ADVANCED ORGANIC CHEMISTRY CHEM601 BY DR GHULAM ABBAS
ADVANCED ORGANIC CHEMISTRY (CHEM-601) BY DR. GHULAM ABBAS
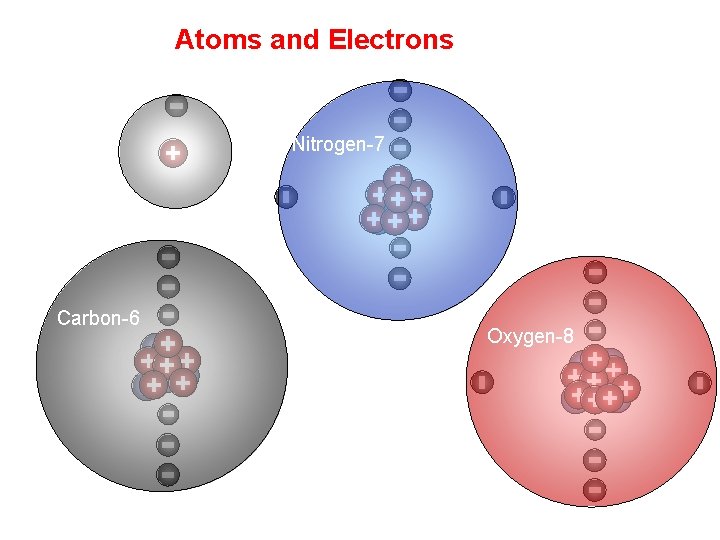
Atoms and Electrons Hydrogen Carbon-6 Nitrogen-7 Oxygen-8
CHEMICAL BONDING • Bonding is the force of attraction that holds atoms together in an compound (CO 2 or Na. Cl). • The distances between bonded atoms are less than those between non-bonded atoms. • The forces between bonded atoms are greater than those between non-bonded atoms. • The principal types of bonding are ionic, covalent, and metallic.
• A chemical bond links two atoms or groups of atoms when the forces acting between them are sufficient to lead to the formation of an aggregate (a molecule) with sufficient stability to make it convenient for the chemist to consider it as an independent "molecular species” Paraphrased from Linus Pauling (1967).
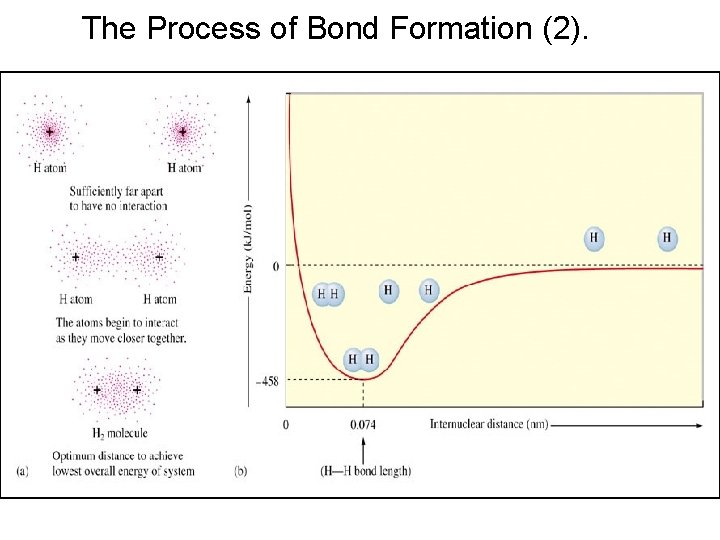
The Process of Bond Formation (2).

Core Electrons vs Valence Electrons • Electrons can be divided into: – Valence electrons (e- in unfilled shells, outermost electrons). Valence electrons participate in bonding. – Core electrons (e- in a filled shells). Core electrons do not participate in bonding. Valence electrons in molecules are usually distributed in such a way that each maingroup element is surrounded by eight electrons (an octet of electrons). Hydrogen is surrounded by two valence electrons.
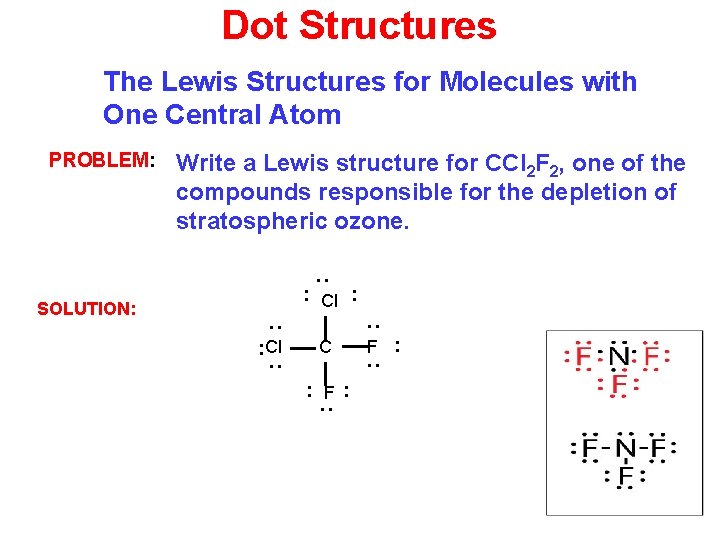
Dot Structures The Lewis Structures for Molecules with One Central Atom Write a Lewis structure for CCl 2 F 2, one of the compounds responsible for the depletion of stratospheric ozone. : PROBLEM: : Cl : SOLUTION: F : : C : : Cl : : F:
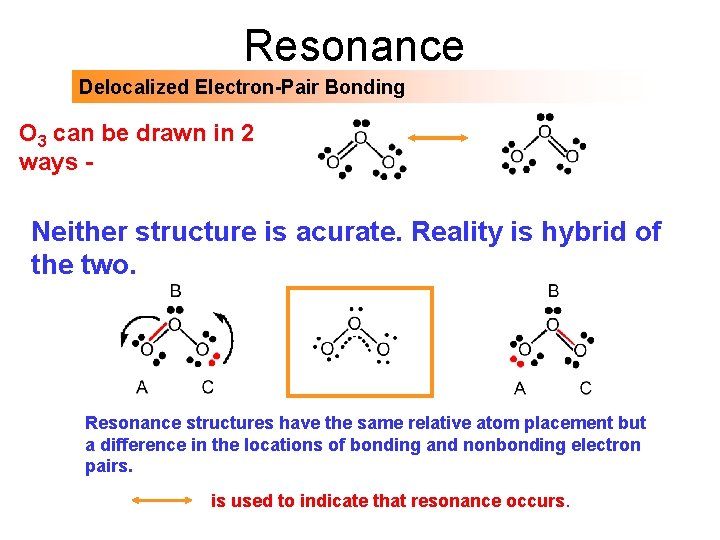
Resonance Delocalized Electron-Pair Bonding O 3 can be drawn in 2 ways - Neither structure is acurate. Reality is hybrid of the two. Resonance structures have the same relative atom placement but a difference in the locations of bonding and nonbonding electron pairs. is used to indicate that resonance occurs.

