Advanced Fractionation Techniques for Complex Polyolefins Harald Pasch




![Crystallization Analysis Fractionation IR detector d. W/d. T W [%] 6 100 4 60 Crystallization Analysis Fractionation IR detector d. W/d. T W [%] 6 100 4 60](https://slidetodoc.com/presentation_image_h/e64da352de7ea9f98591d8ff87aea9e8/image-22.jpg)

![Separation System for PE-PP Blends PP PE M signal % TCB Time [min] 40 Separation System for PE-PP Blends PP PE M signal % TCB Time [min] 40](https://slidetodoc.com/presentation_image_h/e64da352de7ea9f98591d8ff87aea9e8/image-40.jpg)

- Slides: 54

Advanced Fractionation Techniques for Complex Polyolefins Harald Pasch SASOL Chair of Analytical Polymer Science Department of Chemistry and Polymer Science, University of Stellenbosch, South Africa 1

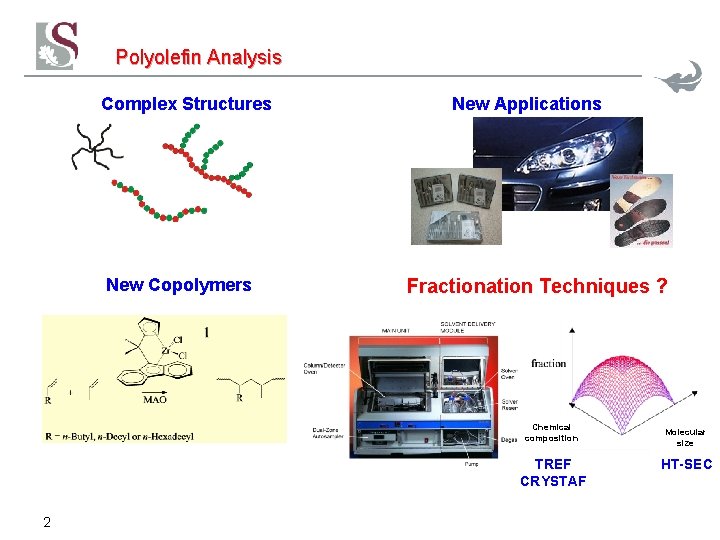
Polyolefin Analysis Complex Structures New Copolymers New Applications Fractionation Techniques ? Chemical composition TREF CRYSTAF 2 Molecular size HT-SEC

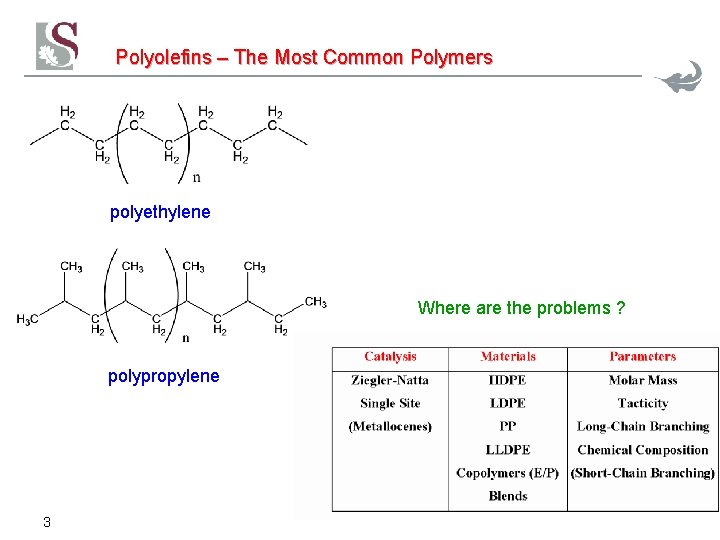
Polyolefins – The Most Common Polymers polyethylene Where are the problems ? polypropylene 3

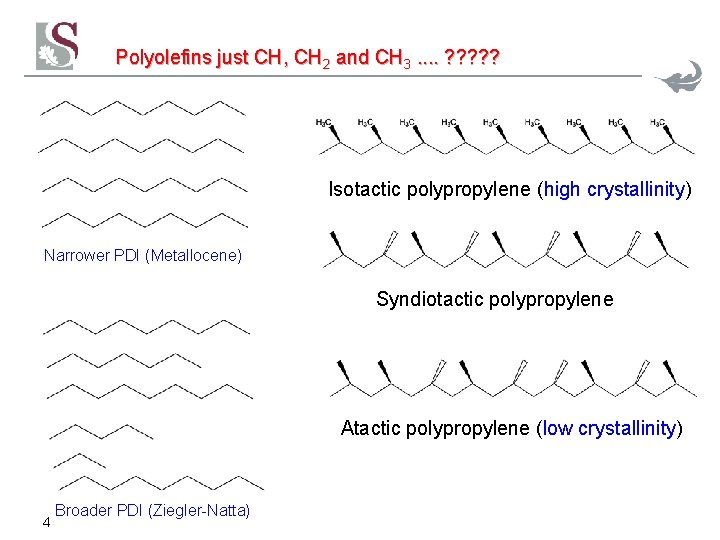
Polyolefins just CH, CH 2 and CH 3. . ? ? ? Isotactic polypropylene (high crystallinity) Narrower PDI (Metallocene) Syndiotactic polypropylene Atactic polypropylene (low crystallinity) 4 Broader PDI (Ziegler-Natta)

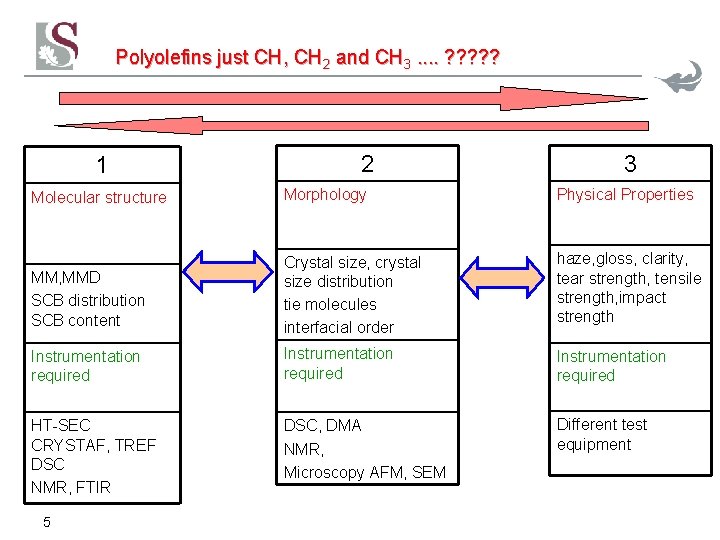
Polyolefins just CH, CH 2 and CH 3. . ? ? ? 1 2 3 Molecular structure Morphology Physical Properties MM, MMD SCB distribution SCB content Crystal size, crystal size distribution tie molecules interfacial order haze, gloss, clarity, tear strength, tensile strength, impact strength Instrumentation required HT-SEC CRYSTAF, TREF DSC NMR, FTIR DSC, DMA NMR, Microscopy AFM, SEM Different test equipment 5

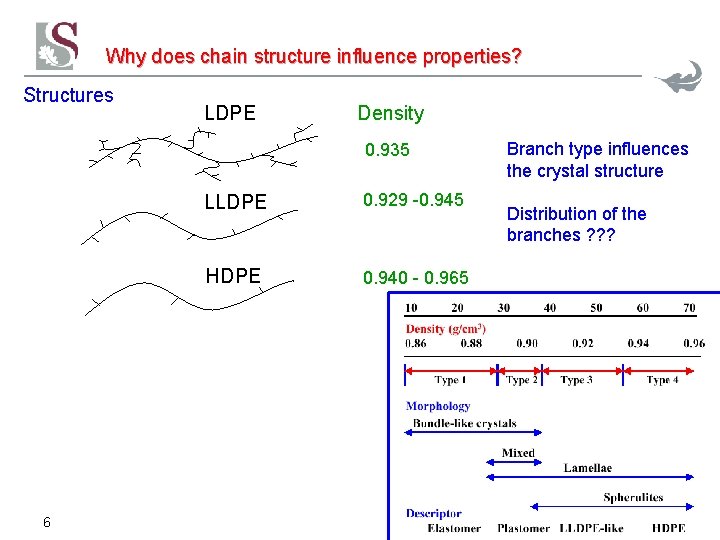
Why does chain structure influence properties? Structures LDPE Density 0. 935 6 LLDPE 0. 929 -0. 945 HDPE 0. 940 - 0. 965 Branch type influences the crystal structure Distribution of the branches ? ? ?

