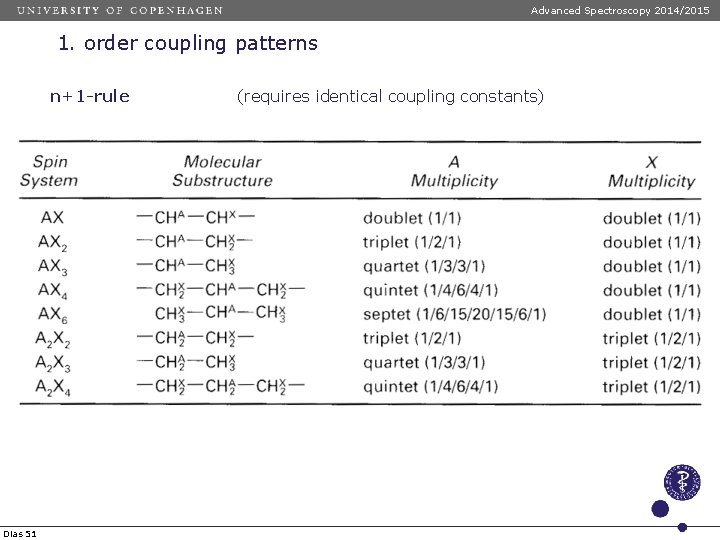
Advanced Spectroscopy 2014/2015 Coupling – Pascal’s triangel singlet. . . s doublet. . . . . d triplet. . . . t quartet. . . q pentet. . p n+1 -rule – requires identical coupling constants between coupled hydrogens Dias 47

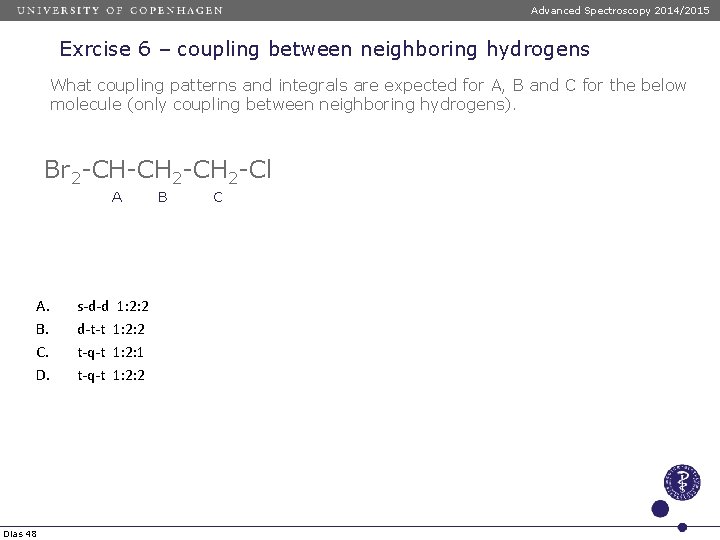
Advanced Spectroscopy 2014/2015 Exrcise 6 – coupling between neighboring hydrogens What coupling patterns and integrals are expected for A, B and C for the below molecule (only coupling between neighboring hydrogens). Br 2 -CH-CH 2 -Cl A A. B. C. D. Dias 48 s-d-d 1: 2: 2 d-t-t 1: 2: 2 t-q-t 1: 2: 1 t-q-t 1: 2: 2 B C

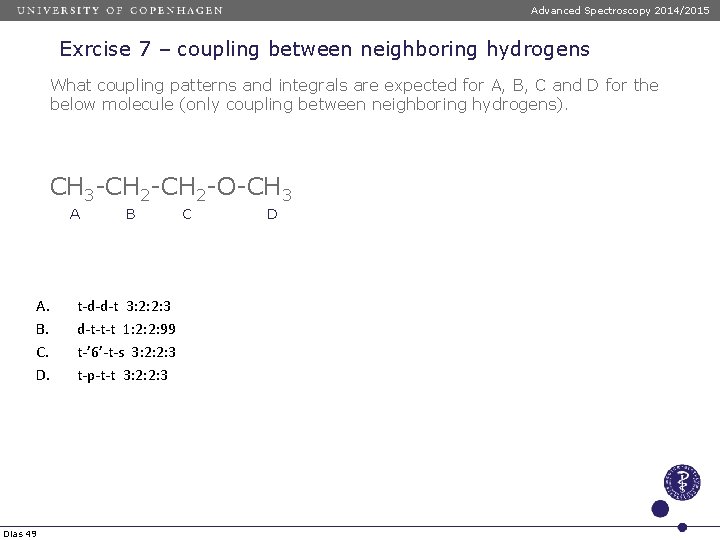
Advanced Spectroscopy 2014/2015 Exrcise 7 – coupling between neighboring hydrogens What coupling patterns and integrals are expected for A, B, C and D for the below molecule (only coupling between neighboring hydrogens). CH 3 -CH 2 -O-CH 3 A A. B. C. D. Dias 49 B t-d-d-t 3: 2: 2: 3 d-t-t-t 1: 2: 2: 99 t-’ 6’-t-s 3: 2: 2: 3 t-p-t-t 3: 2: 2: 3 C D