BOND LENGTHS • • sp 3–sp 3: 154 Pm (Picometer 1 x 10 -12) Alkene: 134 Pm Alkyne: 120 Pm Benzene: 140 Pm
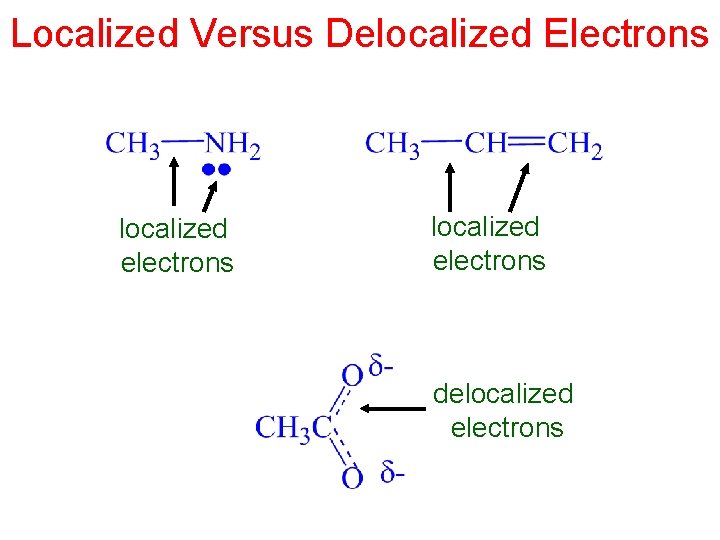
Localized Versus Delocalized Electrons localized electrons delocalized electrons
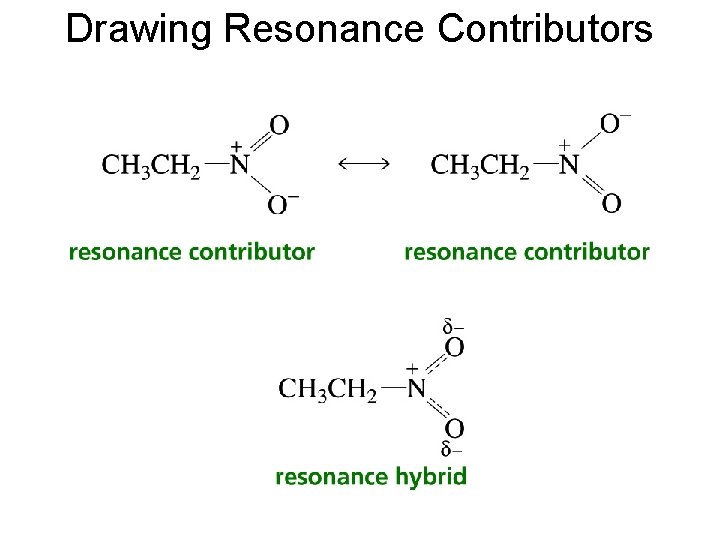
Drawing Resonance Contributors
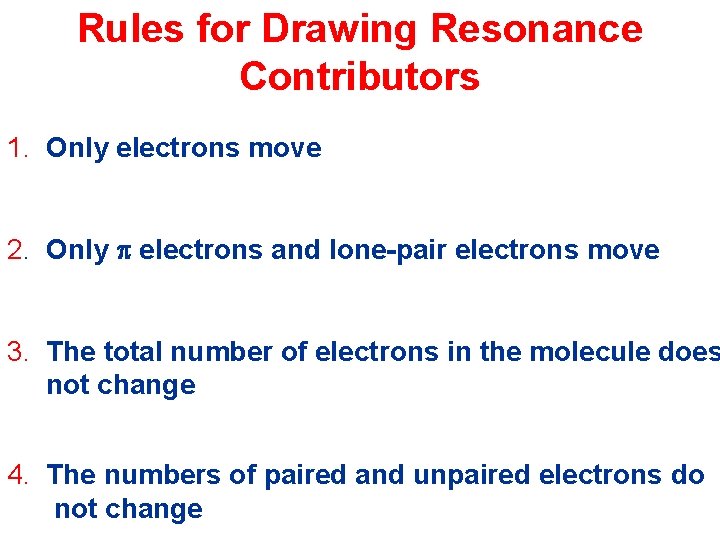
Rules for Drawing Resonance Contributors 1. Only electrons move 2. Only p electrons and lone-pair electrons move 3. The total number of electrons in the molecule does not change 4. The numbers of paired and unpaired electrons do not change

The electrons can be moved in one of the following ways: 1. Move p electrons toward a positive charge or toward a p bond 2. Move lone-pair electrons toward a p bond 3. Move a single nonbonding electron toward a p bon
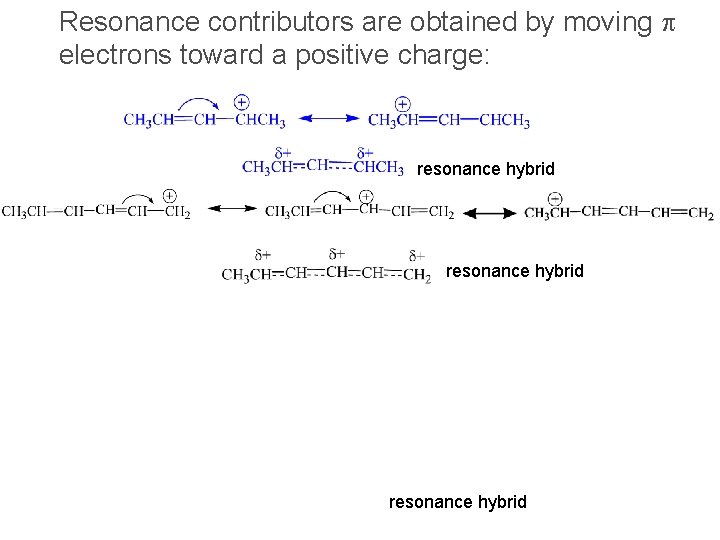
Resonance contributors are obtained by moving p electrons toward a positive charge: resonance hybrid
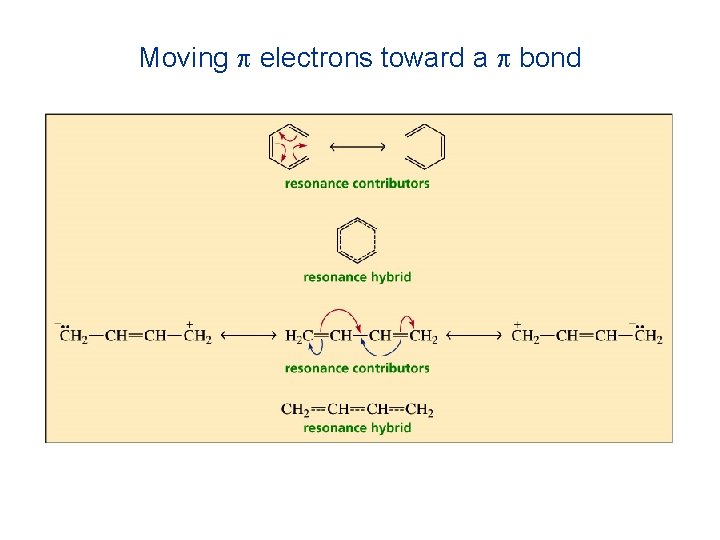
Moving p electrons toward a p bond
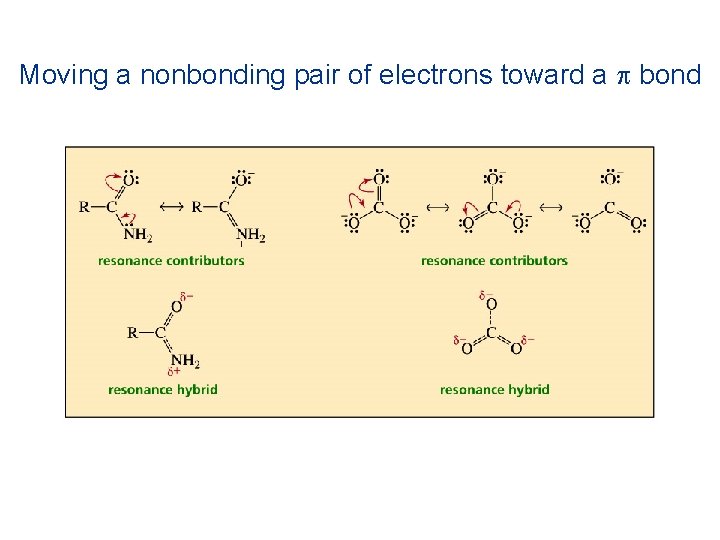
Moving a nonbonding pair of electrons toward a p bond
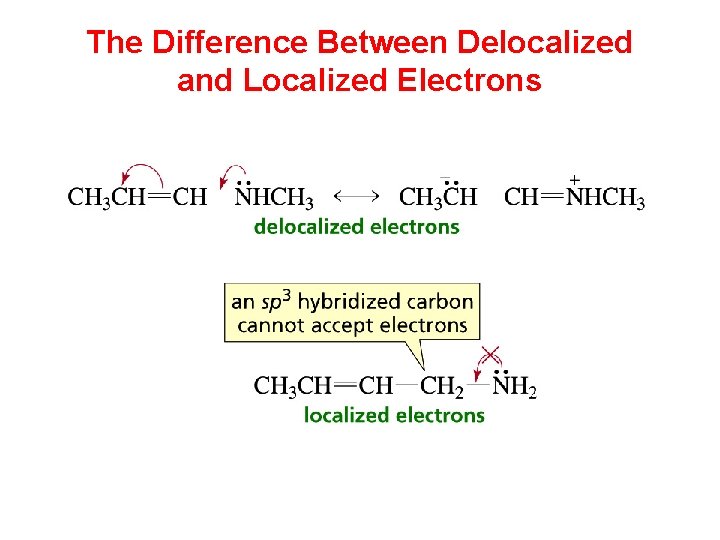
The Difference Between Delocalized and Localized Electrons
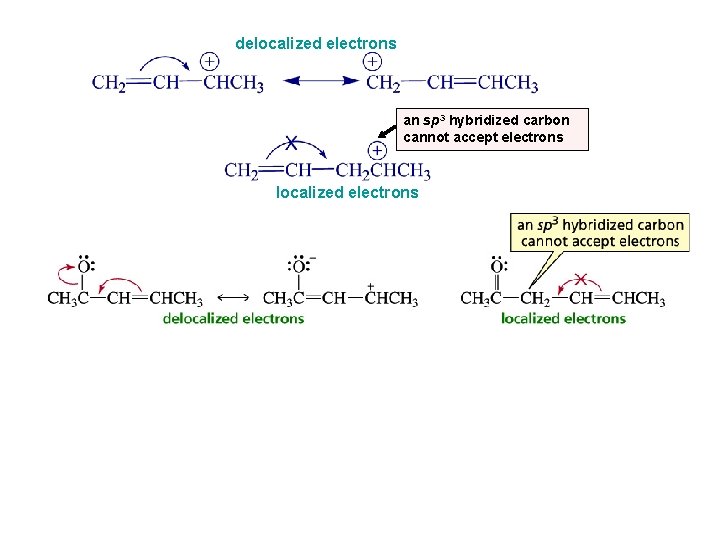