Change in Crystal Results: SEM Morphology as a Result of Blending LDPE 60% LDPE + 40% Plastomer poly(ethylene-1 -octene) 7

Molar Mass Analysis 8

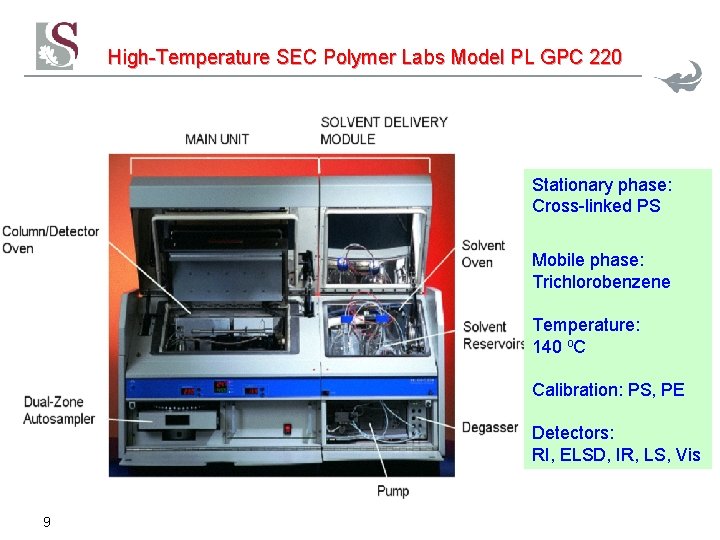
High-Temperature SEC Polymer Labs Model PL GPC 220 Stationary phase: Cross-linked PS Mobile phase: Trichlorobenzene Temperature: 140 o. C Calibration: PS, PE Detectors: RI, ELSD, IR, LS, Vis 9

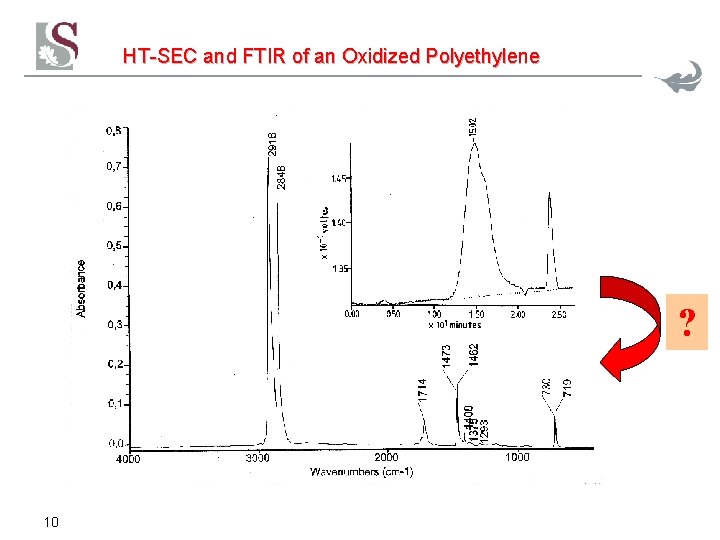
HT-SEC and FTIR of an Oxidized Polyethylene ? 10

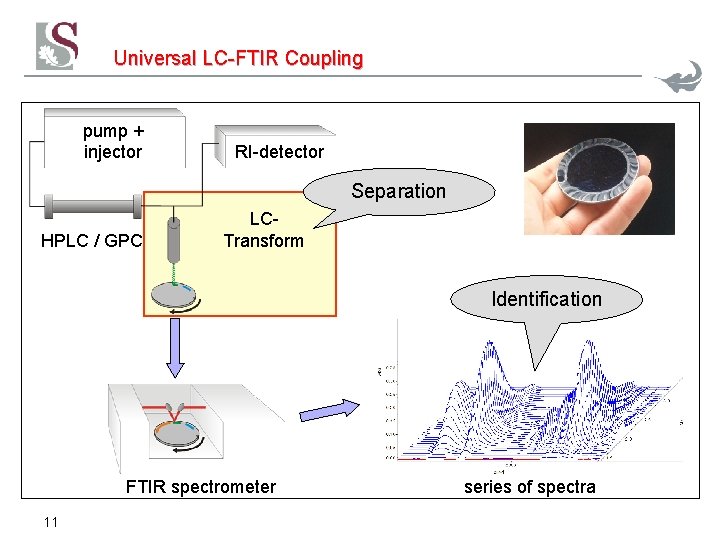
Universal LC-FTIR Coupling pump + injector RI-detector Separation HPLC / GPC LCTransform Identification FTIR spectrometer 11 series of spectra

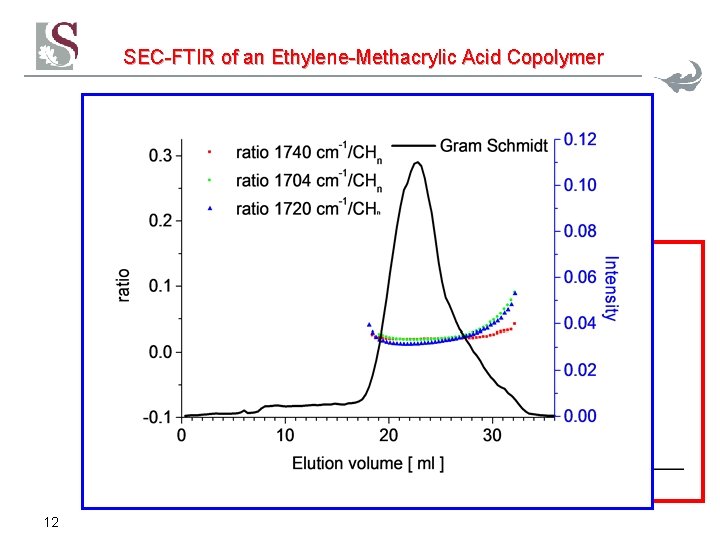
SEC-FTIR of an Ethylene-Methacrylic Acid Copolymer 12

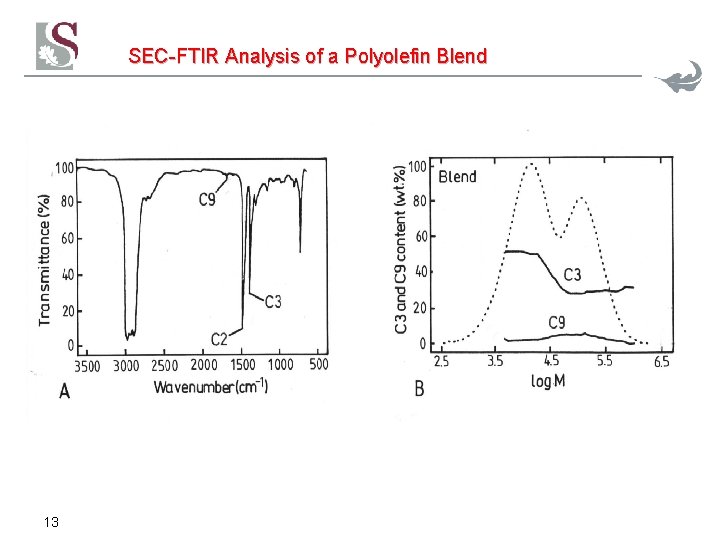
SEC-FTIR Analysis of a Polyolefin Blend 13

