Advanced InOffice PCR Testing UTI Respiratory Sinus COVID19






- Slides: 13

Advanced In-Office PCR Testing UTI • Respiratory • Sinus • COVID-19 • Vaginitis Wound Care • Nail Fungus • Antibiotic Resistance Ancillary Revenue Opportunity

IN-OFFICE POINT OF CARE PCR TESTING = RAPIDLY INCREASED MONTHLY REVENUE Our Point of Care Turn-Key Program is revolutionizing the way large practices increase monthly revenue. Many practices are still using offsite laboratories to process their patients UTI, Vaginitis, wound and respiratory related cultures. Often these facilities are not only billing the insurance carrier, they also send patients to collections. www. preventiondiagnostics. com The Integration of this system will improve patient care, leverage your in-network billing privileges and better control your patient’s financial and treatment outcome. 2

TESTING BENEFITS Rapid same day test results Antibiotic resistance read out Test your patients in-office and get test results during the same visit! Determine if your patient needs antibiotics. Test results will provide the antibiotic resistance of the organism that presents as positive in your patients test results. The Joint Venture program is a complete turnkey opportunity. Compliant with the Antibiotic stewardship program We have designed this program to be effortless on your part. We provide • The setup (including your C. L. I. A license) • The device • The technician • The supplies • The Billing Stop using antibiotics that don’t cure your patient. Test your patients on a clinical level just like the Laboratories. By integrating the point of care system into your office. Your medical practice will elevate with robust services, increased treatment capabilities and monthly revenue. The CDChas started to mandate the use of antibiotic alternatives for long-term managed care. By reducing the use of antibiotics patients will have less resistance when it is truly needed. We have created a program that keeps you compliant with monthly reports. PCRtesting will help reduce the use of antibiotics and increase your facility health score. It is only a matter of time before it is mandated at the clinical level. www. preventiondagnostics. com 3

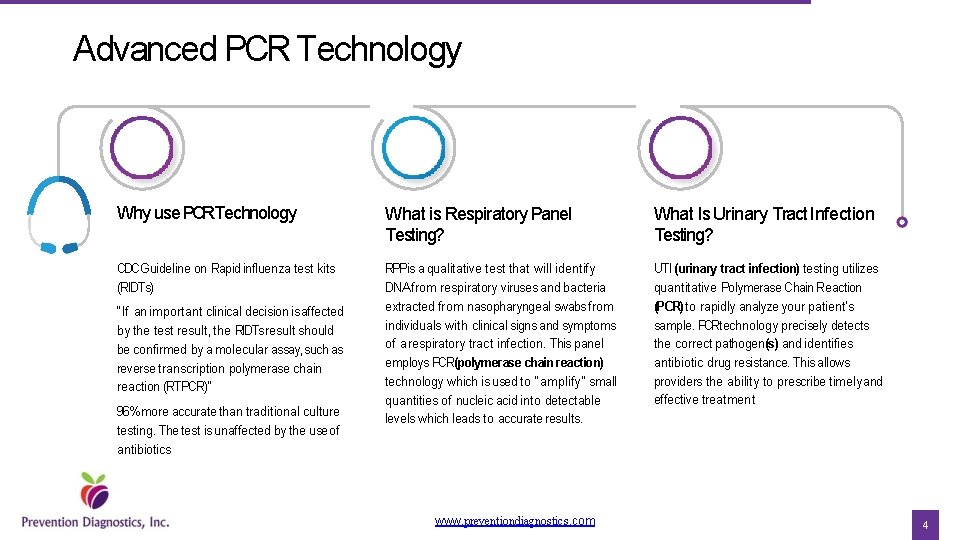
Advanced PCR Technology Why use PCRTechnology What is Respiratory Panel Testing? What Is Urinary Tract Infection Testing? CDCGuideline on Rapid influenza test kits (RIDTs) RPPis a qualitative test that will identify DNA from respiratory viruses and bacteria extracted from nasopharyngeal swabs from individuals with clinical signs and symptoms of a respiratory tract infection. This panel employs PCR(polymerase chain reaction) technology which is used to “amplify” small quantities of nucleic acid into detectable levels which leads to accurate results. UTI (urinary tract infection) testing utilizes quantitative Polymerase Chain Reaction (PCR) to rapidly analyze your patient’s sample. PCRtechnology precisely detects the correct pathogen(s) and identifies antibiotic drug resistance. This allows providers the ability to prescribe timely and effective treatment “If an important clinical decision is affected by the test result, the RIDTsresult should be confirmed by a molecular assay, such as reverse transcription polymerase chain reaction (RTPCR)” 96% more accurate than traditional culture testing. The test is unaffected by the useof antibiotics www. preventiondiagnostics. com 4