delocalized electrons an sp 3 hybridized carbon cannot accept electrons localized electrons
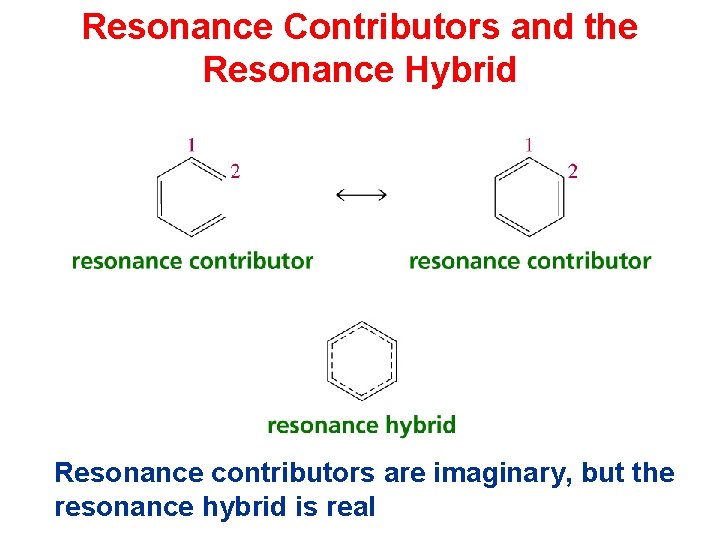
Resonance Contributors and the Resonance Hybrid Resonance contributors are imaginary, but the resonance hybrid is real
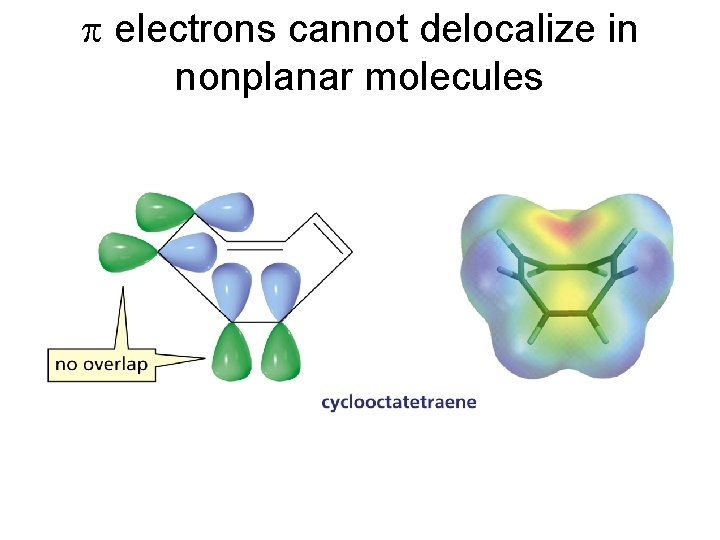
p electrons cannot delocalize in nonplanar molecules
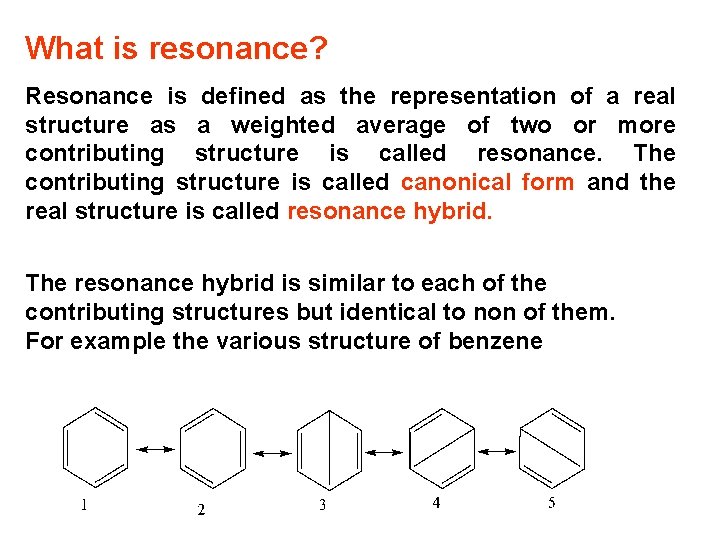
What is resonance? Resonance is defined as the representation of a real structure as a weighted average of two or more contributing structure is called resonance. The contributing structure is called canonical form and the real structure is called resonance hybrid. The resonance hybrid is similar to each of the contributing structures but identical to non of them. For example the various structure of benzene
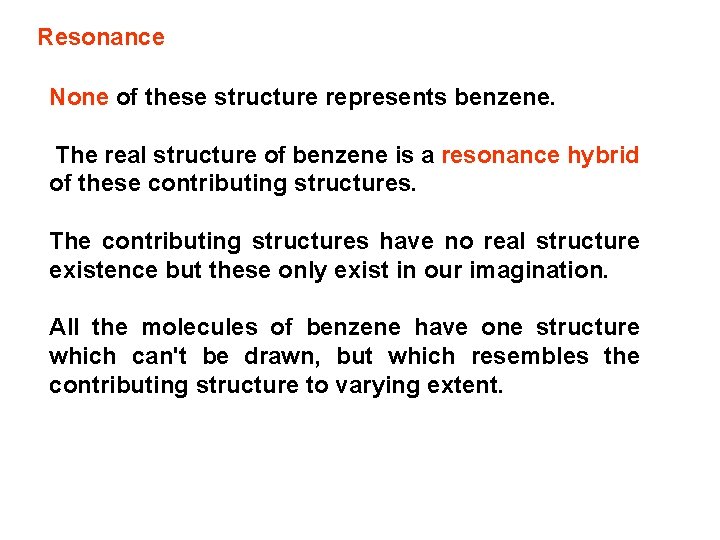
Resonance None of these structure represents benzene. The real structure of benzene is a resonance hybrid of these contributing structures. The contributing structures have no real structure existence but these only exist in our imagination. All the molecules of benzene have one structure which can't be drawn, but which resembles the contributing structure to varying extent.