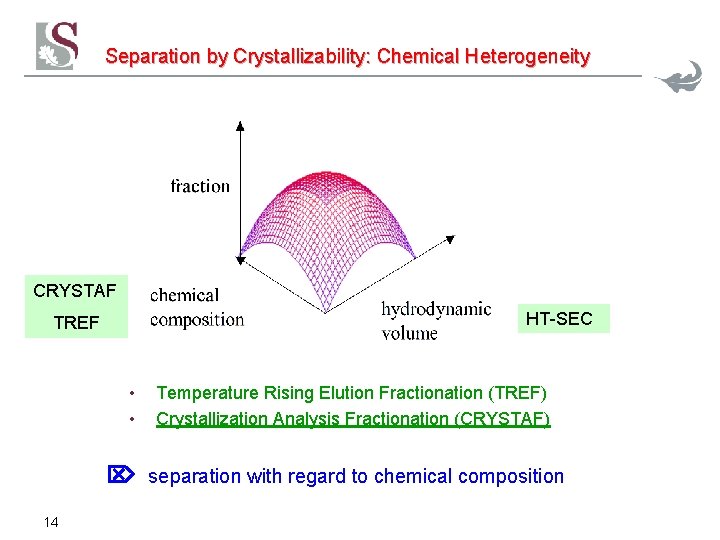
Separation by Crystallizability: Chemical Heterogeneity CRYSTAF HT-SEC TREF • • Temperature Rising Elution Fractionation (TREF) Crystallization Analysis Fractionation (CRYSTAF) separation with regard to chemical composition 14

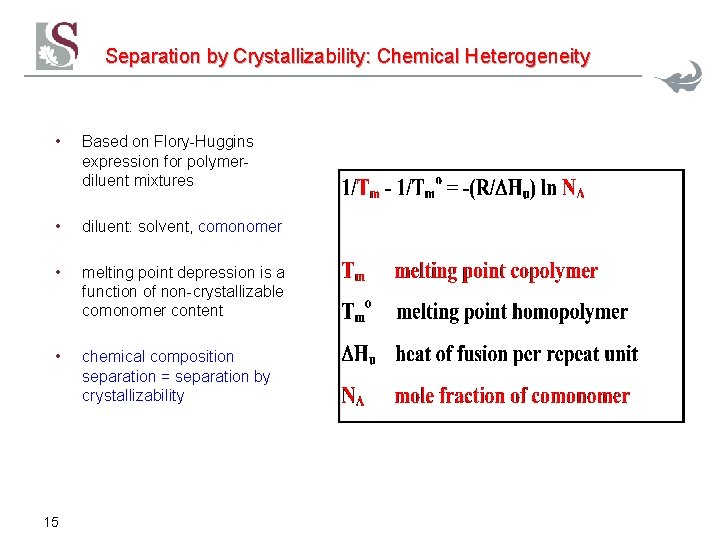
Separation by Crystallizability: Chemical Heterogeneity • Based on Flory-Huggins expression for polymerdiluent mixtures • diluent: solvent, comonomer • melting point depression is a function of non-crystallizable comonomer content • chemical composition separation = separation by crystallizability 15

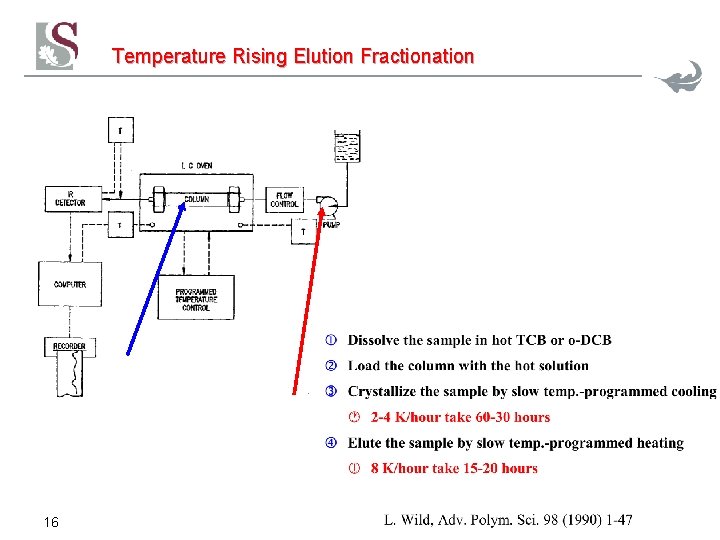
Temperature Rising Elution Fractionation 16

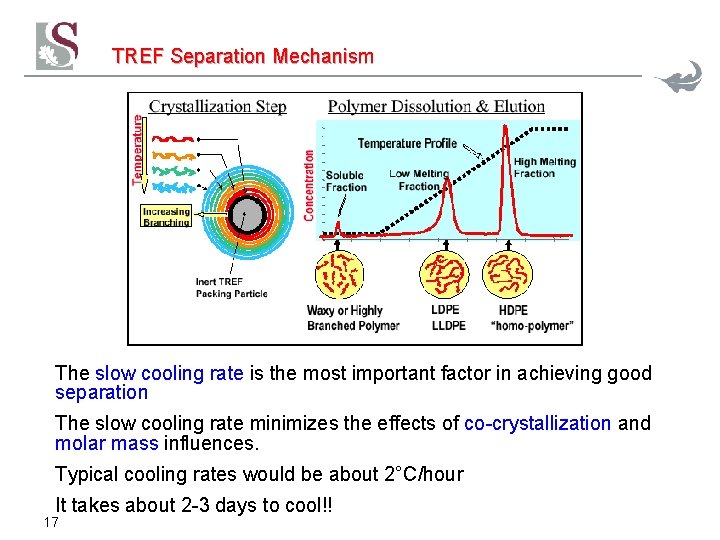
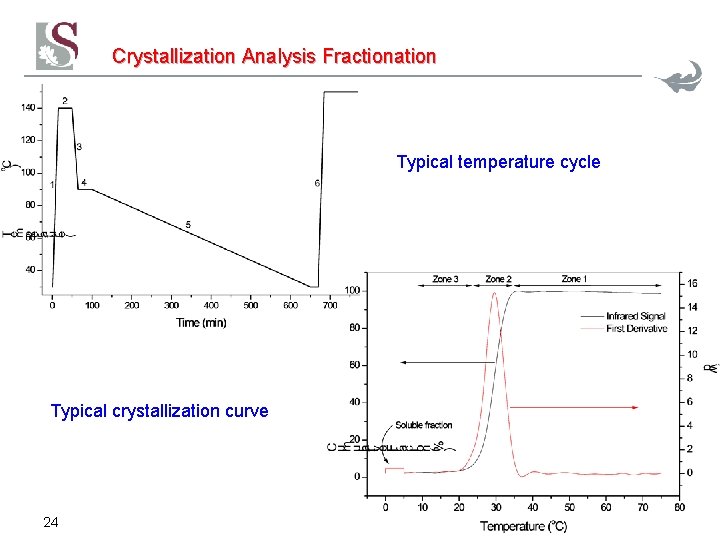
TREF Separation Mechanism The slow cooling rate is the most important factor in achieving good separation The slow cooling rate minimizes the effects of co-crystallization and molar mass influences. Typical cooling rates would be about 2°C/hour It takes about 2 -3 days to cool!! 17

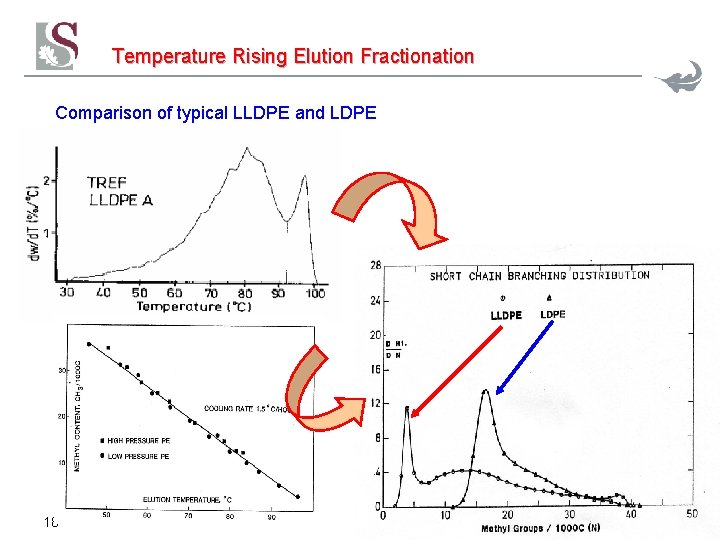
Temperature Rising Elution Fractionation Comparison of typical LLDPE and LDPE 18