This test will prove if a patient has a virus and does not require antibiotics, thereby affirming a provider’s decision to avoid drugs that are unnecessary and can have significant side effects. (THIS IS A CONSTANTSTRUGGLEFORALL PHYSICIANS. ) www. preventiondiagnostics. com 5

What does this mean for Your Practice? 100% 0% 45 MINUTES IMMEDIATELY Increased Clinical Value Over prescription of Antibiotics Timely, actionable results Stop guessing and start treating Minimal upfront costs SUCCESS Just 10 tests a day can generate over $140, 000+ per month in net revenue www. preventiondiagnostics. com 6

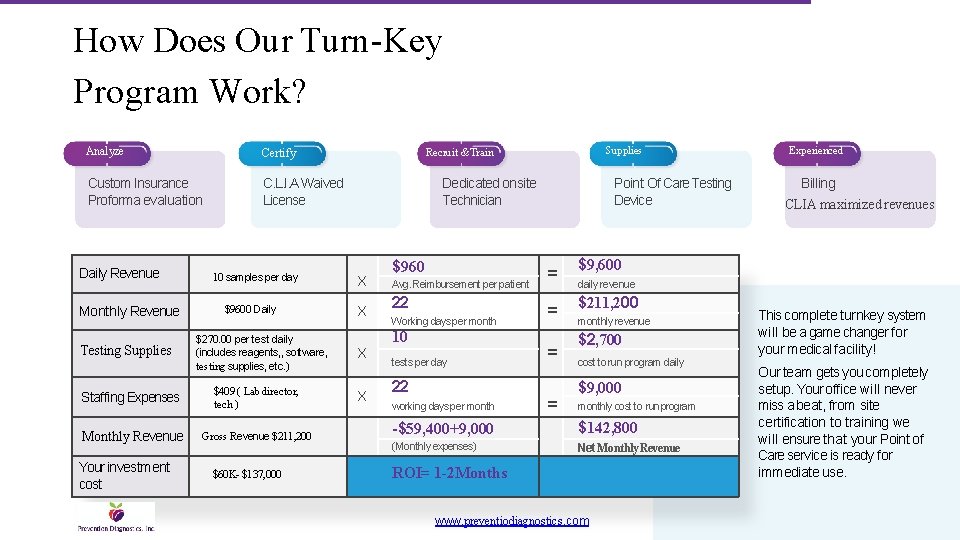
How Does Our Turn-Key Program Work? Analyze Certify Custom Insurance Proforma evaluation C. L. I. A Waived License Daily Revenue Monthly Revenue Testing Supplies 10 samples per day $9600 Daily $270. 00 per test daily (includes reagents, , software, testing supplies, etc. ) Staffing Expenses $409 ( Lab director, tech ) Monthly Revenue Gross Revenue $211, 200 Your investment cost $60 K- $137, 000 Supplies Recruit & Train Dedicated onsite Technician X X $960 Avg. Reimbursement per patient 22 Working days per month 10 tests per day 22 working days per month Point Of Care Testing Device = $9, 600 = $211, 200 = = Experienced Billing CLIA maximized revenues daily revenue monthly revenue $2, 700 cost to run program daily $9, 000 monthly cost to runprogram -$59, 400+9, 000 $142, 800 (Monthly expenses) Net Monthly Revenue ROI= 1 -2 Months www. preventiodiagnostics. com This complete turnkey system will be a game changer for your medical facility! Our team gets you completely setup. Your office will never miss a beat, from site certification to training we will ensure that your Point of Care service is ready for immediate use. 7