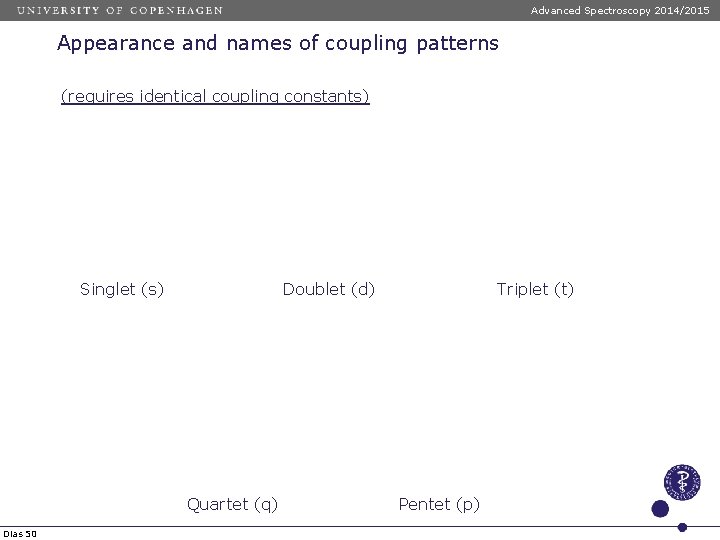
Advanced Spectroscopy 2014/2015 Appearance and names of coupling patterns (requires identical coupling constants) Singlet (s) Doublet (d) Quartet (q) Dias 50 Triplet (t) Pentet (p)

Advanced Spectroscopy 2014/2015 1. order coupling patterns n+1 -rule Dias 51 (requires identical coupling constants)

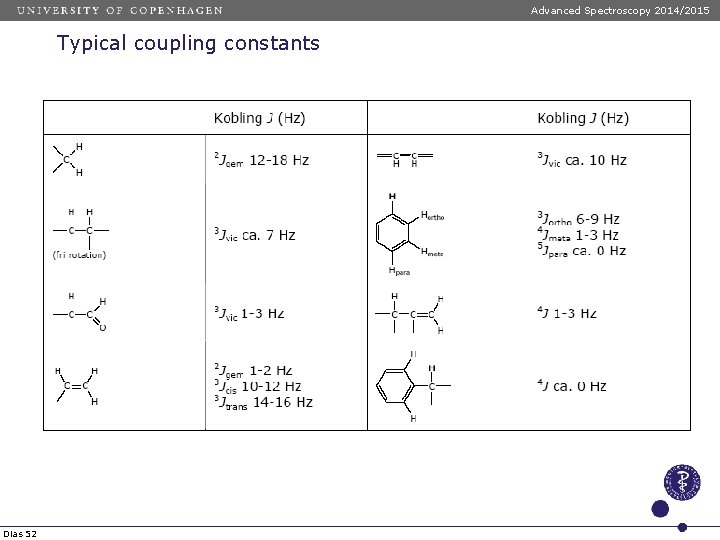
Advanced Spectroscopy 2014/2015 Typical coupling constants Dias 52