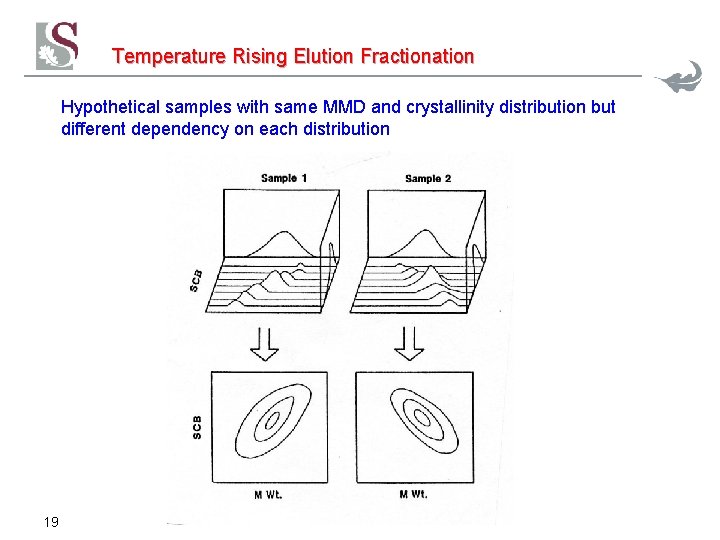
Temperature Rising Elution Fractionation Hypothetical samples with same MMD and crystallinity distribution but different dependency on each distribution 19

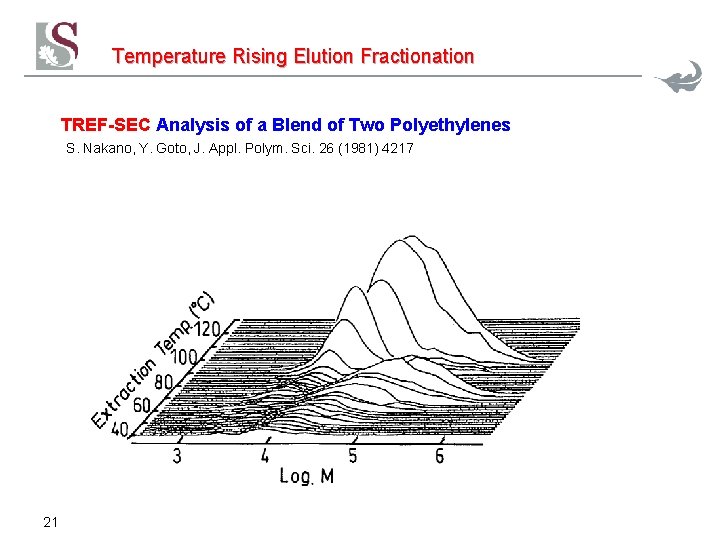
Temperature Rising Elution Fractionation Automatic Cross-Fractionation System TREF-SEC S. Nakano, Y. Goto, J. Appl. Polym. Sci. 26 (1981) 4217 20

Temperature Rising Elution Fractionation TREF-SEC Analysis of a Blend of Two Polyethylenes S. Nakano, Y. Goto, J. Appl. Polym. Sci. 26 (1981) 4217 21
![Crystallization Analysis Fractionation IR detector d Wd T W 6 100 4 60 Crystallization Analysis Fractionation IR detector d. W/d. T W [%] 6 100 4 60](https://slidetodoc.com/presentation_image_h/e64da352de7ea9f98591d8ff87aea9e8/image-22.jpg)
Crystallization Analysis Fractionation IR detector d. W/d. T W [%] 6 100 4 60 40 2 20 0 20 22 30 40 50 60 70 Temperature [°C] 80 0 90 W [%] d. W/d. T 80

Crystallization Analysis Fractionation 23

Crystallization Analysis Fractionation Typical temperature cycle Typical crystallization curve 24

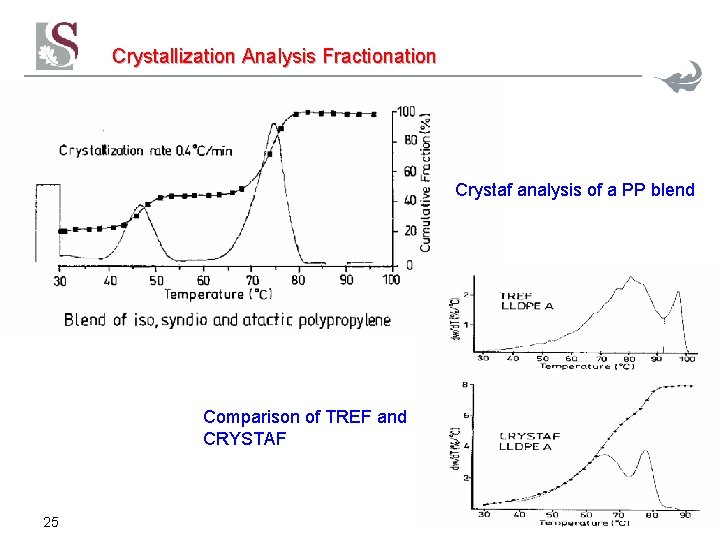
Crystallization Analysis Fractionation Crystaf analysis of a PP blend Comparison of TREF and CRYSTAF 25

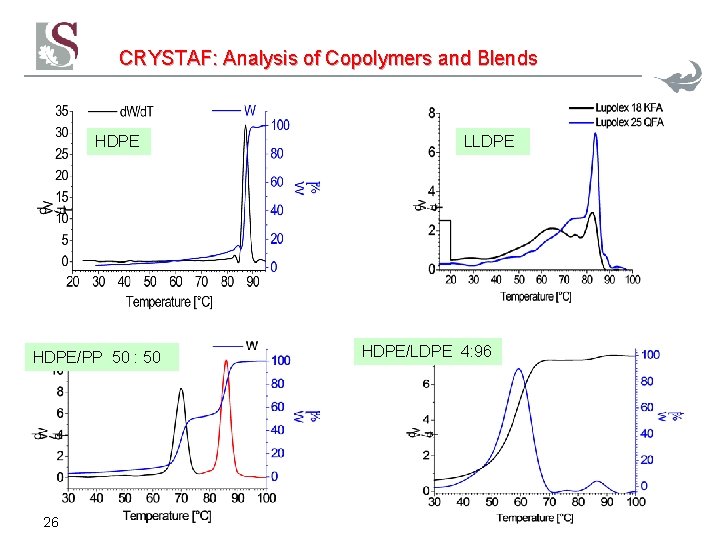
CRYSTAF: Analysis of Copolymers and Blends HDPE/PP 50 : 50 26 LLDPE HDPE/LDPE 4: 96

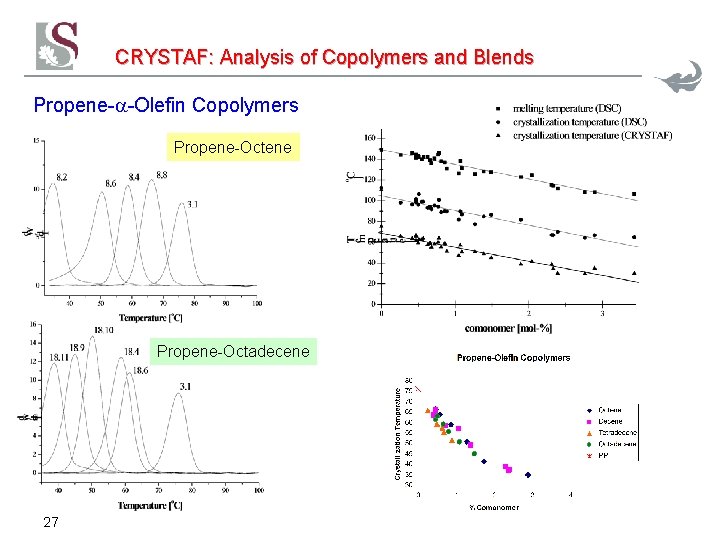
CRYSTAF: Analysis of Copolymers and Blends Propene- -Olefin Copolymers Propene-Octene Propene-Octadecene 27

