Adult Immunization and Quality Improvement for Residents Module

![Disclosures § [insert your disclosures here] 3 Disclosures § [insert your disclosures here] 3](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-3.jpg)

![Adult Vaccination Rates = POOR! Data: NHIS 2014 Vaccine [Population] 2013 2014 Influenza – Adult Vaccination Rates = POOR! Data: NHIS 2014 Vaccine [Population] 2013 2014 Influenza –](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-5.jpg)
![Disparities and Adult Vaccination Rates Data: NHIS 2014 Vaccine [Population] Rate Pneumococcal [>65 years] Disparities and Adult Vaccination Rates Data: NHIS 2014 Vaccine [Population] Rate Pneumococcal [>65 years]](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-6.jpg)

![Vaccine Groups “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap Vaccine Groups “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-14.jpg)

![Vaccine Groups “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap Vaccine Groups “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-16.jpg)
![Influenza § Influenza: Orthomyxoviridae family [enveloped RNA virus] • 3 types based on surface Influenza § Influenza: Orthomyxoviridae family [enveloped RNA virus] • 3 types based on surface](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-17.jpg)




![PPS 23 Vaccine Effectiveness § 7 Meta-Analyses of RCT [Most recent Cochrane 1/2013] • PPS 23 Vaccine Effectiveness § 7 Meta-Analyses of RCT [Most recent Cochrane 1/2013] •](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-25.jpg)

![Pneumococcal Immunization HIGHEST Risk PCV 13 + PPSV 23 Immune compromised [IC], ‘Anatomic Risk’ Pneumococcal Immunization HIGHEST Risk PCV 13 + PPSV 23 Immune compromised [IC], ‘Anatomic Risk’](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-27.jpg)
![Pneumococcal ‘Nuts and Bolts’ § § PCV 13 [1 dose in adults] PPSV 23 Pneumococcal ‘Nuts and Bolts’ § § PCV 13 [1 dose in adults] PPSV 23](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-30.jpg)



![Vaccine Groups “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap Vaccine Groups “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-37.jpg)
![HPV § Cervical Cancer is consequence of a STD [HPV] • Second most common HPV § Cervical Cancer is consequence of a STD [HPV] • Second most common](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-38.jpg)



![Vaccine Groups “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap Vaccine Groups “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-45.jpg)

![Vaccine Groups… “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap Vaccine Groups… “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-48.jpg)



![Zoster § § § Vaccinate Healthy 60+ adults [ACIP: Not immunocompromised] • • • Zoster § § § Vaccinate Healthy 60+ adults [ACIP: Not immunocompromised] • • •](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-53.jpg)


- Slides: 55

Adult Immunization and Quality Improvement for Residents Module 1 – The Science of Adult Immunization

Overview § Module 1 – Science of Adult Immunization • Adult immunization rates • ACIP recommended adult vaccine schedule • Vaccination among special populations: • • Diabetics Healthcare workers Pregnant women The elderly § Module 2 – Quality Improvement in Adult Immunization 2
![Disclosures insert your disclosures here 3 Disclosures § [insert your disclosures here] 3](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-3.jpg)
Disclosures § [insert your disclosures here] 3

Opportunity and Reward § Immunization rates are far below HP 2020 goals § Common measure of quality preventive care • Inpatient, outpatient • Adult, obstetric, pediatric • Primary, specialty care § Many elements in process which can be improved • • Front desk Nursing/MA Physician Checkout HP 2020 = Healthy People 2020 www. healthypeople. gov 4
![Adult Vaccination Rates POOR Data NHIS 2014 Vaccine Population 2013 2014 Influenza Adult Vaccination Rates = POOR! Data: NHIS 2014 Vaccine [Population] 2013 2014 Influenza –](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-5.jpg)
Adult Vaccination Rates = POOR! Data: NHIS 2014 Vaccine [Population] 2013 2014 Influenza – All Adults 42. 7 % 43. 2 % [All] 19 – 49 years 30. 4 % 31. 5 % [All] 50 – 64 years 48. 0 % 47. 7 % > 65 years 71. 7% 71. 5 % HCW [All] 75. 2 % 77. 3% High risk 19 – 49 years 21. 2 % 20. 3 % > 65 years 59. 7 % 61. 3 % Tetanus [19 – 49 years, received past 10 years] 62. 9 % 62. 6 % Tetanus/Pertussis [19+, received in past 8 years] 17. 2 % 20. 1 % Shingles – Zoster[Age 60+] 24. 3 % 27. 9 % Hepatitis B Vaccine [High risk 19 – 49 years] 32. 6 % 32. 2 % HPV Vaccine [Women 19 -26 >1 dose] 36. 8% 40. 2% HPV Vaccine [Men 19 -26, >1 dose] 5. 9% 8. 2% Influenza PPS 23 & PCV 13 MMWR Feb 5, 2016/ Vol 65(1). http: //www. cdc. gov/mmwr/preview/mmwrhtml/mm 6436 a 1. htm 5
![Disparities and Adult Vaccination Rates Data NHIS 2014 Vaccine Population Rate Pneumococcal 65 years Disparities and Adult Vaccination Rates Data: NHIS 2014 Vaccine [Population] Rate Pneumococcal [>65 years]](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-6.jpg)
Disparities and Adult Vaccination Rates Data: NHIS 2014 Vaccine [Population] Rate Pneumococcal [>65 years] All Adults 61. 3 % Hispanic 45. 2 % White 64. 7 % Black 49. 8 % Asian 47. 7 % “…and, unfortunately, there are similar disparities for most adult vaccines. This is absolutely unacceptable in the United States in 2015!!” -RHH, MD 2/15/2015 MMWR Feb 5, 2016/ Vol 65(1). 6