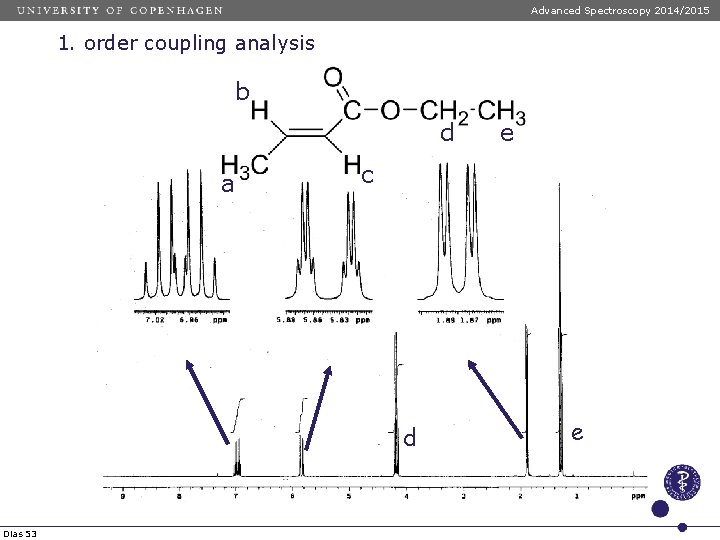
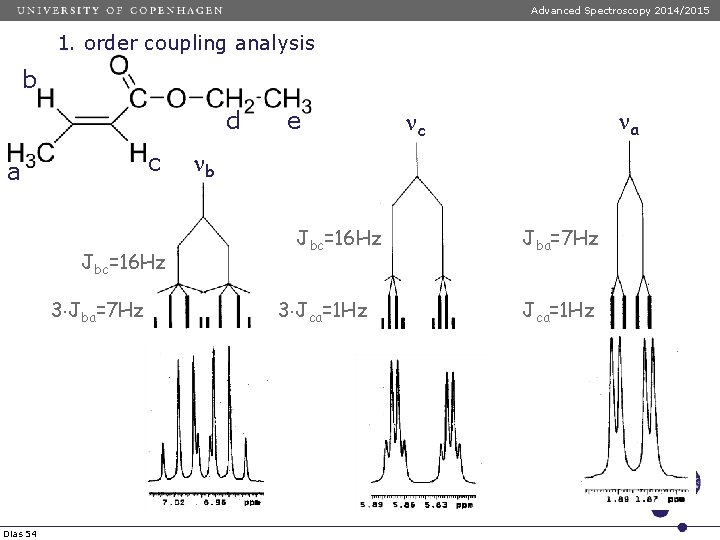
Advanced Spectroscopy 2014/2015 1. order coupling analysis b d a c d Dias 53 e e

Advanced Spectroscopy 2014/2015 1. order coupling analysis b d c a Jbc=16 Hz 3 Jba=7 Hz Dias 54 e na nc nb Jbc=16 Hz 3 Jca=1 Hz Jba=7 Hz Jca=1 Hz

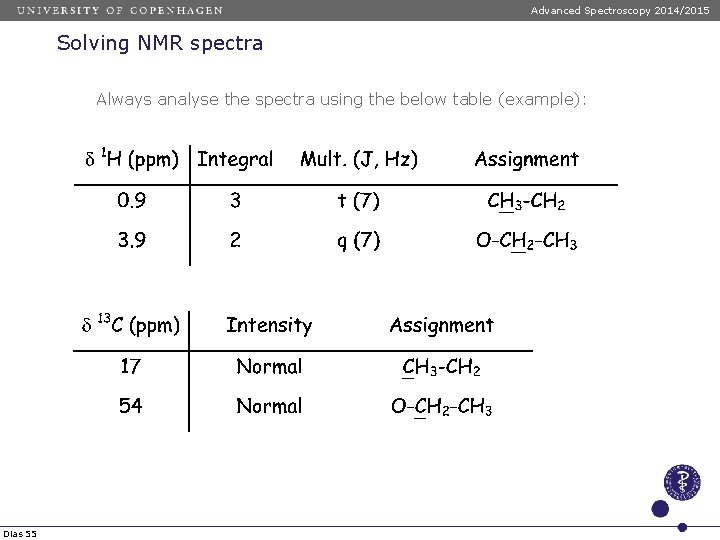
Advanced Spectroscopy 2014/2015 Solving NMR spectra Always analyse the spectra using the below table (example): Dias 55