Resonance The energy of the actual molecule is less than the contributing structures. The contributing structure has different energies. Some have high and some have low energies. The contributing structure which has the lowest energy is more resemble to the actual molecule and is called major contributor. The contributing structure which has the highest energy is less contributing to the hybrid one and is called the minor contributor.
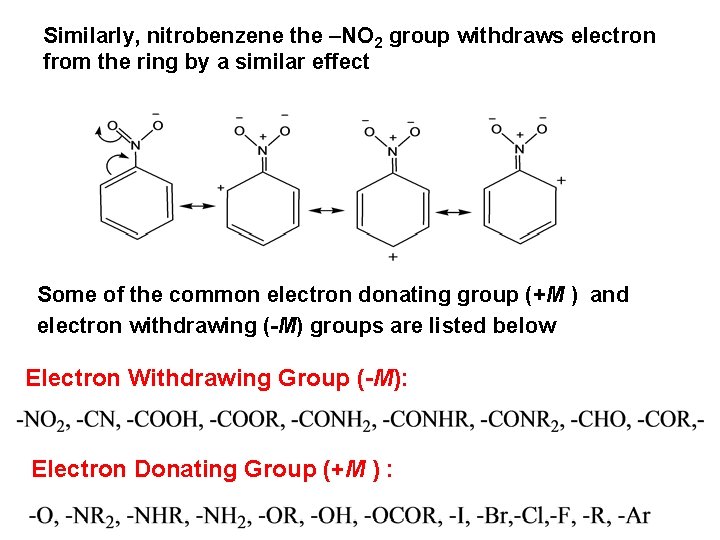
Similarly, nitrobenzene the –NO 2 group withdraws electron from the ring by a similar effect Some of the common electron donating group (+M ) and electron withdrawing (-M) groups are listed below Electron Withdrawing Group (-M): Electron Donating Group (+M ) :
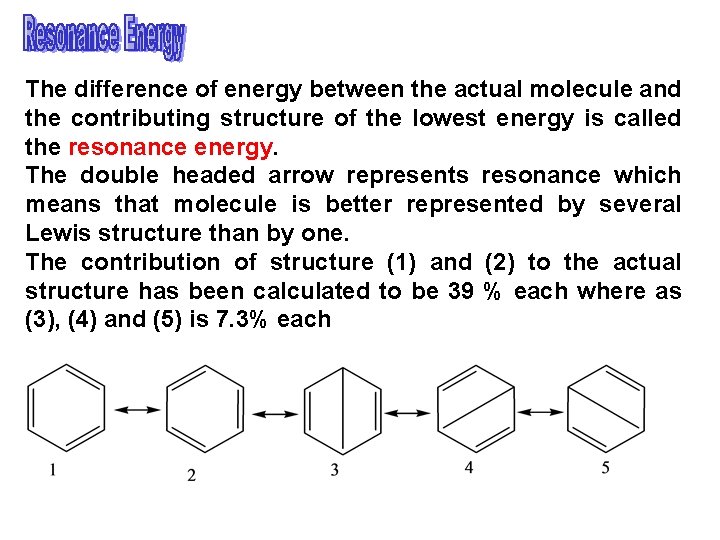
The difference of energy between the actual molecule and the contributing structure of the lowest energy is called the resonance energy. The double headed arrow represents resonance which means that molecule is better represented by several Lewis structure than by one. The contribution of structure (1) and (2) to the actual structure has been calculated to be 39 % each where as (3), (4) and (5) is 7. 3% each
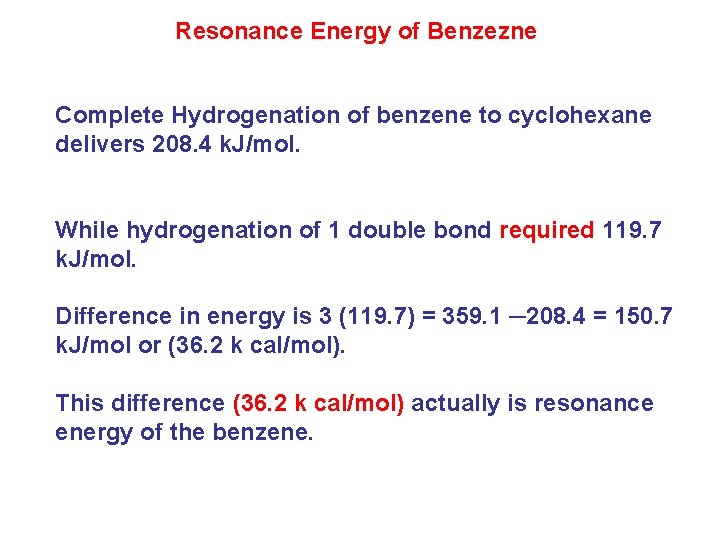
Resonance Energy of Benzezne Complete Hydrogenation of benzene to cyclohexane delivers 208. 4 k. J/mol. While hydrogenation of 1 double bond required 119. 7 k. J/mol. Difference in energy is 3 (119. 7) = 359. 1 ─208. 4 = 150. 7 k. J/mol or (36. 2 k cal/mol). This difference (36. 2 k cal/mol) actually is resonance energy of the benzene.