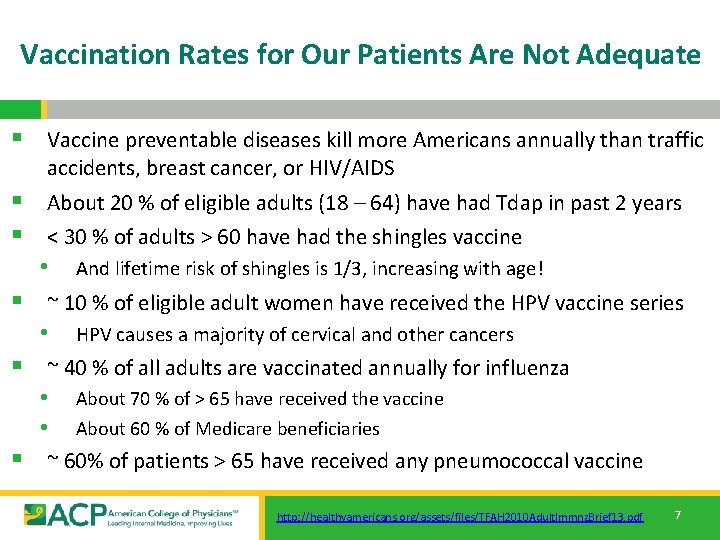
Vaccination Rates for Our Patients Are Not Adequate § Vaccine preventable diseases kill more Americans annually than traffic accidents, breast cancer, or HIV/AIDS § About 20 % of eligible adults (18 – 64) have had Tdap in past 2 years § < 30 % of adults > 60 have had the shingles vaccine • And lifetime risk of shingles is 1/3, increasing with age! § ~ 10 % of eligible adult women have received the HPV vaccine series • HPV causes a majority of cervical and other cancers § ~ 40 % of all adults are vaccinated annually for influenza • About 70 % of > 65 have received the vaccine • About 60 % of Medicare beneficiaries § ~ 60% of patients > 65 have received any pneumococcal vaccine http: //healthyamericans. org/assets/files/TFAH 2010 Adult. Immnz. Brief 13. pdf 7

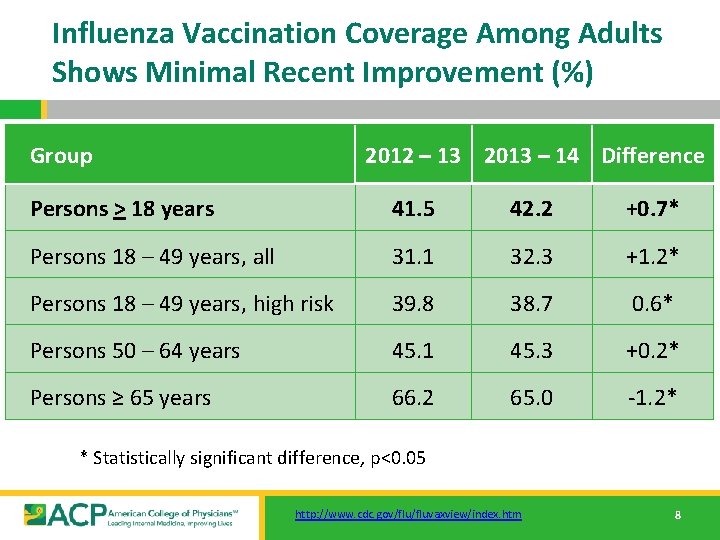
Influenza Vaccination Coverage Among Adults Shows Minimal Recent Improvement (%) Group 2012 – 13 2013 – 14 Difference Persons > 18 years 41. 5 42. 2 +0. 7* Persons 18 – 49 years, all 31. 1 32. 3 +1. 2* Persons 18 – 49 years, high risk 39. 8 38. 7 0. 6* Persons 50 – 64 years 45. 1 45. 3 +0. 2* Persons ≥ 65 years 66. 2 65. 0 -1. 2* * Statistically significant difference, p<0. 05 http: //www. cdc. gov/fluvaxview/index. htm 8