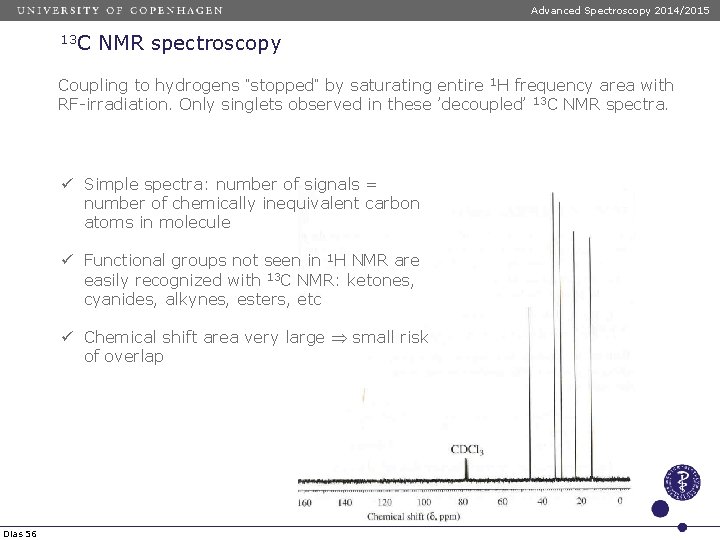
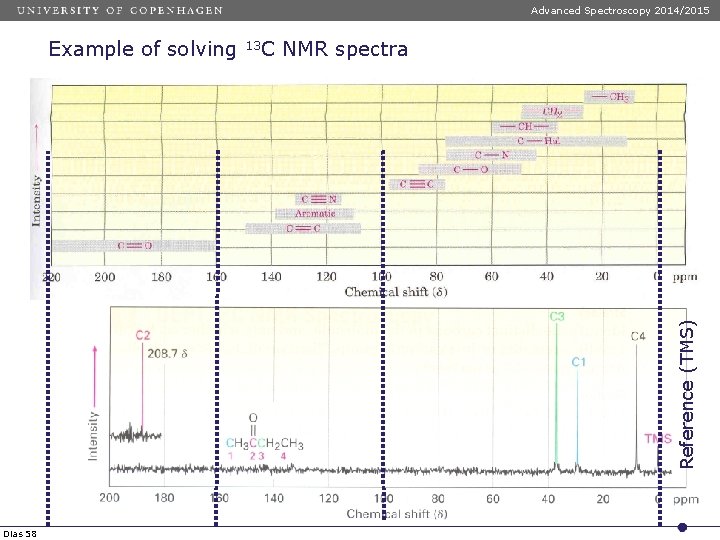
Advanced Spectroscopy 2014/2015 13 C NMR spectroscopy Coupling to hydrogens ”stopped” by saturating entire 1 H frequency area with RF-irradiation. Only singlets observed in these ’decoupled’ 13 C NMR spectra. ü Simple spectra: number of signals = number of chemically inequivalent carbon atoms in molecule ü Functional groups not seen in 1 H NMR are easily recognized with 13 C NMR: ketones, cyanides, alkynes, esters, etc ü Chemical shift area very large small risk of overlap Dias 56

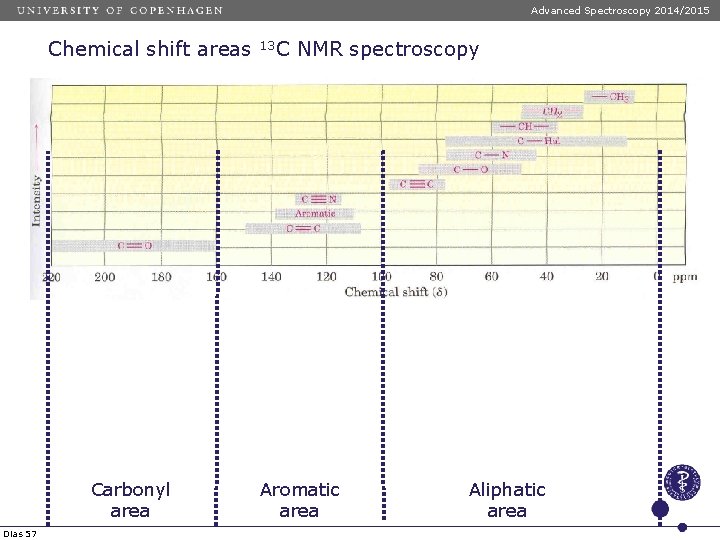
Advanced Spectroscopy 2014/2015 Chemical shift areas Carbonyl area Dias 57 13 C NMR spectroscopy Aromatic area Aliphatic area

Advanced Spectroscopy 2014/2015 13 C NMR spectra Reference (TMS) Example of solving Dias 58