In-Office RPPand UTI Testing Device This well-known manufacture provides hardware for laboratories and hospitals nationwide. Integrate the Point of Care UTI and RPP device today! The program requires just 6 feet of dedicated desk space and a CLIA Certificate. The CLIA licensing is part of the program and does not require any upfront costs. Start increasing your monthly revenue with no out of pocket expenses! www. preventiondiagnostics. com 8

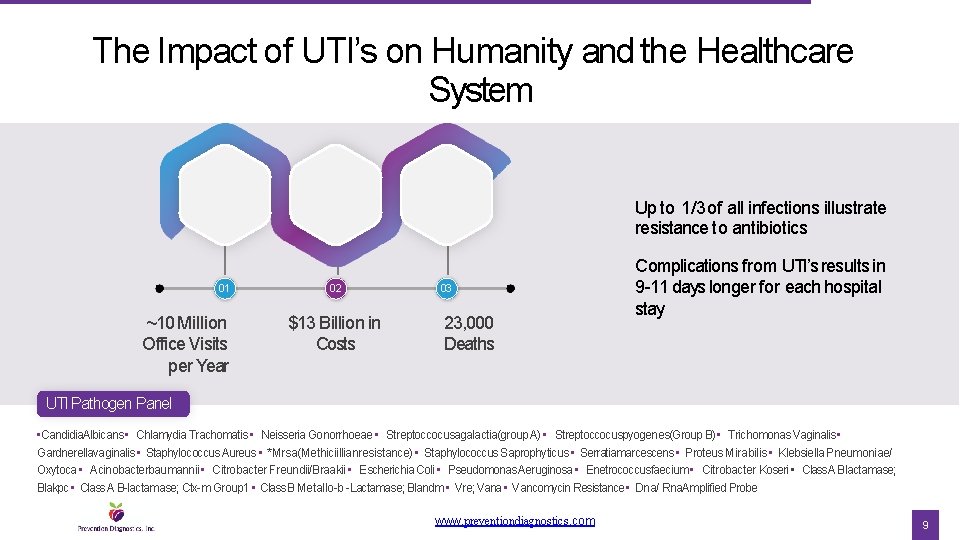
The Impact of UTI’s on Humanity and the Healthcare System Up to 1/3 of all infections illustrate resistance to antibiotics 01 ~10 Million Office Visits per Year 02 $13 Billion in Costs 03 23, 000 Deaths Complications from UTI’s results in 9 -11 days longer for each hospital stay UTI Pathogen Panel • Candidia. Albicans • Chlamydia Trachomatis • Neisseria Gonorrhoeae • Streptoccocusagalactia(group A) • Streptoccocuspyogenes(Group B) • Trichomonas Vaginalis • Gardnerellavaginalis • Staphylococcus Aureus • *Mrsa(Methiciillianresistance) • Staphylococcus Saprophyticus • Serratiamarcescens • Proteus Mirabilis • Klebsiella Pneumoniae/ Oxytoca • Acinobacterbaumannii • Citrobacter Freundii/Braakii • Escherichia Coli • Pseudomonas Aeruginosa • Enetrococcusfaecium • Citrobacter Koseri • Class A Blactamase; Blakpc • Class A B-lactamase; Ctx-m Group 1 • Class B Metallo-b -Lactamase; Blandm • Vre; Vana • Vancomycin Resistance • Dna/ Rna. Amplified Probe www. preventiondiagnostics. com 9