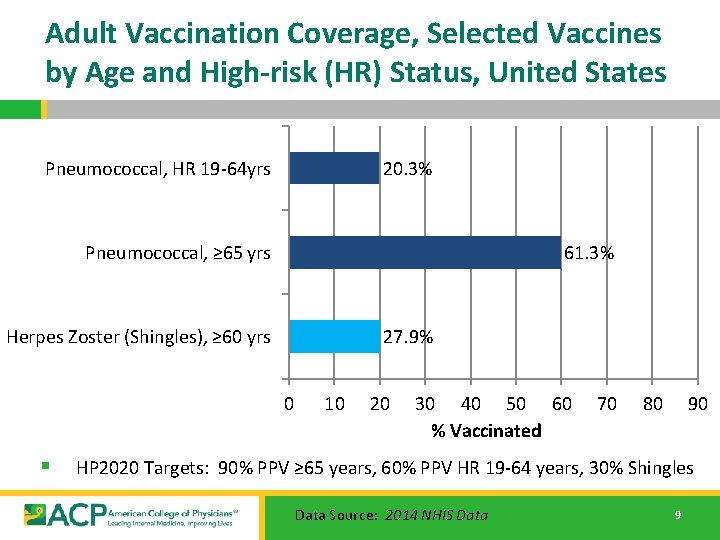
Adult Vaccination Coverage, Selected Vaccines by Age and High-risk (HR) Status, United States Pneumococcal, HR 19 -64 yrs 20. 3% Pneumococcal, ≥ 65 yrs 61. 3% Herpes Zoster (Shingles), ≥ 60 yrs 27. 9% 0 § 10 20 30 40 50 60 % Vaccinated 70 80 90 HP 2020 Targets: 90% PPV ≥ 65 years, 60% PPV HR 19 -64 years, 30% Shingles Data Source: 2014 NHIS Data 9

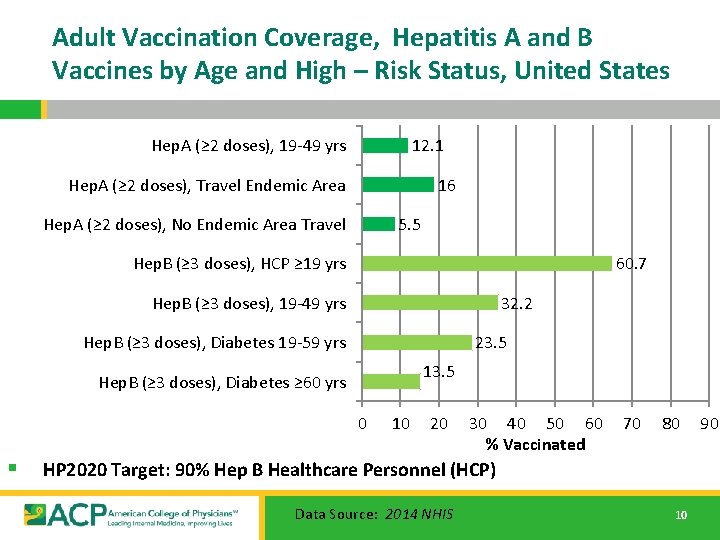
Adult Vaccination Coverage, Hepatitis A and B Vaccines by Age and High – Risk Status, United States Hep. A (≥ 2 doses), 19 -49 yrs 12. 1 Hep. A (≥ 2 doses), Travel Endemic Area 16 Hep. A (≥ 2 doses), No Endemic Area Travel 5. 5 Hep. B (≥ 3 doses), HCP ≥ 19 yrs 60. 7 Hep. B (≥ 3 doses), 19 -49 yrs 32. 2 Hep. B (≥ 3 doses), Diabetes 19 -59 yrs 23. 5 13. 5 Hep. B (≥ 3 doses), Diabetes ≥ 60 yrs 0 § 10 20 30 40 50 60 % Vaccinated 70 80 HP 2020 Target: 90% Hep B Healthcare Personnel (HCP) Data Source: 2014 NHIS 10 90

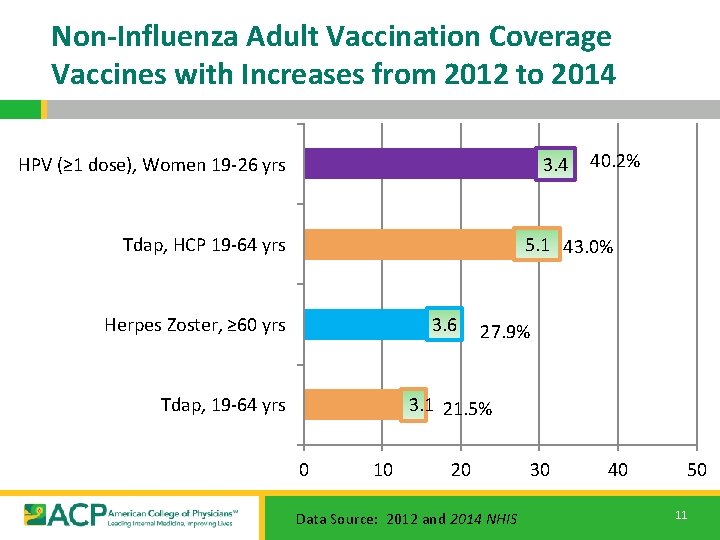
Non-Influenza Adult Vaccination Coverage Vaccines with Increases from 2012 to 2014 HPV (≥ 1 dose), Women 19 -26 yrs 3. 4 40. 2% 5. 1 43. 0% Tdap, HCP 19 -64 yrs Herpes Zoster, ≥ 60 yrs 3. 6 27. 9% 3. 1 21. 5% Tdap, 19 -64 yrs 0 10 20 Data Source: 2012 and 2014 NHIS 30 40 50 11