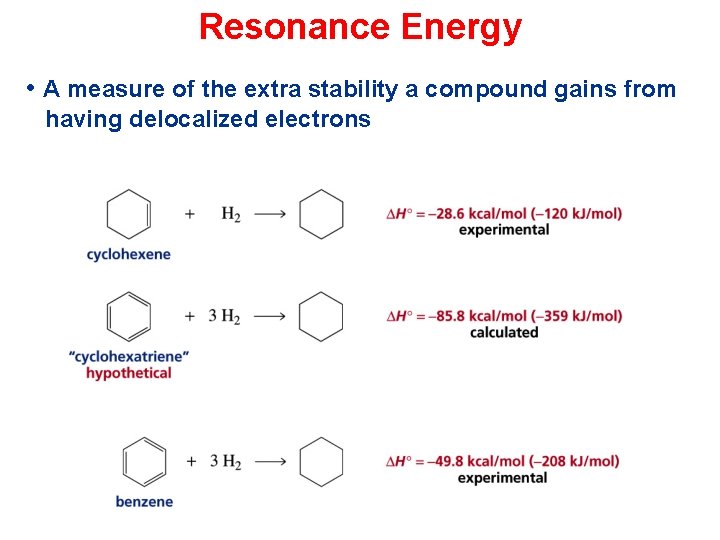
Resonance Energy • A measure of the extra stability a compound gains from having delocalized electrons
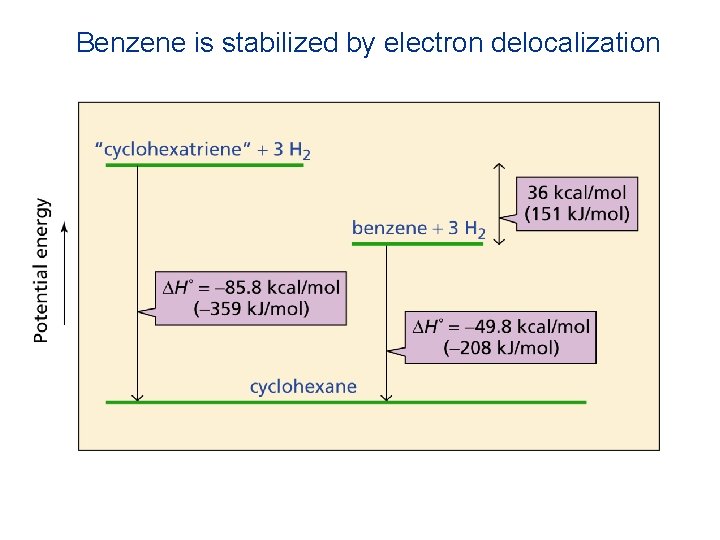
Benzene is stabilized by electron delocalization
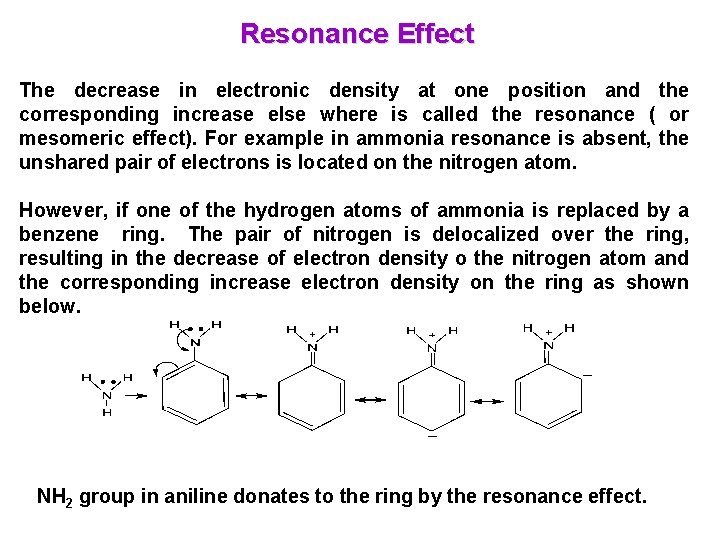
Resonance Effect The decrease in electronic density at one position and the corresponding increase else where is called the resonance ( or mesomeric effect). For example in ammonia resonance is absent, the unshared pair of electrons is located on the nitrogen atom. However, if one of the hydrogen atoms of ammonia is replaced by a benzene ring. The pair of nitrogen is delocalized over the ring, resulting in the decrease of electron density o the nitrogen atom and the corresponding increase electron density on the ring as shown below. NH 2 group in aniline donates to the ring by the resonance effect.
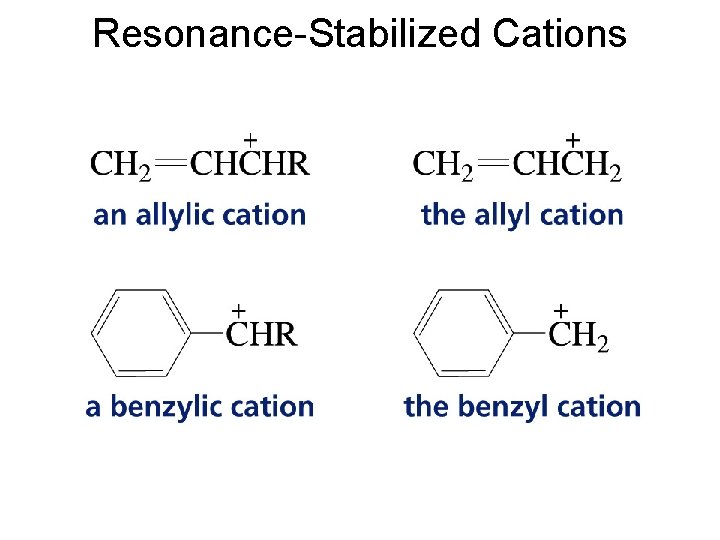
Resonance-Stabilized Cations
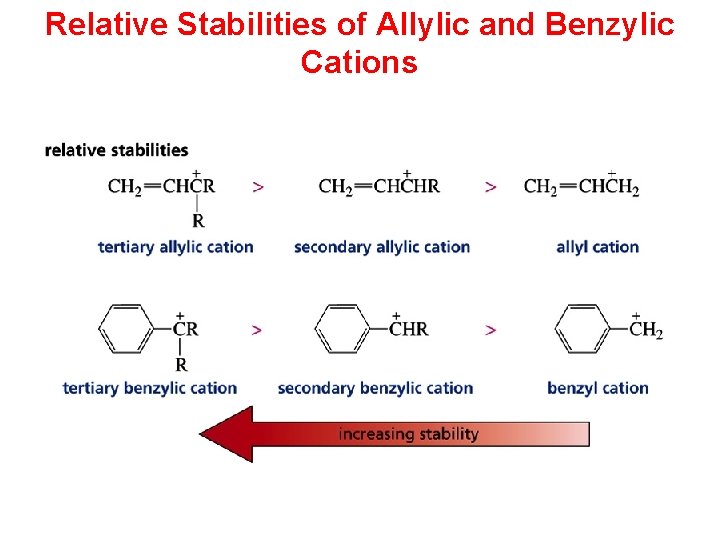
Relative Stabilities of Allylic and Benzylic Cations
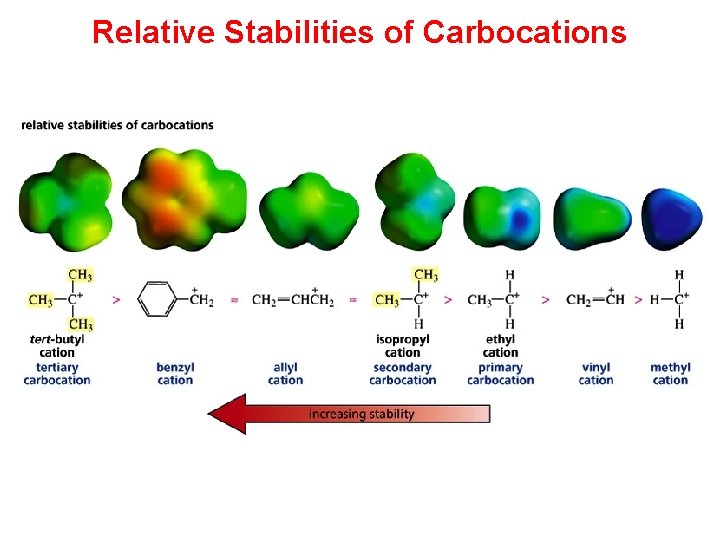
Relative Stabilities of Carbocations
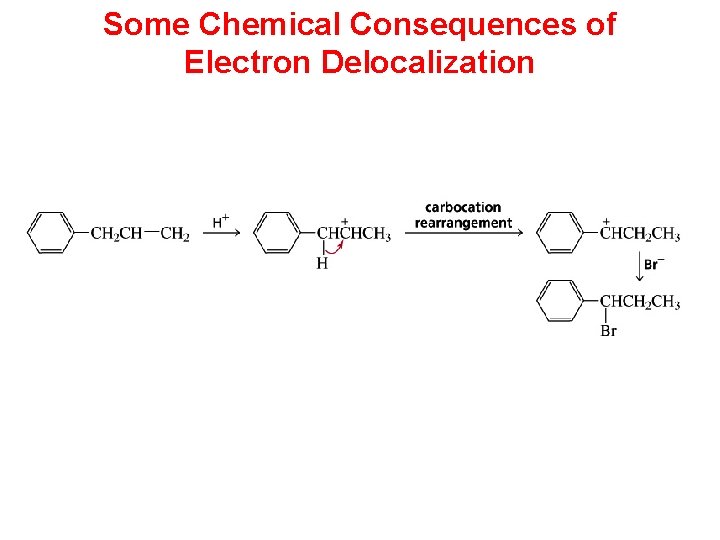