CRYSTAF (in particular when coupled to IR sensor) excellent technique but very time consuming Fast and selective techniques are required liquid chromatography is a good candidate Polyolefins are soluble only at high temperatures unconventional stationary and mobile phases ? ? ? 28

Elution Behaviour of Polyolefins in High Temperature Chromatography (HT-HPLC) Using Interactive Stationary Phases polyolefin must dissolve in the mobile phase screening of solubility polyolefin must interact with the phase system screening of mobile and stationary phases 29

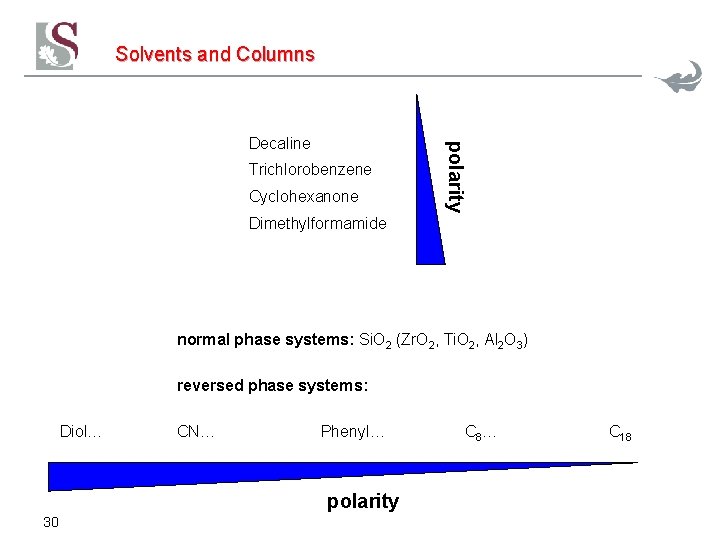
Solvents and Columns Trichlorobenzene Cyclohexanone polarity Decaline Dimethylformamide normal phase systems: Si. O 2 (Zr. O 2, Ti. O 2, Al 2 O 3) reversed phase systems: Diol… CN… Phenyl… polarity 30 C 8 … C 18

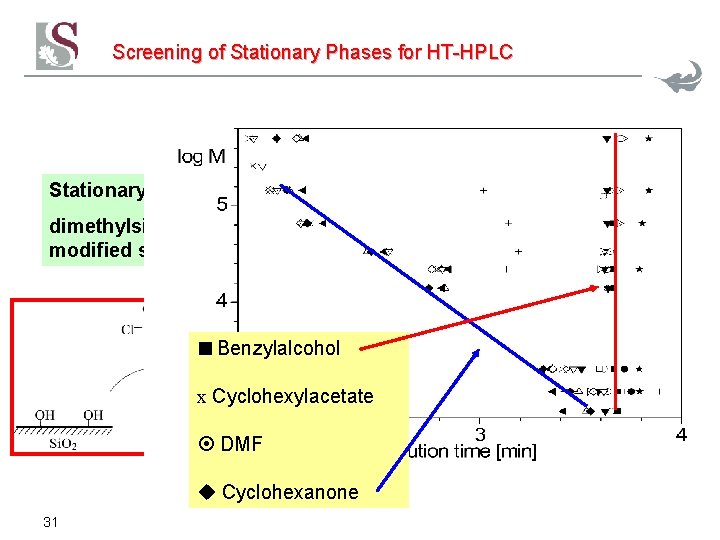
Screening of Stationary Phases for HT-HPLC Stationary phase: dimethylsiloxanemodified silica gel Benzylalcohol x Cyclohexylacetate DMF Cyclohexanone 31

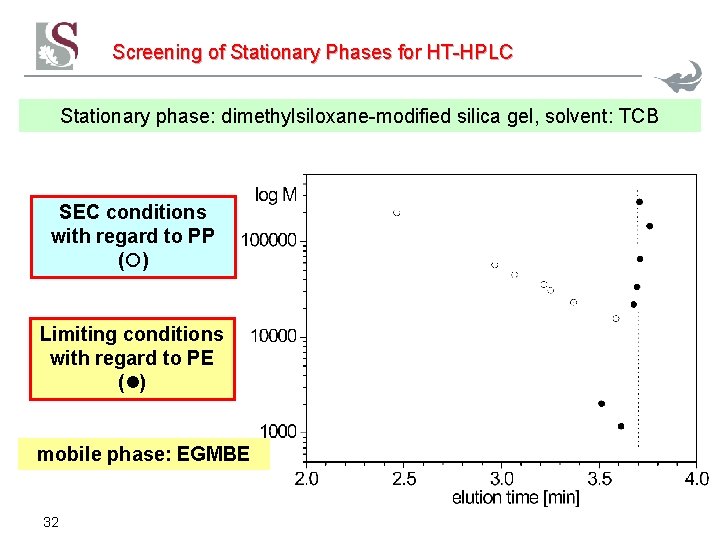
Screening of Stationary Phases for HT-HPLC Stationary phase: dimethylsiloxane-modified silica gel, solvent: TCB SEC conditions with regard to PP ( ) Limiting conditions with regard to PE ( ) mobile phase: EGMBE 32

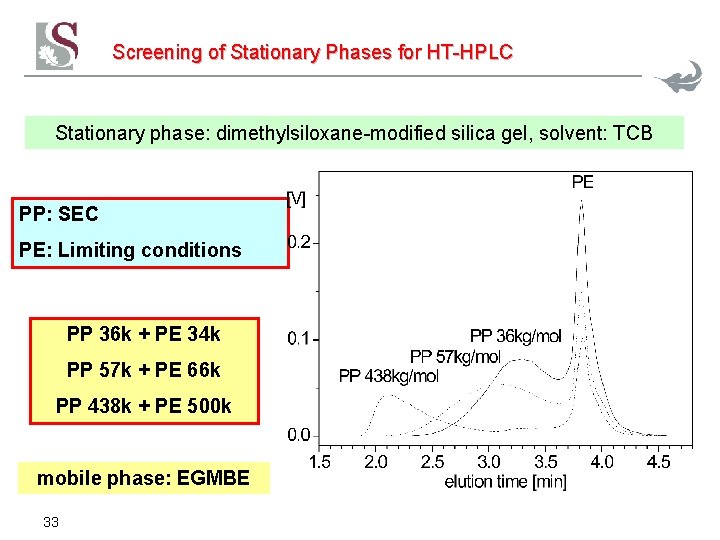
Screening of Stationary Phases for HT-HPLC Stationary phase: dimethylsiloxane-modified silica gel, solvent: TCB PP: SEC PE: Limiting conditions PP 36 k + PE 34 k PP 57 k + PE 66 k PP 438 k + PE 500 k mobile phase: EGMBE 33

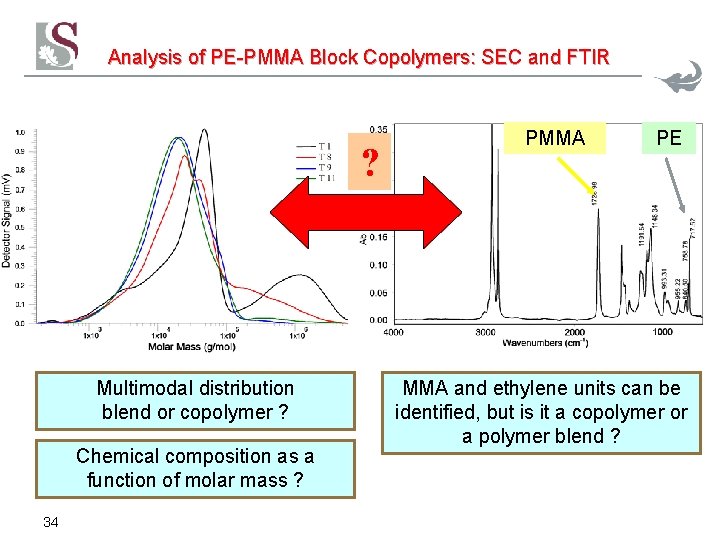
Analysis of PE-PMMA Block Copolymers: SEC and FTIR ? Multimodal distribution blend or copolymer ? Chemical composition as a function of molar mass ? 34 PMMA PE MMA and ethylene units can be identified, but is it a copolymer or a polymer blend ?