Adult Immunization Schedule – 2016, By Age http: //www. cdc. gov/vaccines/schedules/hcp/imz/adult-conditions. html 12

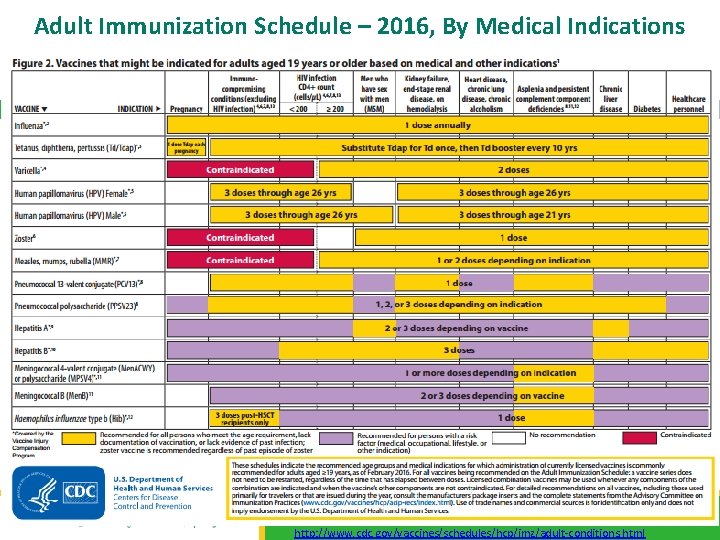
Adult Immunization Schedule – 2016, By Medical Indications 13 http: //www. cdc. gov/vaccines/schedules/hcp/imz/adult-conditions. html
![Vaccine Groups Universals Influenza Pneumococcal PCV 13 PPSV 23 Tdap Vaccine Groups “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-14.jpg)
Vaccine Groups “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap § Zoster “Selectives” § HPV § § § • [HPV 9, HPV 4, HPV 2] MMR Varicella Meningococcal • Quad [MCV 4, MPSV 4] • Men. B Hepatitis A Hepatitis B 14

Case 1 § John Francis is a 42 year old man with a history of diabetes and hypertension presenting for a routine diabetes visit. § While reviewing his immunization history, you note that he had all of his childhood vaccinations [documented in record]. His last booster shot was over 10 years ago. § Which vaccines should you strongly recommend that he receive today? 15
![Vaccine Groups Universals Influenza Pneumococcal PCV 13 PPSV 23 Tdap Vaccine Groups “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-16.jpg)
Vaccine Groups “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap § Zoster “Selectives” § HPV § § § • [HPV 9, HPV 4, HPV 2] MMR Varicella Meningococcal • Quad [MCV 4, MPSV 4] • Men. B Hepatitis A Hepatitis B 16
![Influenza Influenza Orthomyxoviridae family enveloped RNA virus 3 types based on surface Influenza § Influenza: Orthomyxoviridae family [enveloped RNA virus] • 3 types based on surface](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-17.jpg)
Influenza § Influenza: Orthomyxoviridae family [enveloped RNA virus] • 3 types based on surface Ag [HA, NA] + internal structure • A: Multiple hosts – Birds, Mammals [Man]. Many HA, NA types ‘Highly Pathogenic’ and ‘Mild’ strains B: Human host. 1 HA and 1 NA C: Human host. Mild illness ‘URI’ • • • § 30 – 50 K deaths annually in US from Influenza • 200 K+ assoc. hospitalizations, chronic illnesses exacerbations • > 90% seasonal influenza M&M in people > 65 years § Vaccination is most effective intervention to reduce illness and death. • Multiple vaccines avail. in US [effectiveness variable] http: //www. cdc. gov/flu/avian/gen-info/flu-viruses. htm 17