Some Chemical Consequences of Electron Delocalization
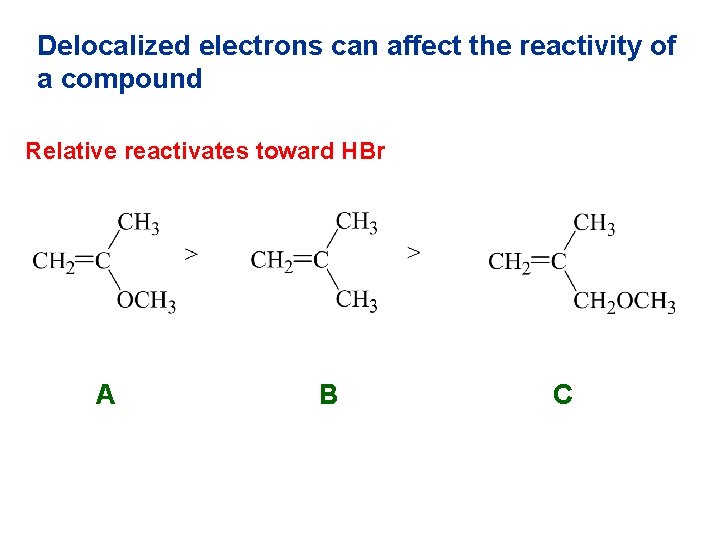
Delocalized electrons can affect the reactivity of a compound Relative reactivates toward HBr A B C
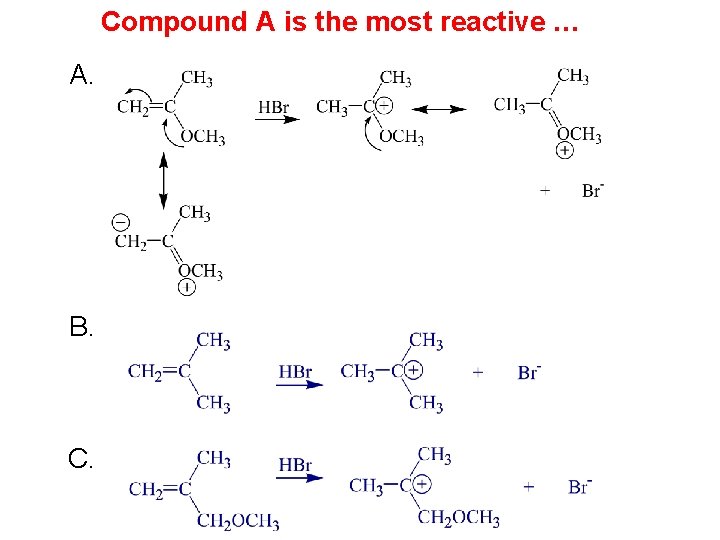
Compound A is the most reactive … A. B. C.
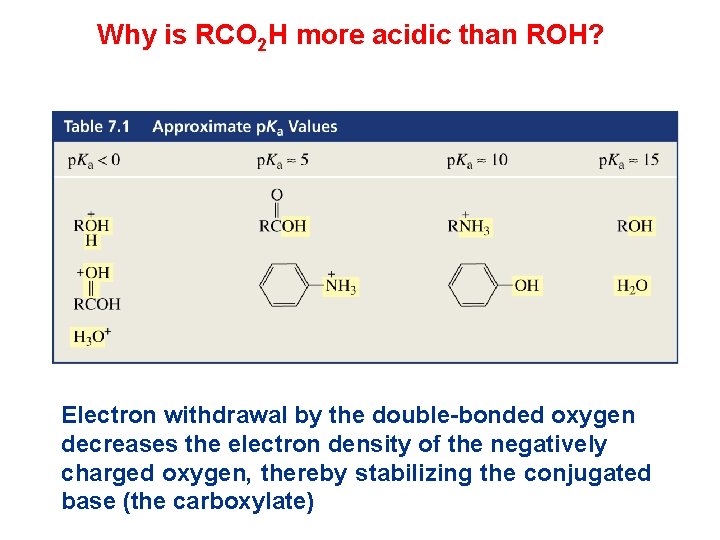
Why is RCO 2 H more acidic than ROH? Electron withdrawal by the double-bonded oxygen decreases the electron density of the negatively charged oxygen, thereby stabilizing the conjugated base (the carboxylate)
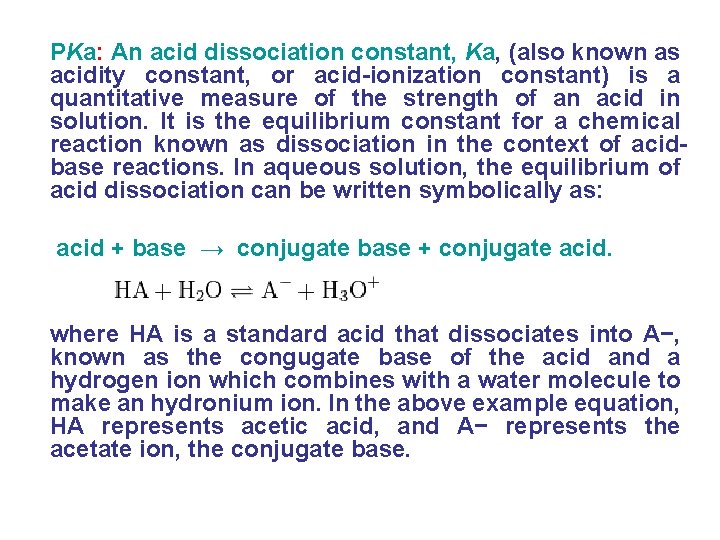
PKa: An acid dissociation constant, Ka, (also known as acidity constant, or acid-ionization constant) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acidbase reactions. In aqueous solution, the equilibrium of acid dissociation can be written symbolically as: acid + base → conjugate base + conjugate acid. where HA is a standard acid that dissociates into A−, known as the congugate base of the acid and a hydrogen ion which combines with a water molecule to make an hydronium ion. In the above example equation, HA represents acetic acid, and A− represents the acetate ion, the conjugate base.