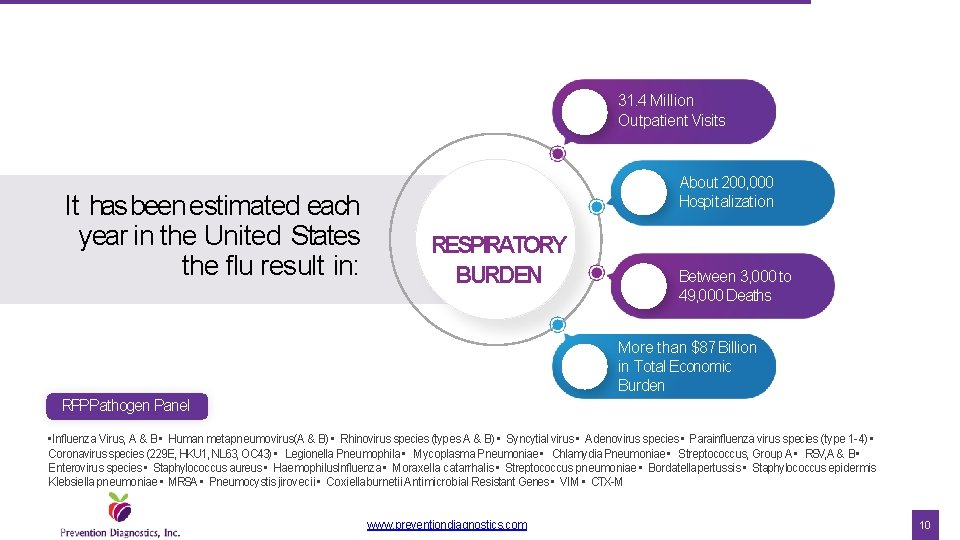
31. 4 Million Outpatient Visits It has been estimated each year in the United States the flu result in: About 200, 000 Hospitalization RESPIRATORY BURDEN Between 3, 000 to 49, 000 Deaths More than $87 Billion in Total Economic Burden RPPPathogen Panel • Influenza Virus, A & B • Human metapneumovirus(A & B) • Rhinovirus species (types A & B) • Syncytial virus • Adenovirus species • Parainfluenza virus species (type 1 -4) • Coronavirus species (229 E, HKU 1, NL 63, OC 43) • Legionella Pneumophila • Mycoplasma Pneumoniae • Chlamydia Pneumoniae • Streptococcus, Group A • RSV, A & B • Enterovirus species • Staphylococcus aureus • Haemophilus. Influenza • Moraxella catarrhalis • Streptococcus pneumoniae • Bordatellapertussis • Staphylococcus epidermis Klebsiella pneumoniae • MRSA • Pneumocystis jirovecii • Coxiellaburnetii Antimicrobial Resistant Genes • VIM • CTX-M www. preventiondiagnostics. com 10