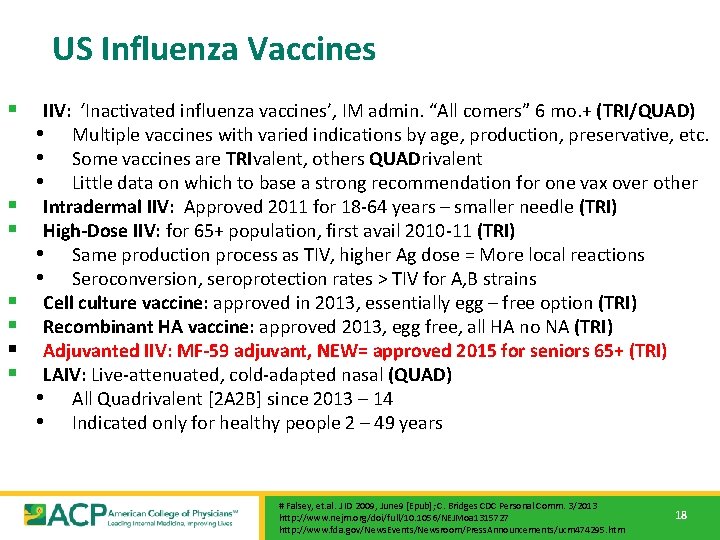
US Influenza Vaccines § § § § IIV: ‘Inactivated influenza vaccines’, IM admin. “All comers” 6 mo. + (TRI/QUAD) • Multiple vaccines with varied indications by age, production, preservative, etc. • Some vaccines are TRIvalent, others QUADrivalent • Little data on which to base a strong recommendation for one vax over other Intradermal IIV: Approved 2011 for 18 -64 years – smaller needle (TRI) High-Dose IIV: for 65+ population, first avail 2010 -11 (TRI) • Same production process as TIV, higher Ag dose = More local reactions • Seroconversion, seroprotection rates > TIV for A, B strains Cell culture vaccine: approved in 2013, essentially egg – free option (TRI) Recombinant HA vaccine: approved 2013, egg free, all HA no NA (TRI) Adjuvanted IIV: MF-59 adjuvant, NEW= approved 2015 for seniors 65+ (TRI) LAIV: Live-attenuated, cold-adapted nasal (QUAD) • All Quadrivalent [2 A 2 B] since 2013 – 14 • Indicated only for healthy people 2 – 49 years # Falsey, et. al. J ID 2009, June 9 [Epub]; C. Bridges CDC Personal Comm. 3/2013 http: //www. nejm. org/doi/full/10. 1056/NEJMoa 1315727 http: //www. fda. gov/News. Events/Newsroom/Press. Announcements/ucm 474295. htm 18

Influenza Vaccine Priorities § ALL 6 MONTHS AND OLDER + DON’T WANT THE FLU § HEALTHCARE WORKERS • High risk for disease (symptomatic and asymptomatic) • High risk for transmission • If sick not available to provide healthcare… § PATIENTS @ HIGHEST RISK – SEVERE ILLNESS/SPREAD • Pregnant women • Newborns and children < 2 years • Elderly • “Medical Comorbidities” (including obesity) • Household contacts of high-risk • Long-term care/institutionalized, crowded living conditions http: //www. cdc. gov/vaccines/pubs/vis/downloads/vis-flu. pdf 19

Influenza Vaccines 2015 - 2016 20

Influenza ‘Nuts and Bolts’ § IIV: 1 dose for adults • Incl: QIV, TIV, sq. TIV, hd. TIV, LAIV, cc. TIV, r. HA (Flublock), Adjuvanted (Fluad) Kids < 9 years, first vaccine season: 2 doses 4+ weeks apart LAIV can be safely used in MOST HC settings as alt. to TIV 2 • • § No CLEAR indication to choose 1 IIV over another • Potential Exception: • HD vs TIV in seniors 65+ based NEJM Aug 2014 • No data [quadrivalent] IIV v [trivalent] IIV. • LAIV reasonable but not preferred. • Vaccine allergy, availability… § Egg allergy: ACIP, AAAI: NO contraindication • Consider Recombinant vaccine if anaphylactic egg sensitivity http: //www. cdc. gov/vaccines/pubs/vis/downloads/vis-flu. pdf http: //www. premierinc. com/all/safety-share/12 -05 -downloads/03 -shea-hcw-flu-position-paper. pdf. Diaz. Granados, et. al. NEJM 371(7), August 14, 2014. https: //cc. readytalk. com/cc/s/meeting. Archive? event. Id=rtv 7 rz 2 szhrw 21

Influenza § Vaccine effectiveness is multifactorial • Match with ‘disease’ strains • Vaccine availability and timing • • 2015 – 2016 vaccine efficacy ~ 60% Patient ‘substrate’: • ‘Healthy young < 65’ @ ~60 – 80% v. ‘Sick older > 65’ @ 30 -40% § Ongoing vaccine research • • Adjuvants Newer production methods Higher Ag content New delivery devices • Surveillance and candidate vaccine development: § ‘New Flu’s’ • H 5 N 1, H 7 N 3, H 9 and others http: //www. cdc. gov/flu/professionals/antivirals/index. htm http: //www. cdc. gov/flu/professionals/diagnosis/ 22

Pneumococcal Disease § > 2000 Adults/yr. 65+ die from invasive pneumococcal disease (IPD) • Bacteremia, sepsis, meningitis § PPSV 23 = ‘adult standard’ vaccine = purified capsule polysaccharide • 23 types cause of 88 % bacteremic PNC disease • PPSV 23 has 60 -70% efficacy vs. IPD • Immunity lasts at least 5 years following 1 dose • Local reactions – only common AE • REVACCINATION if imm. before age 65; NOT ‘routine’ 65+ immunized § PCV 13=‘pediatric standard’ vaccine = conjugated to protein • 13 types ~50% IPD in immunocompromised adults • No published efficacy studies in adults [PCV 7 data in HIV, reports, etc. ] • Useful/ACIP recommended – combined strategy with PPSV 23 – in adults http: //www. cdc. gov/vaccines/hcp/acip-recs/vacc-specific/pneumo. html 23