• For many practical purposes it is more convenient to discuss the logarithmic constant, p. Ka • The larger the value of p. Ka, the smaller the extent of dissociation at any given p. H that is, the weaker the acid. A weak has a p. Ka value in the approximate range − 2 to 12 in water. Acids with a p. Ka value of less than about − 2 are said to be strong acids; the dissociation of a strong acid is effectively complete such that concentration of the undissociated acid is too small to be measured.
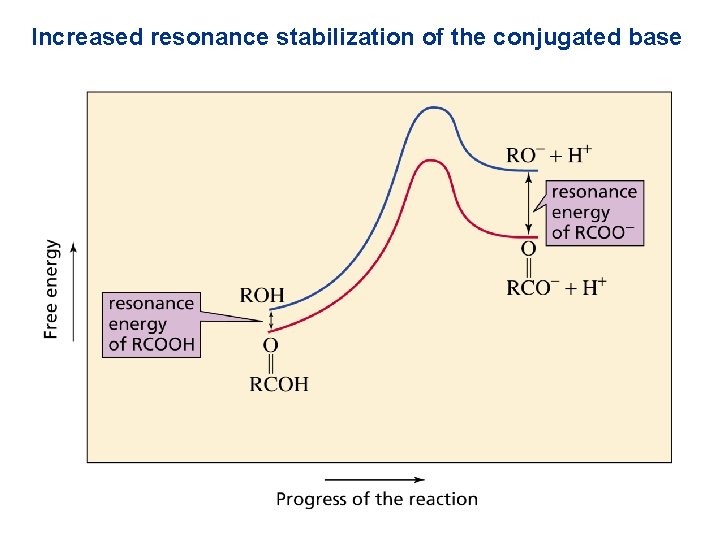
Increased resonance stabilization of the conjugated base
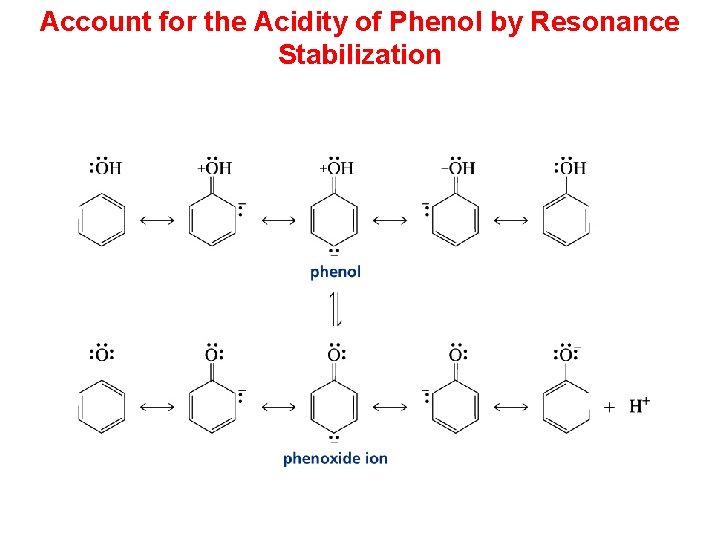
Account for the Acidity of Phenol by Resonance Stabilization
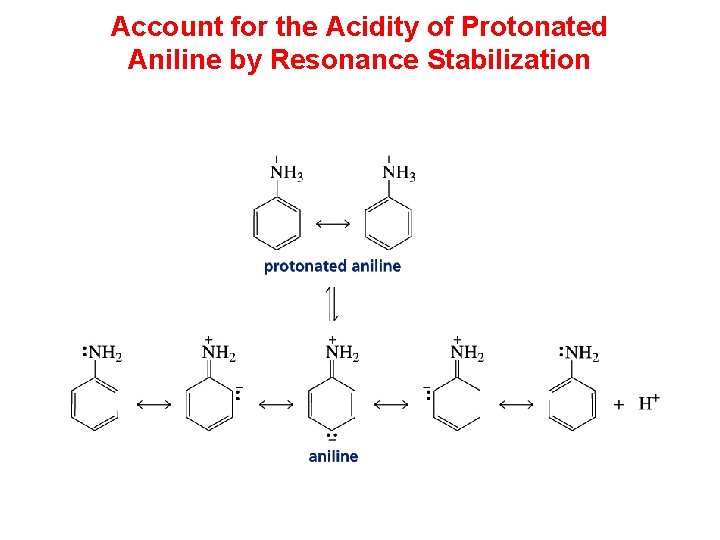
Account for the Acidity of Protonated Aniline by Resonance Stabilization
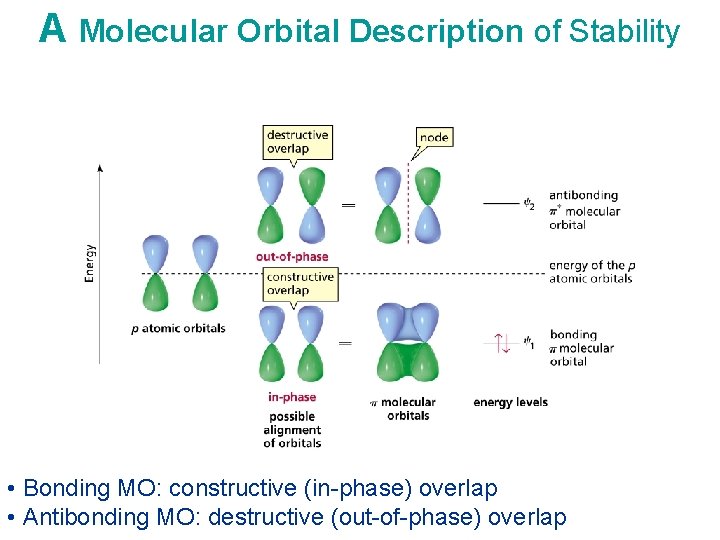
A Molecular Orbital Description of Stability • Bonding MO: constructive (in-phase) overlap • Antibonding MO: destructive (out-of-phase) overlap
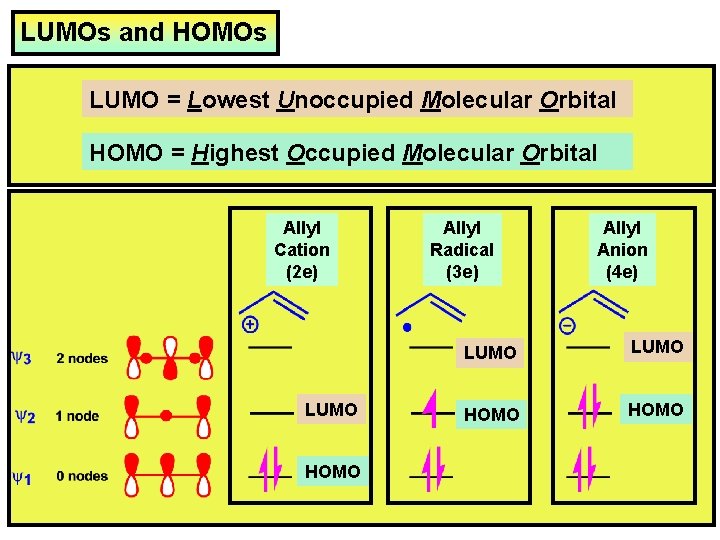
LUMOs and HOMOs LUMO = Lowest Unoccupied Molecular Orbital HOMO = Highest Occupied Molecular Orbital Allyl Cation (2 e) LUMO HOMO Allyl Radical (3 e) Allyl Anion (4 e) LUMO HOMO