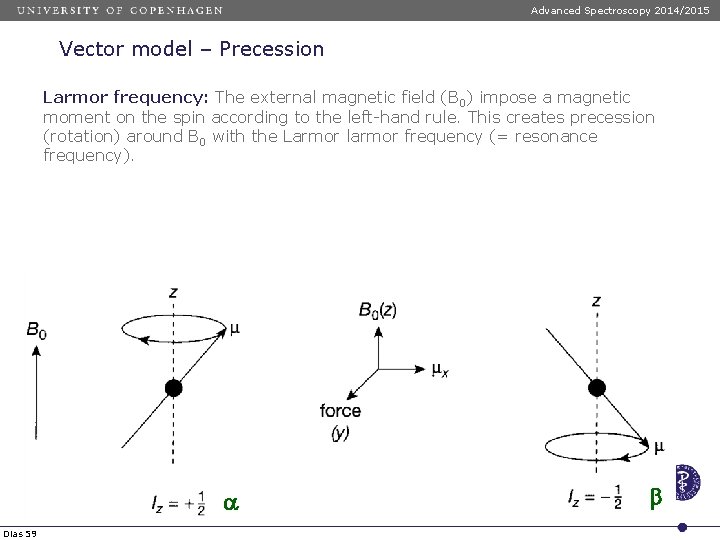
Advanced Spectroscopy 2014/2015 Vector model – Precession Larmor frequency: The external magnetic field (B 0) impose a magnetic moment on the spin according to the left-hand rule. This creates precession (rotation) around B 0 with the Larmor larmor frequency (= resonance frequency). a Dias 59 b

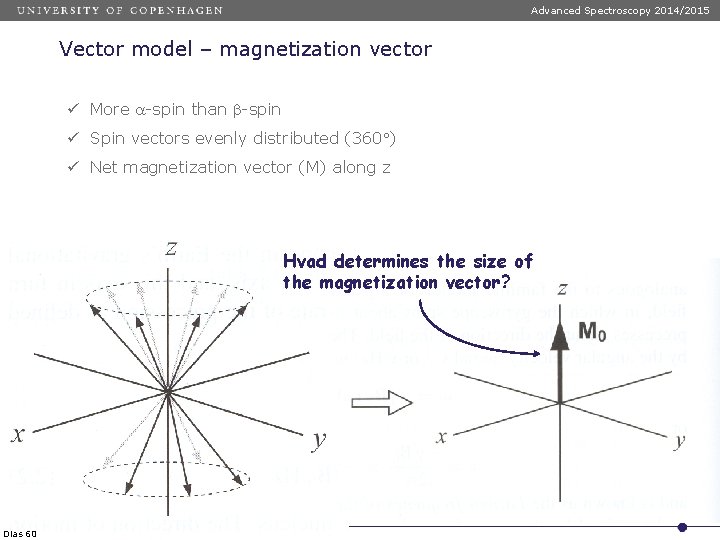
Advanced Spectroscopy 2014/2015 Vector model – magnetization vector ü More a-spin than b-spin ü Spin vectors evenly distributed (360 ) ü Net magnetization vector (M) along z Hvad determines the size of the magnetization vector? Dias 60

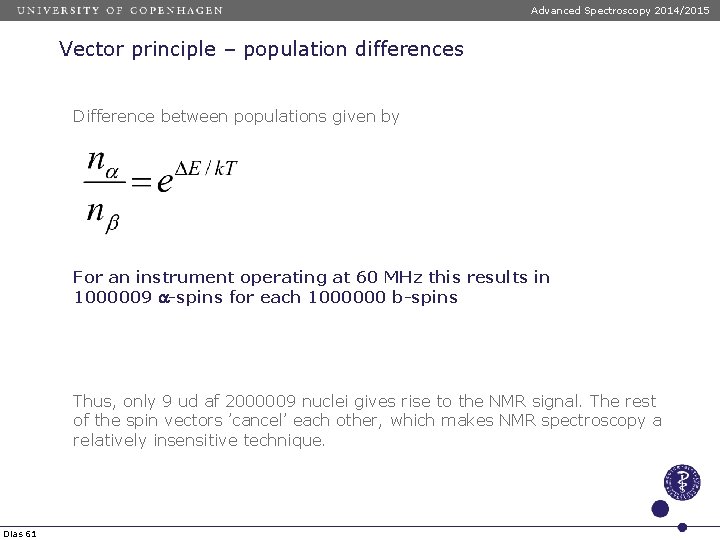
Advanced Spectroscopy 2014/2015 Vector principle – population differences Difference between populations given by For an instrument operating at 60 MHz this results in 1000009 a-spins for each 1000000 b-spins Thus, only 9 ud af 2000009 nuclei gives rise to the NMR signal. The rest of the spin vectors ’cancel’ each other, which makes NMR spectroscopy a relatively insensitive technique. Dias 61