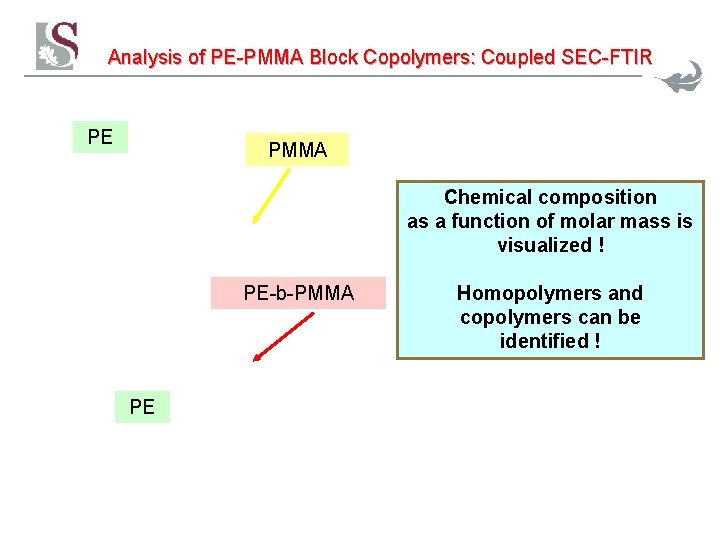
Analysis of PE-PMMA Block Copolymers: Coupled SEC-FTIR PE PMMA Chemical composition as a function of molar mass is visualized ! PE-b-PMMA PE 35 Homopolymers and copolymers can be identified !

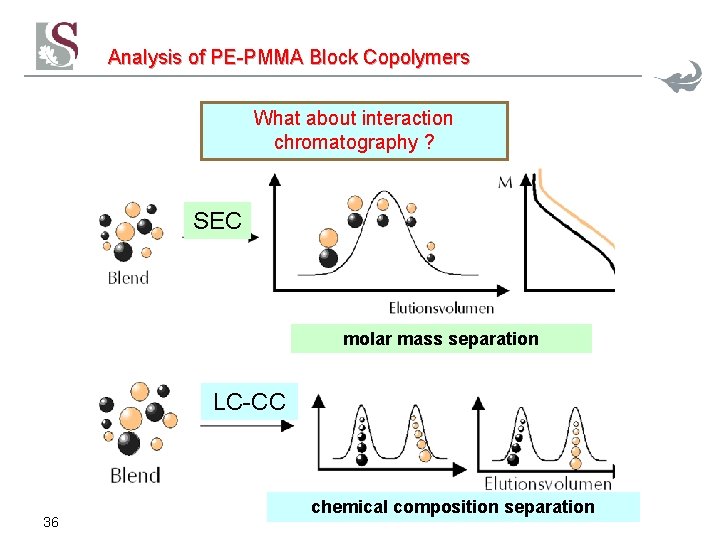
Analysis of PE-PMMA Block Copolymers What about interaction chromatography ? SEC molar mass separation LC-CC 36 chemical composition separation

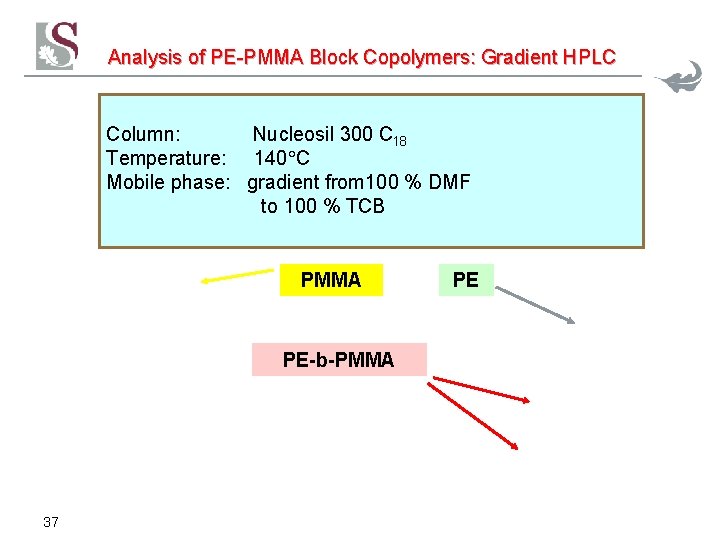
Analysis of PE-PMMA Block Copolymers: Gradient HPLC Column: Nucleosil 300 C 18 Temperature: 140 C Mobile phase: gradient from 100 % DMF to 100 % TCB PMMA PE-b-PMMA 37 PE

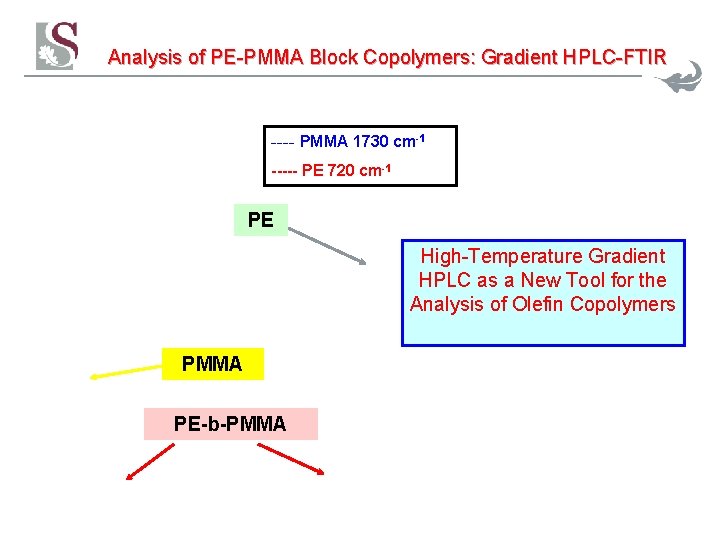
Analysis of PE-PMMA Block Copolymers: Gradient HPLC-FTIR ---- PMMA 1730 cm-1 ----- PE 720 cm-1 PE High-Temperature Gradient HPLC as a New Tool for the Analysis of Olefin Copolymers PMMA PE-b-PMMA 38

Polymer Labs‘ High-Temperature Gradient HPLC System 39
![Separation System for PEPP Blends PP PE M signal TCB Time min 40 Separation System for PE-PP Blends PP PE M signal % TCB Time [min] 40](https://slidetodoc.com/presentation_image_h/e64da352de7ea9f98591d8ff87aea9e8/image-40.jpg)
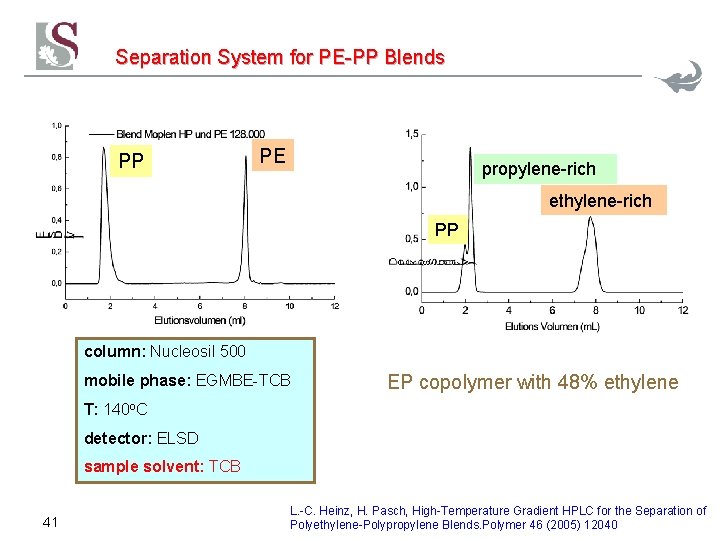
Separation System for PE-PP Blends PP PE M signal % TCB Time [min] 40

Separation System for PE-PP Blends PP PE propylene-rich ethylene-rich PP column: Nucleosil 500 mobile phase: EGMBE-TCB EP copolymer with 48% ethylene T: 140 o. C detector: ELSD sample solvent: TCB 41 L. -C. Heinz, H. Pasch, High-Temperature Gradient HPLC for the Separation of Polyethylene-Polypropylene Blends. Polymer 46 (2005) 12040