US Pneumococcal Vaccines Vaccine Body Year Population Indication Comments PPS 14 FDA 1977 High risk Prevent IPD 1 st lic PNC vax PPS 23 FDA/ 1983; ’ 89; ACIP ’ 97; 9/2010 High risk adult, child Prevent IPD Initial then updated recs. PCV 7 FDA/ ACIP 2/2000 Children < 24 months Prevent PNC Infection New Vaccine PCV 13 FDA/ ACIP 3/2010 PCV 13 FDA 12/2011 Adults 50+ years Prevent IPD, Pneumonia Immunogenicity and safety data ACIP 10/2012 Highest Risk Adults Prevent IPD PCV 13/PPS ACIP 12/2012 High Risk Kids > 6 years Prevent IPD PCV 13/PPS ACIP 9/19/2014 Adults 65+ years Prevent IPD Best before PPS 23 Children Prevent PNC Infection Changed, 6 week - 71 months additional types http: //www. cdc. gov/vaccines/hcp/acip-recs/vacc-specific/pneumo. html 24
![PPS 23 Vaccine Effectiveness 7 MetaAnalyses of RCT Most recent Cochrane 12013 PPS 23 Vaccine Effectiveness § 7 Meta-Analyses of RCT [Most recent Cochrane 1/2013] •](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-25.jpg)
PPS 23 Vaccine Effectiveness § 7 Meta-Analyses of RCT [Most recent Cochrane 1/2013] • • Conclusions inconsistent re: cause specific outcomes Agreement: REDUCTION in IPD • NO reduction ALL CAUSE mortality, pneumonia § 3 Meta-Analyses of Observational Studies • Consistent results: vaccine is effective for prevention of IPD • • • Invasive PNC Dz: Odds ratio [consistent] Pneumonia: Odds ratio [signif. heterogeneity] Mortality: Odds ratio • Data = PPS prevents IPD, not compelling for Pneumonia, Mortality § Recent RCT Results § Summary 0. 26 (CI 0. 25 -0. 46) 0. 71 (CI 0. 52 -0. 97) 0. 87 (CI 0. 69 -1. 10) Fine, et. al. Archives. IM 1994(154): 2666. Hutchinson et. al. Can. JFP 1999(45): 2381. Watson, et. al. Vaccine 2002(20): 25 2166. Conaty, et. al. Vaccine 2004(22): 3214. Dear, et. al. Cochrane DB Syst Rev 2004, Issue 3. Moberley , et. al. Cochrane DB Syst Rev 2008, Issue 1. Moberly, et. al. Cochrane DB Syst Rev 2013, Issue 1.

PCV 13 Adult Vaccine Effectiveness § CAPi. TA • PC RCT PCV 13 unimmunized 65+ aged adults, Netherlands • PCV 7 in Dutch infants since 6/2006 -> PCV 10 in March 2011 • 84, 000+ participants PCV 13 v Placebo • Enrolled 9/2008 -1/2010, follow-up ended 8/2013 • Primary: 1 st bacteremic CAP with vaccine – type PNC-> MET • Secondary: 1 st nonbacteremic 1 st CAP, other IPD-> MET • Serologic and urinary Ag used to identify PNC infection • Considered by ACIP in making current Pneumococcal recs. § No published study to date on sequential PCV 13/PPSV 23 immunization in adults http: //www. nejm. org/doi/full/10. 1056/NEJMoa 1408544 26
![Pneumococcal Immunization HIGHEST Risk PCV 13 PPSV 23 Immune compromised IC Anatomic Risk Pneumococcal Immunization HIGHEST Risk PCV 13 + PPSV 23 Immune compromised [IC], ‘Anatomic Risk’](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-27.jpg)
Pneumococcal Immunization HIGHEST Risk PCV 13 + PPSV 23 Immune compromised [IC], ‘Anatomic Risk’ Adults 65+ [NEW 9/2014] INCREASED Risk NO PPSV 23 ONLY Smokers, Chronic Medical Conditions – Not Immunocompromised NO NO PNEUMOCOCCAL VACCINE AVERAGE Risk Young [< 65], No Chronic Medical Conditions 27

Pneumococcal Immunization I PPSV 23 ALONE for INCREASED RISK 28 All cigarette smokers ≥ 19 years old Chronic conditions • • • ≥ 19 years old Diabetes Lung disease: asthma, COPD Cardiovascular disease Liver disease Kidney disease (except ESRD, nephrotic syndrome – PCV 13 recommended) § § Immunity lasts at least 5 years following 1 dose REVACCINATION ONCE after age 65 [AND >5 years after initial dose] for those vaccinated prior to age 65 -------------------------------§ Adults 65 years and older are in highest risk group- addressed in next slides. http: //www. cdc. gov/mmwr/preview/mmwrhtml/mm 5934 a 3. htm http: //www. cdc. gov/mmwr/preview/mmwrhtml/mm 6140 a 4. htm 28