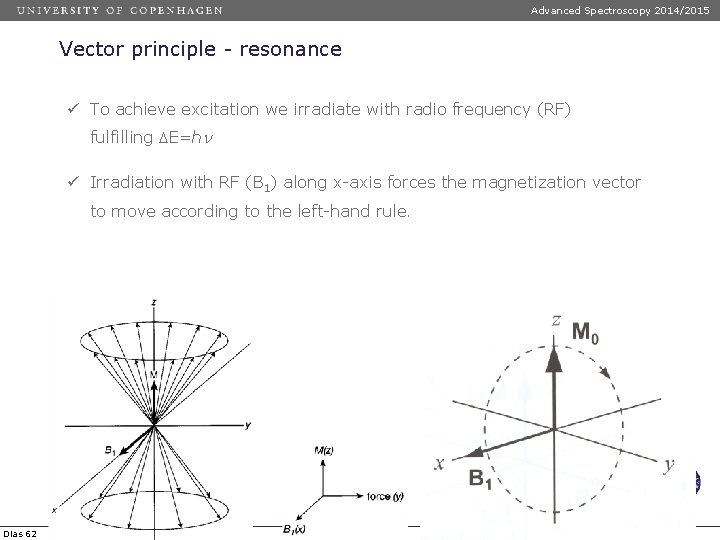
Advanced Spectroscopy 2014/2015 Vector principle - resonance ü To achieve excitation we irradiate with radio frequency (RF) fulfilling DE=hn ü Irradiation with RF (B 1) along x-axis forces the magnetization vector to move according to the left-hand rule. Dias 62

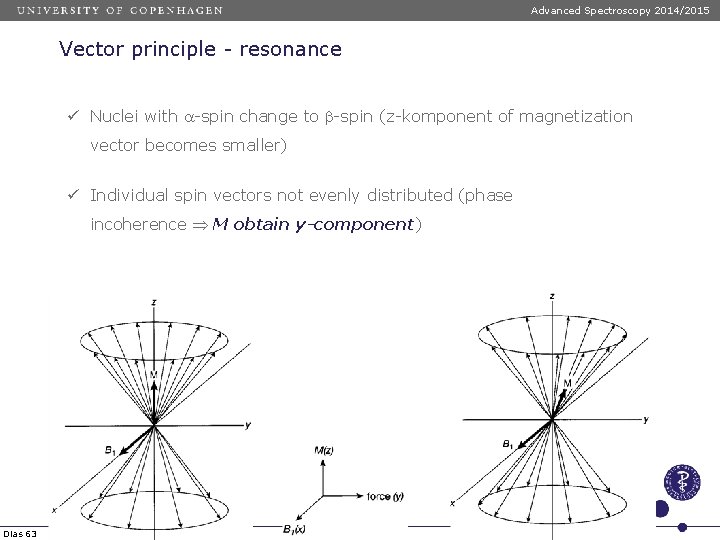
Advanced Spectroscopy 2014/2015 Vector principle - resonance ü Nuclei with a-spin change to b-spin (z-komponent of magnetization vector becomes smaller) ü Individual spin vectors not evenly distributed (phase incoherence M obtain y-component) Dias 63

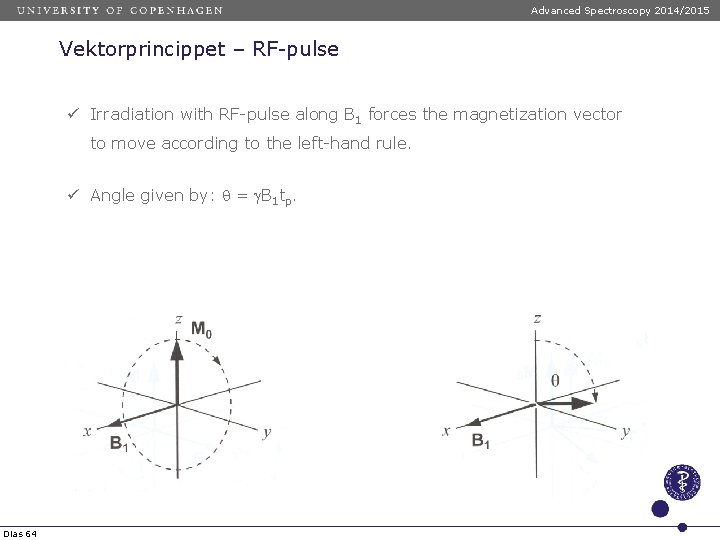
Advanced Spectroscopy 2014/2015 Vektorprincippet – RF-pulse ü Irradiation with RF-pulse along B 1 forces the magnetization vector to move according to the left-hand rule. ü Angle given by: q = g. B 1 tp. Dias 64