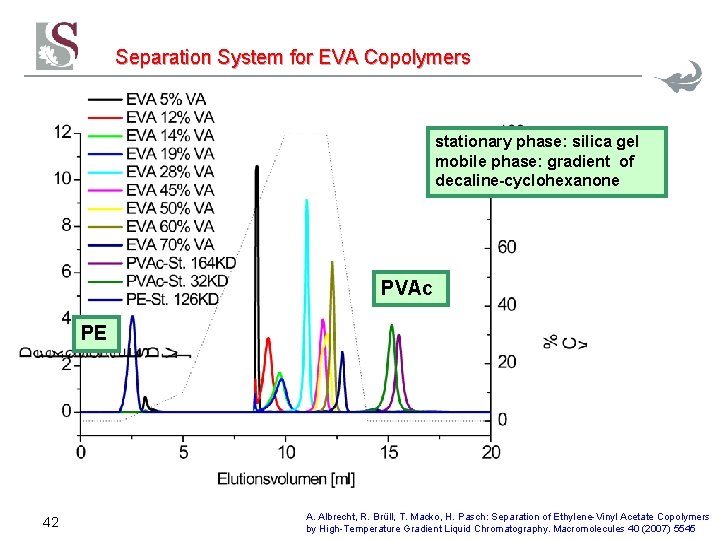
Separation System for EVA Copolymers stationary phase: silica gel mobile phase: gradient of decaline-cyclohexanone PVAc PE 42 A. Albrecht, R. Brüll, T. Macko, H. Pasch: Separation of Ethylene-Vinyl Acetate Copolymers by High-Temperature Gradient Liquid Chromatography. Macromolecules 40 (2007) 5545

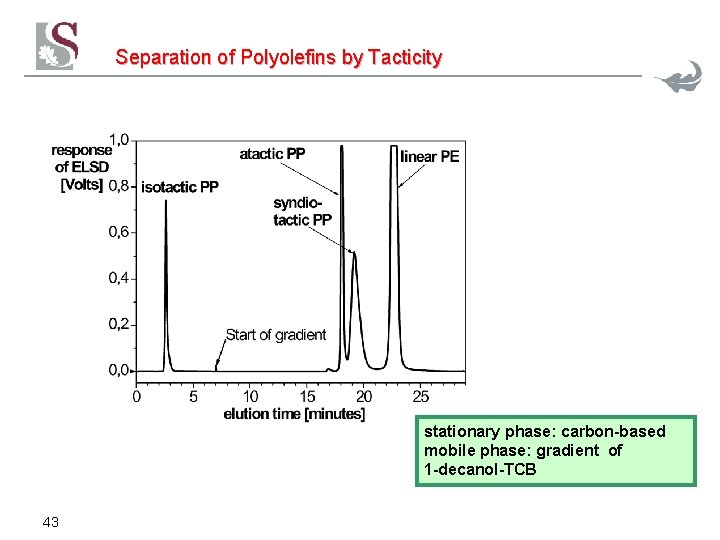
Separation of Polyolefins by Tacticity stationary phase: carbon-based mobile phase: gradient of 1 -decanol-TCB 43

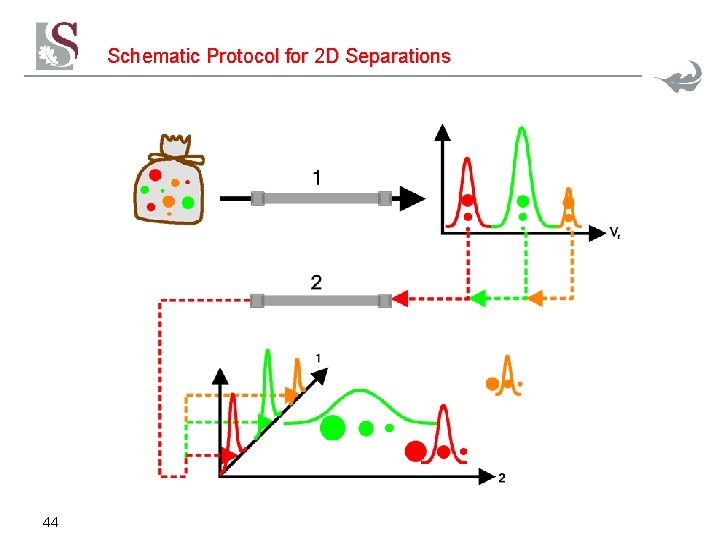
Schematic Protocol for 2 D Separations 44

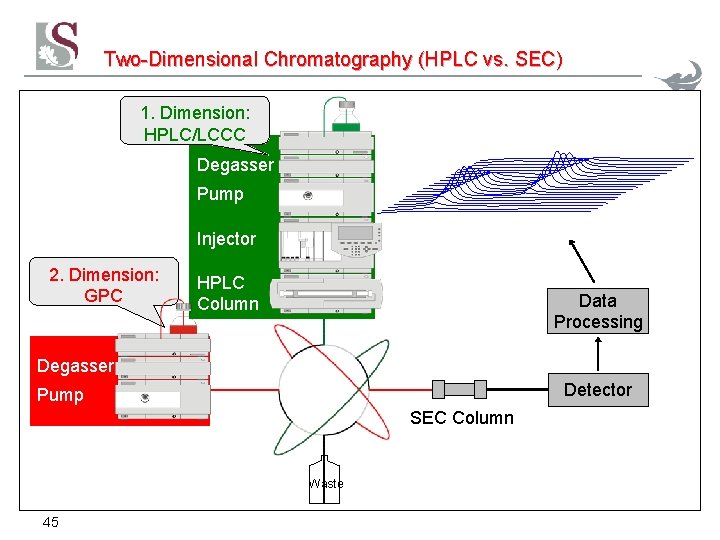
Two-Dimensional Chromatography (HPLC vs. SEC) 1. Dimension: HPLC/LCCC Degasser Pump Injector 2. Dimension: GPC HPLC Column Data Processing Degasser Detector Pump SEC Column Waste 45

High-Temperature 2 D-HPLC in Stellenbosch 46

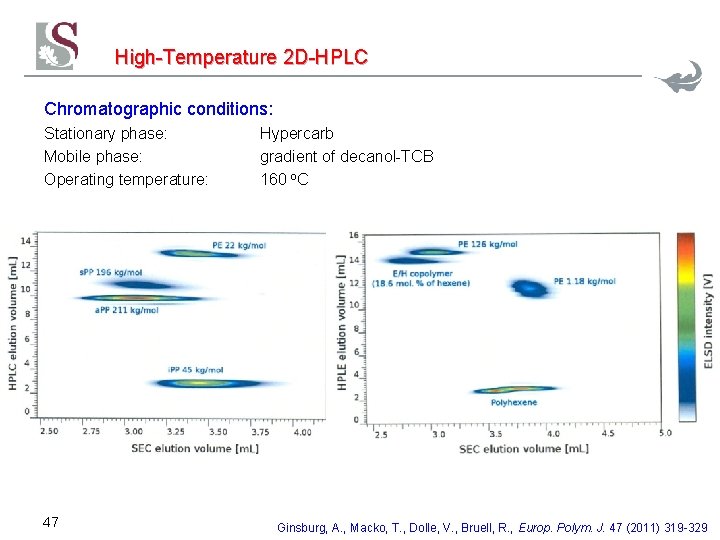
High-Temperature 2 D-HPLC Chromatographic conditions: Stationary phase: Mobile phase: Operating temperature: 47 Hypercarb gradient of decanol-TCB 160 o. C Ginsburg, A. , Macko, T. , Dolle, V. , Bruell, R. , Europ. Polym. J. 47 (2011) 319 -329