Pneumococcal Immunization II SEQUENTIAL PCV 13 + PPSV 23 for HIGHEST RISK Adults 65 and older AGE Indication ‘Immunocompromised’ MEDICAL Indications 1. Disease: • CA: solid tumors, hematologic malignancies, myeloma, etc. • HIV • Inherited and other immune deficiency (CVID, etc. ) • End-stage kidney disease ESRD, nephrotic syndrome 2. Latrogenic: • MEDS: Steroids (20 mg/d or greater), biologic immunomodulators, other • TRANSPLANTS: solid organ, bone marrow, stem cell 3. Asplenia: • ANATOMIC: splenectomy (best if immunized prior to) • FUNCTIONAL: hemoglobinopathy, sickle cell, other 4. Anatomic: • CSF leak, cochlear implant, splenectomy http: //www. cdc. gov/mmwr/preview/mmwrhtml/mm 6140 a 4. htm http: //www. cdc. gov/mmwr/preview/mmwrhtml/mm 6337 a 4. htm 29
![Pneumococcal Nuts and Bolts PCV 13 1 dose in adults PPSV 23 Pneumococcal ‘Nuts and Bolts’ § § PCV 13 [1 dose in adults] PPSV 23](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-30.jpg)
Pneumococcal ‘Nuts and Bolts’ § § PCV 13 [1 dose in adults] PPSV 23 [1 – 3 doses based on risk] • • § • • • Increased Risk Patients: Pneumococcal polysaccharide (PPSV 23) – now PCV/Booster once at 65+ years/5+ years later (only for persons vax before 64) Highest Risk Patients: Pneumococcal (PPSV 23) vaccine-naive patients (best practice): • PCV 13 followed by PPSV 23 at least 8 weeks later • Booster PPSV 23 in 5 years AND final PPSV 23 at 5+ years/65+ years Previously PPSV 23 – vaccinated patients: • PCV 13 at least 1 year after prior dose PPSV 23 • Booster PPSV 23 5 years after prior PPSV 23 (must be 8+ weeks after PCV 13) • Final PPSV 23 after 65+ years and at least 5 years after last dose 65+ Patients: Give PCV 13 first, [if pneumococcal vaccine-naive] followed 1 year by PPSV 23 If any prior PPSV 23, PCV 13 must be given at least 1 year after the last PPSV 23 No additional/booster doses if sole indication is age > 65 years ACIP recommends PPSV 23 12 months after PCV 13, CMS [M’CARE] will only pay if administered 11+ months between doses http: //www. cdc. gov/mmwr/preview/mmwrhtml/mm 6140 a 4. htm http: //www. cdc. gov/mmwr/preview/mmwrhtml/mm 6337 a 4. htm 30

c/o R. Hopkins, MD, FACP, FAAP UAMS 31

Adult Pneumococcal Vaccine: By The Numbers § Two vaccines • • PCV 13 PPSV 23 § Three intervals • • 8 weeks between PCV 13 and PPSV 23 in highest risk medical conditions 1 year between PCV 13 and PPSV 23 if PPSV 23 first or age is only ‘highest risk’ indication 5 years minimum between doses of PPSV 23 • § Maximum doses in adult lifetime • 1 PCV 13 • 3 PPSV 23 [If highest risk medical condition and first dose before 59 yr] 32

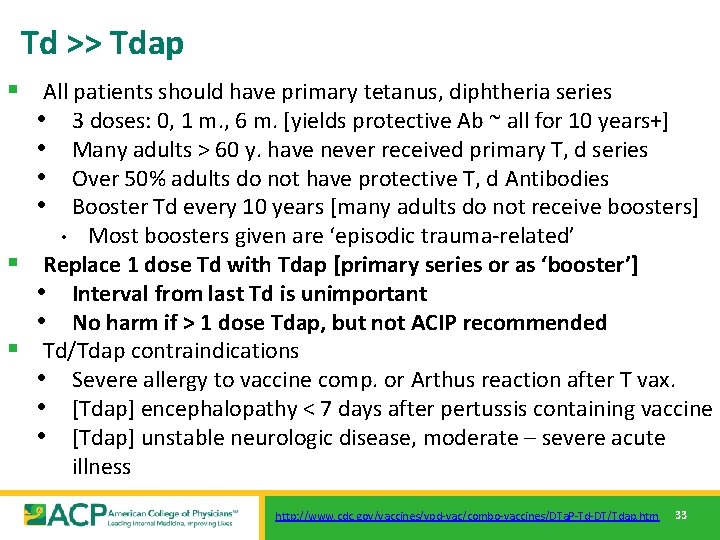
Td >> Tdap § All patients should have primary tetanus, diphtheria series • 3 doses: 0, 1 m. , 6 m. [yields protective Ab ~ all for 10 years+] • Many adults > 60 y. have never received primary T, d series • Over 50% adults do not have protective T, d Antibodies • Booster Td every 10 years [many adults do not receive boosters] Most boosters given are ‘episodic trauma-related’ § Replace 1 dose Td with Tdap [primary series or as ‘booster’] • Interval from last Td is unimportant • No harm if > 1 dose Tdap, but not ACIP recommended § Td/Tdap contraindications • Severe allergy to vaccine comp. or Arthus reaction after T vax. • [Tdap] encephalopathy < 7 days after pertussis containing vaccine • [Tdap] unstable neurologic disease, moderate – severe acute illness • http: //www. cdc. gov/vaccines/vpd-vac/combo-vaccines/DTa. P-Td-DT/Tdap. htm 33