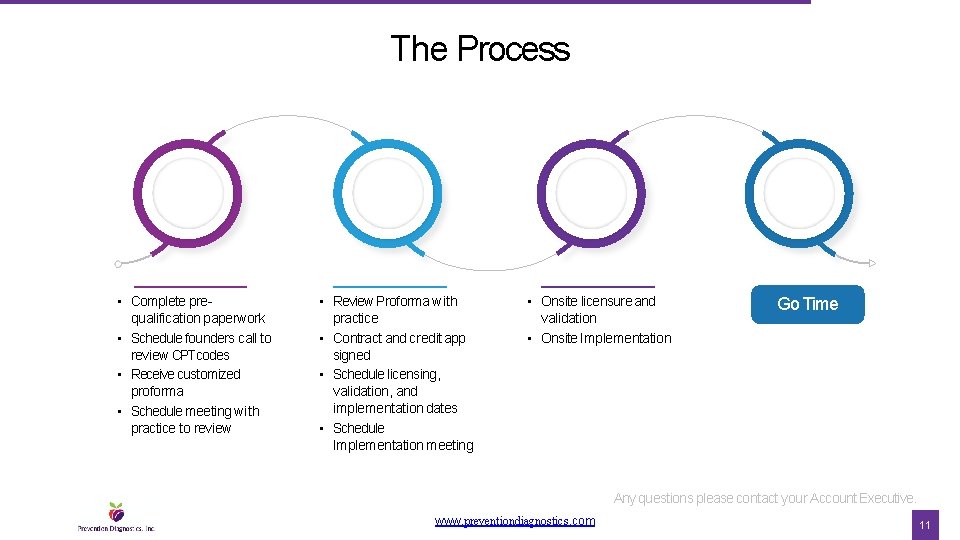
The Process • Complete prequalification paperwork • Schedule founders call to review CPT codes • Receive customized proforma • Schedule meeting with practice to review • Review Proforma with practice • Contract and credit app signed • Schedule licensing, validation, and implementation dates • Schedule Implementation meeting • Onsite licensure and validation • Onsite Implementation Go Time Any questions please contact your Account Executive. www. preventiondiagnostics. com 11

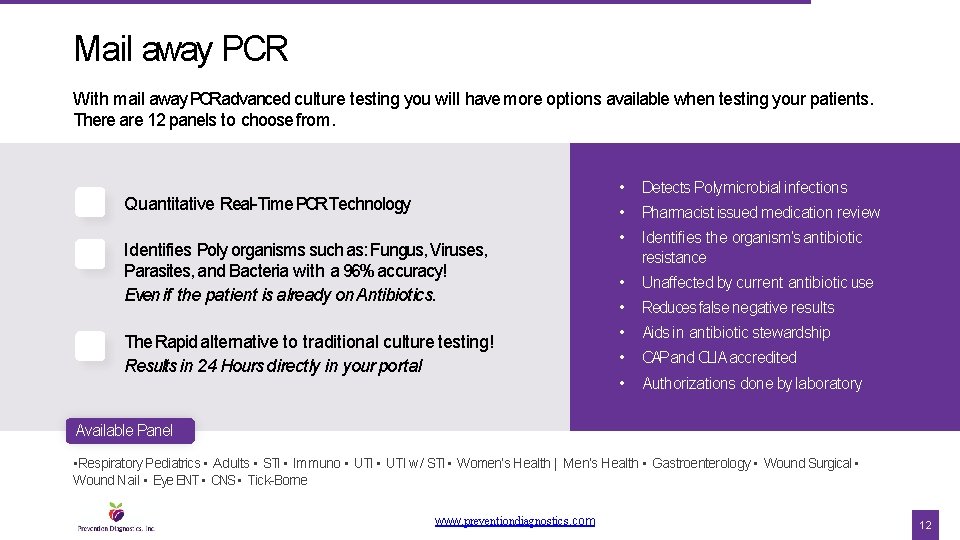
Mail away PCR With mail away PCRadvanced culture testing you will have more options available when testing your patients. There are 12 panels to choose from. Quantitative Real-Time PCRTechnology Identifies Poly organisms such as: Fungus, Viruses, Parasites, and Bacteria with a 96% accuracy! Even if the patient is already on Antibiotics. The Rapid alternative to traditional culture testing! Results in 24 Hours directly in your portal • Detects Polymicrobial infections • Pharmacist issued medication review • Identifies the organism’s antibiotic resistance • Unaffected by current antibiotic use • Reduces false negative results • Aids in antibiotic stewardship • CAPand CLIA accredited • Authorizations done by laboratory Available Panel • Respiratory Pediatrics • Adults • STI • Immuno • UTI w/ STI • Women’s Health | Men’s Health • Gastroenterology • Wound Surgical • Wound Nail • Eye ENT • CNS • Tick-Borne www. preventiondiagnostics. com 12

THANK YOU sales@preventiondiagnostics. com www. preventiondiagnostics. com 800 -353 -9469