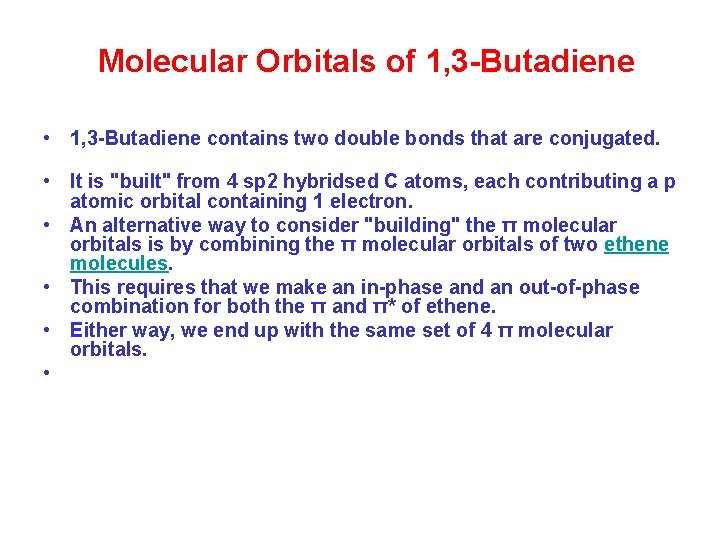
Molecular Orbitals of 1, 3 -Butadiene • 1, 3 -Butadiene contains two double bonds that are conjugated. • It is "built" from 4 sp 2 hybridsed C atoms, each contributing a p atomic orbital containing 1 electron. • An alternative way to consider "building" the π molecular orbitals is by combining the π molecular orbitals of two ethene molecules. • This requires that we make an in-phase and an out-of-phase combination for both the π and π* of ethene. • Either way, we end up with the same set of 4 π molecular orbitals. •
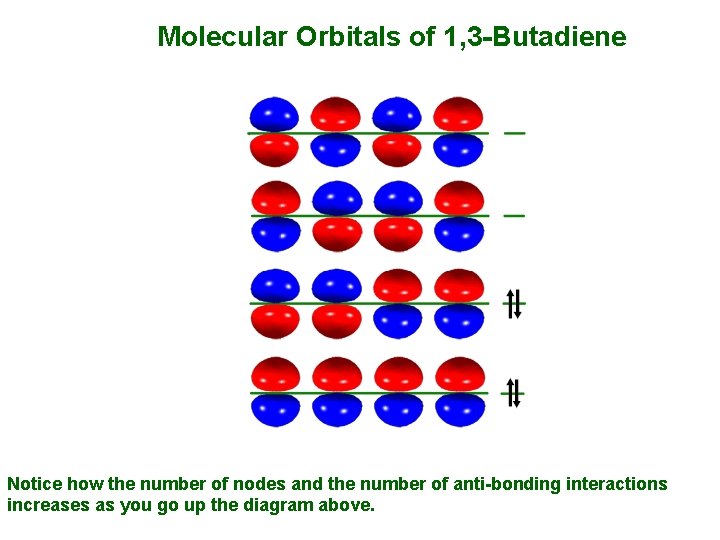
Molecular Orbitals of 1, 3 -Butadiene Notice how the number of nodes and the number of anti-bonding interactions increases as you go up the diagram above.
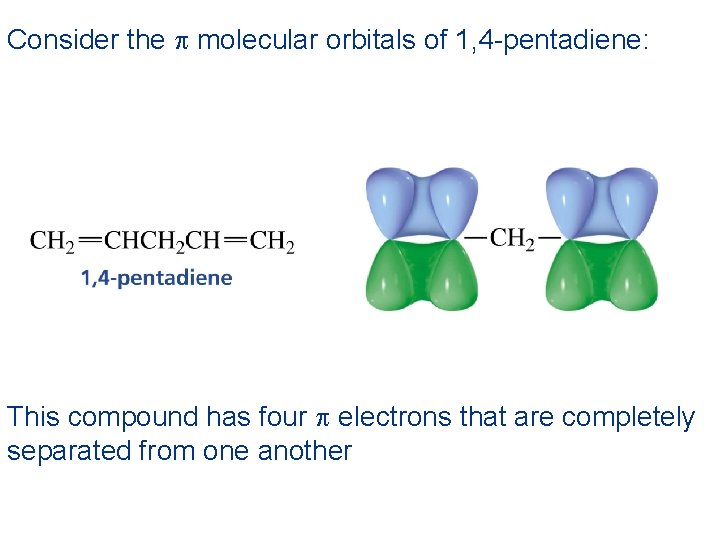
Consider the p molecular orbitals of 1, 4 -pentadiene: This compound has four p electrons that are completely separated from one another
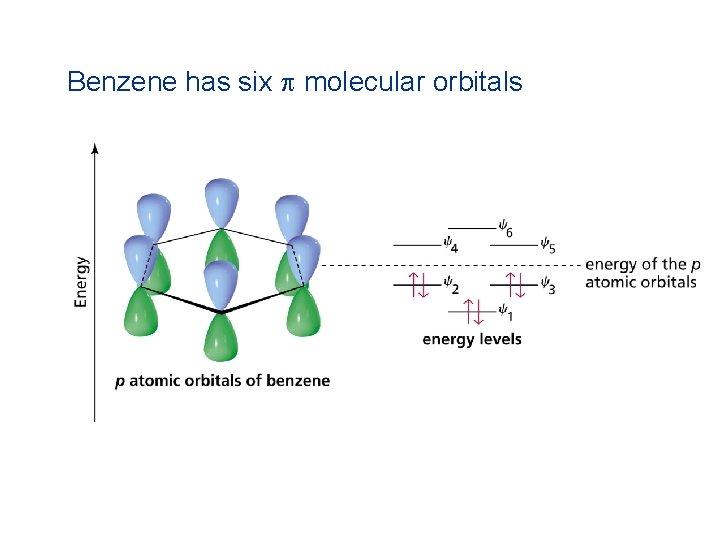
Benzene has six p molecular orbitals
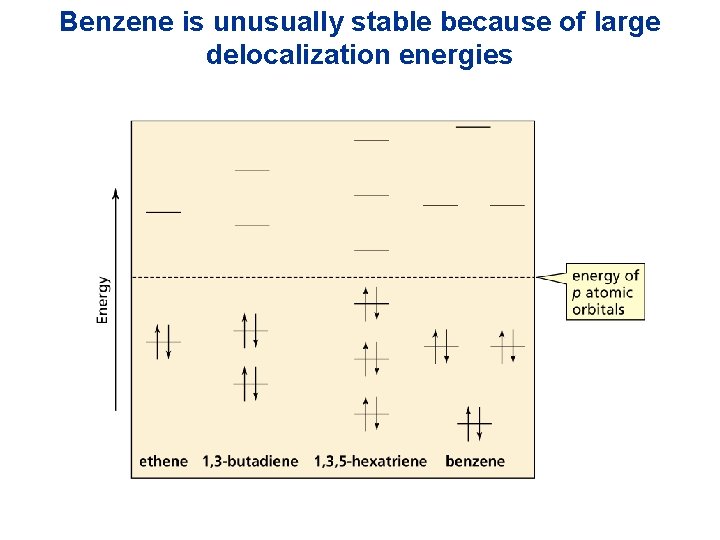
Benzene is unusually stable because of large delocalization energies
- Slides: 48