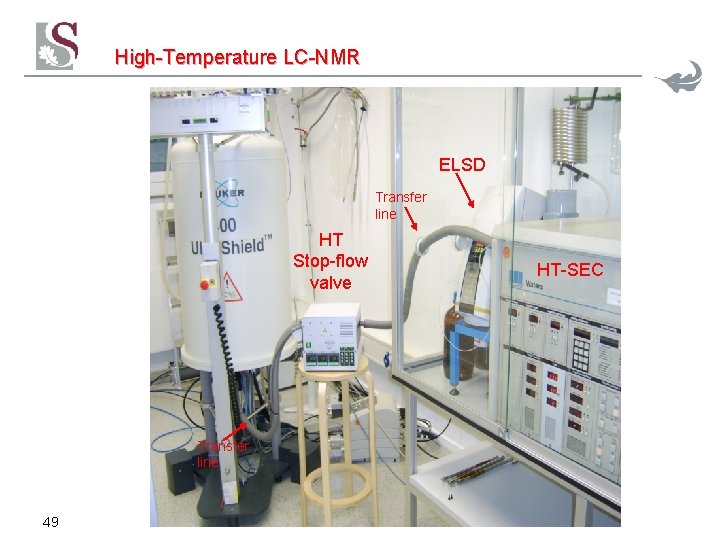
High-Temperature LC-NMR 48

High-Temperature LC-NMR ELSD Transfer line HT Stop-flow valve Transfer line 49 HT-SEC

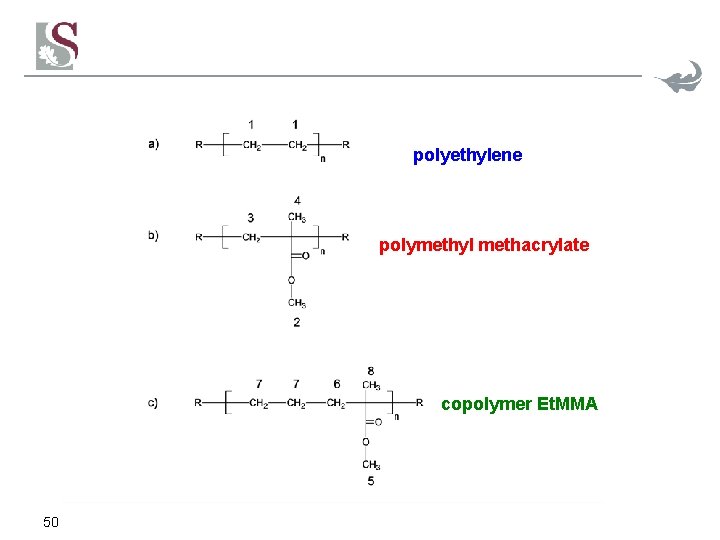
polyethylene polymethyl methacrylate copolymer Et. MMA 50

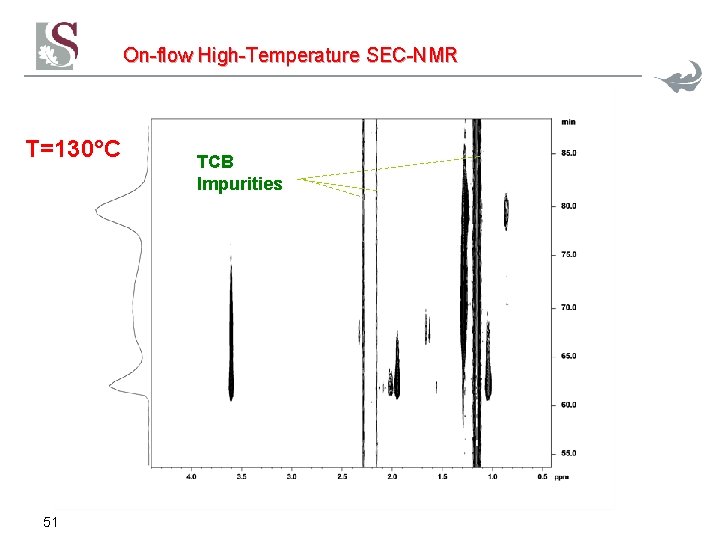
On-flow High-Temperature SEC-NMR T=130°C 51 TCB Impurities

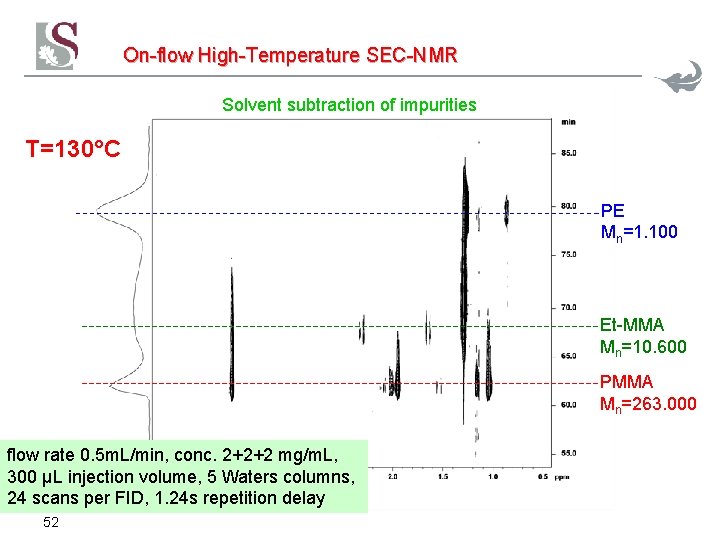
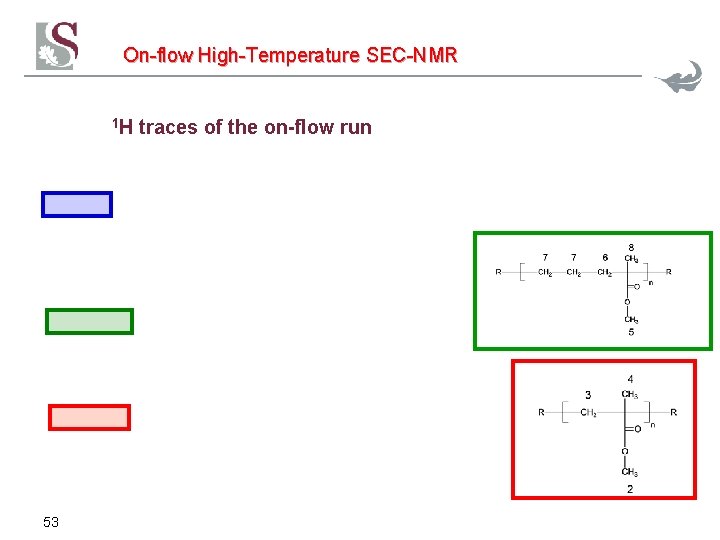
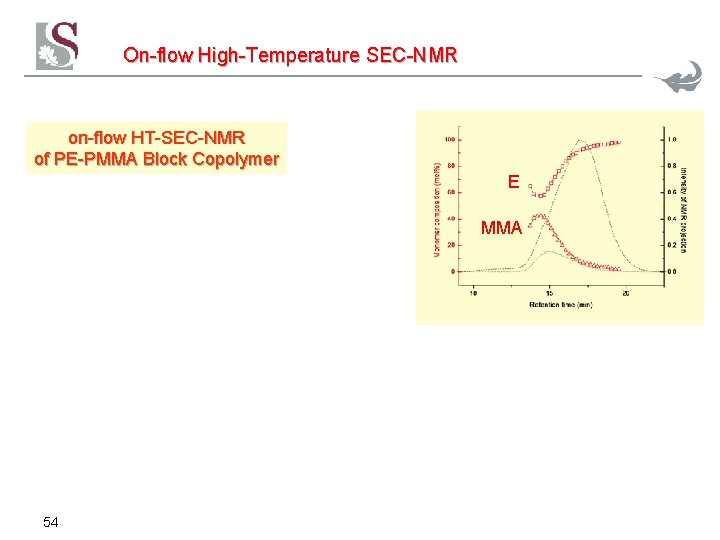
On-flow High-Temperature SEC-NMR Solvent subtraction of impurities T=130°C PE Mn=1. 100 Et-MMA Mn=10. 600 PMMA Mn=263. 000 flow rate 0. 5 m. L/min, conc. 2+2+2 mg/m. L, 300 µL injection volume, 5 Waters columns, 24 scans per FID, 1. 24 s repetition delay 52

On-flow High-Temperature SEC-NMR 1 H 53 traces of the on-flow run

On-flow High-Temperature SEC-NMR on-flow HT-SEC-NMR of PE-PMMA Block Copolymer E MMA 54