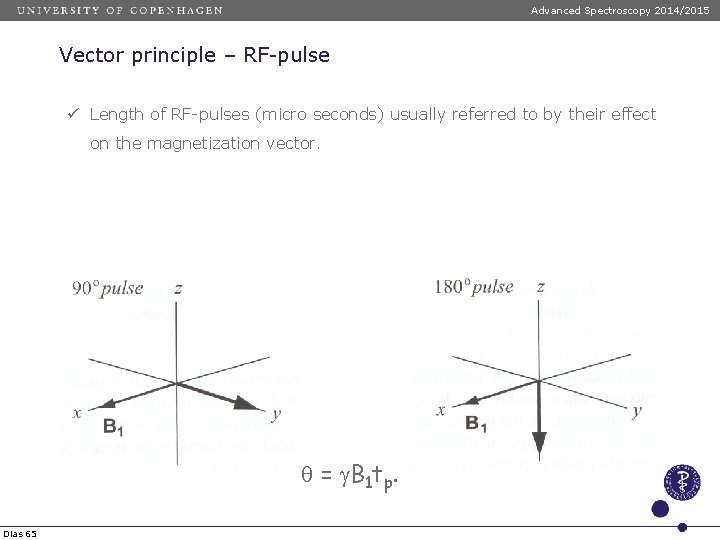
Advanced Spectroscopy 2014/2015 Vector principle – RF-pulse ü Length of RF-pulses (micro seconds) usually referred to by their effect on the magnetization vector. q = g. B 1 tp. Dias 65

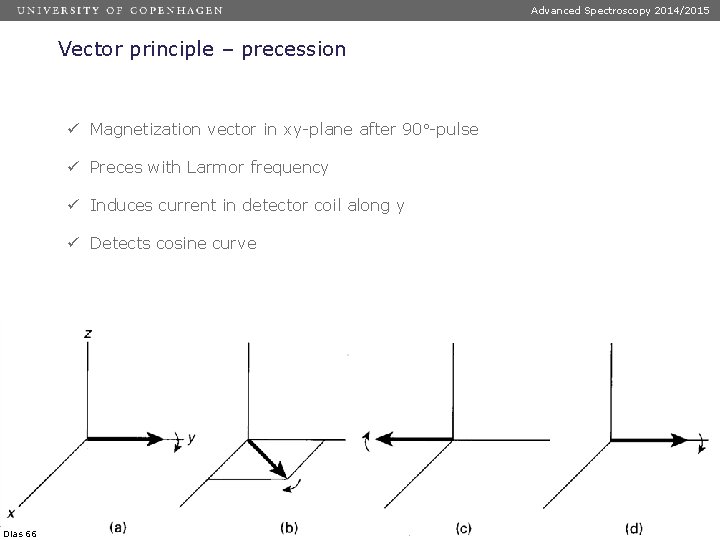
Advanced Spectroscopy 2014/2015 Vector principle – precession ü Magnetization vector in xy-plane after 90 -pulse ü Preces with Larmor frequency ü Induces current in detector coil along y ü Detects cosine curve Dias 66

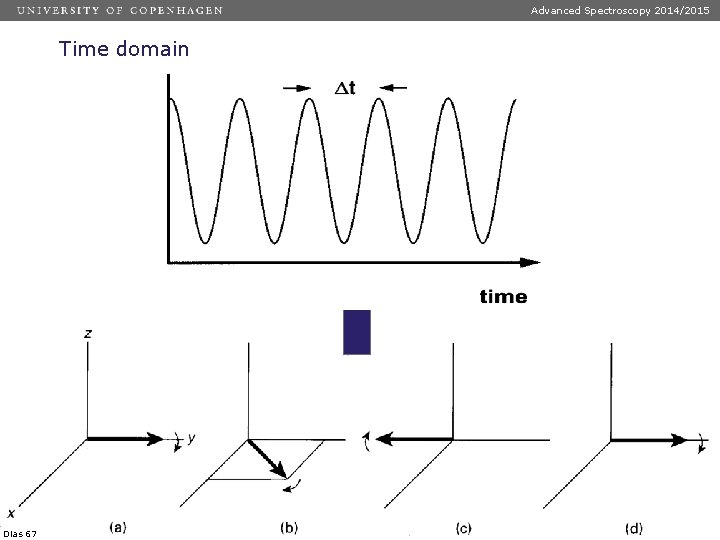
Advanced Spectroscopy 2014/2015 Time domain Dias 67

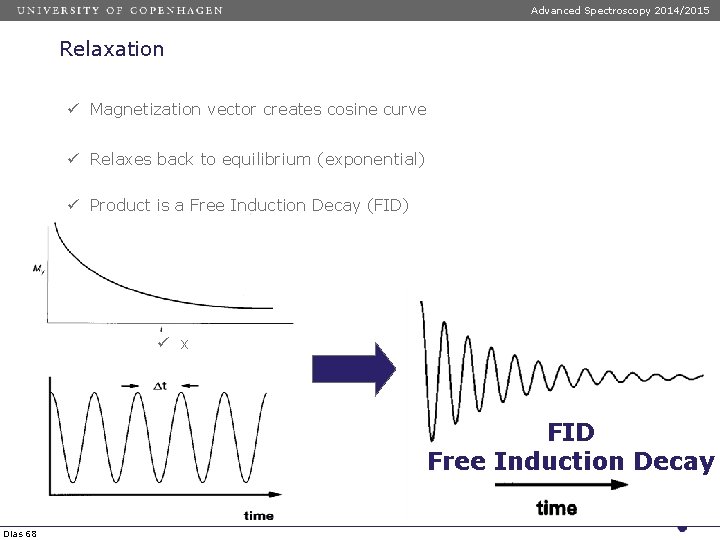
Advanced Spectroscopy 2014/2015 Relaxation ü Magnetization vector creates cosine curve ü Relaxes back to equilibrium (exponential) ü Product is a Free Induction Decay (FID) ü x FID Free Induction Decay Dias 68

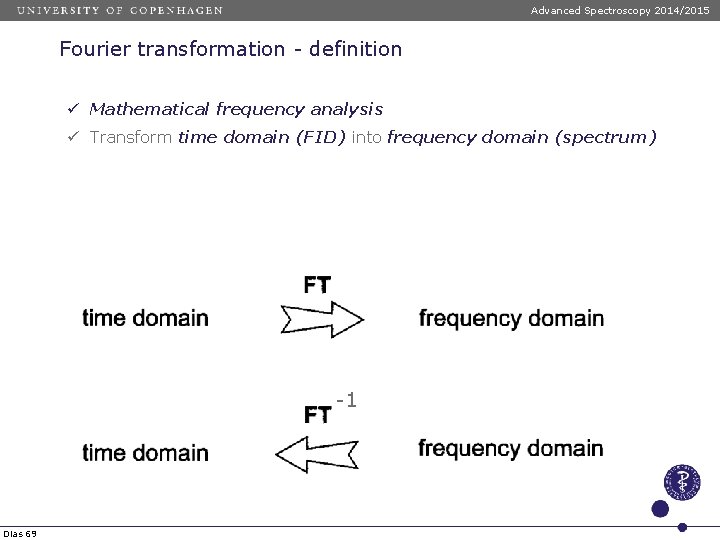
Advanced Spectroscopy 2014/2015 Fourier transformation - definition ü Mathematical frequency analysis ü Transform time domain (FID) into frequency domain (spectrum) -1 Dias 69

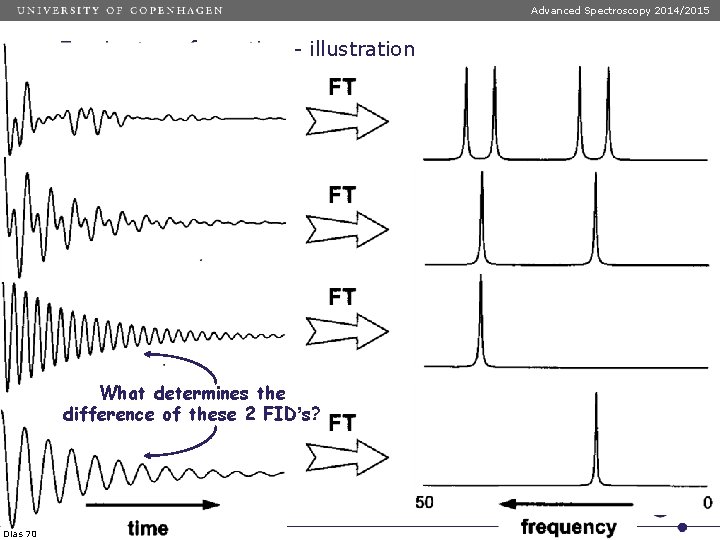
Advanced Spectroscopy 2014/2015 Fourier transformation - illustration What determines the difference of these 2 FID’s? Dias 70