Td >> Tdap § Tdap Recommendation: All Adults • Single dose to replace one dose Td [booster or primary] • Current recommendation: subsequent Td q 10 yr Research on repeated dosing ongoing • May give < 10 years following last Td § Special emphasis: • Adults with close infant contact • HEALTHCARE • Parents • Child Care, etc. § Tdap intrapartum all pregnant, every pregnancy [since 2013] • Regardless of interval/prior Tdap [ideal @ 27 – 35 weeks] • Focus: Protect infants [Highest M&M group] by passive immunity • http: //www. cdc. gov/vaccines/vpd-vac/combo-vaccines/DTa. P-Td-DT/Tdap. htm 34

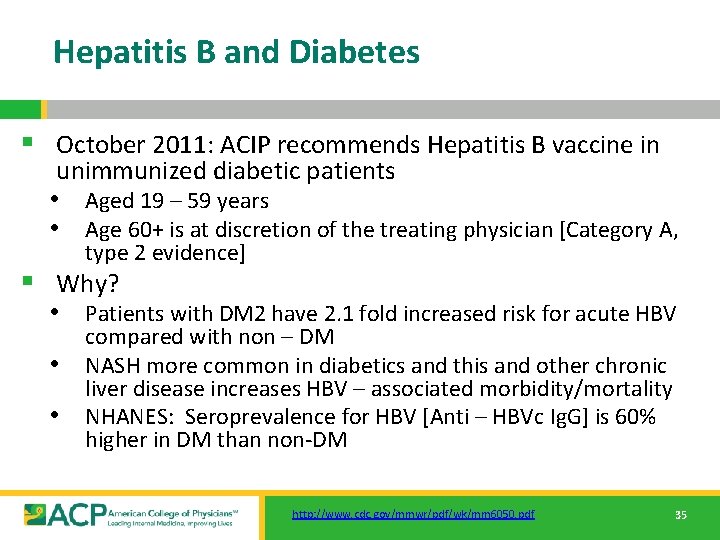
Hepatitis B and Diabetes § October 2011: ACIP recommends Hepatitis B vaccine in unimmunized diabetic patients • Aged 19 – 59 years • Age 60+ is at discretion of the treating physician [Category A, type 2 evidence] § Why? • Patients with DM 2 have 2. 1 fold increased risk for acute HBV • • compared with non – DM NASH more common in diabetics and this and other chronic liver disease increases HBV – associated morbidity/mortality NHANES: Seroprevalence for HBV [Anti – HBVc Ig. G] is 60% higher in DM than non-DM http: //www. cdc. gov/mmwr/pdf/wk/mm 6050. pdf 35

Case 2 § Maria Alvarez is a 24 year old medical student with no significant past medical history presenting for a routine annual exam. § Which vaccines should you make sure she receives? As a healthcare worker in training, what vaccines should she receive? 36
![Vaccine Groups Universals Influenza Pneumococcal PCV 13 PPSV 23 Tdap Vaccine Groups “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-37.jpg)
Vaccine Groups “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap § Zoster “Selectives” § HPV § § § • [HPV 9, HPV 4, HPV 2] MMR Varicella Meningococcal • Quad [MCV 4, MPSV 4] • Men. B Hepatitis A Hepatitis B 37
![HPV Cervical Cancer is consequence of a STD HPV Second most common HPV § Cervical Cancer is consequence of a STD [HPV] • Second most common](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-38.jpg)
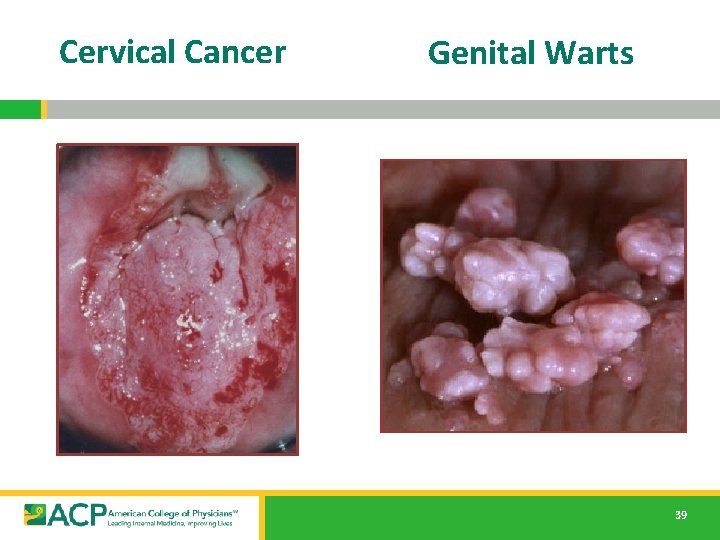
HPV § Cervical Cancer is consequence of a STD [HPV] • Second most common cause CA death in women • 500, 000 cases and 250, 000 deaths per year US: ~10 women die every day of cervical cancer • Many clear spontaneously • 74% in women 15 – 24 years of age • • Cause of anal CA and penile CA in men § 20 million current HPV infections • By age 50, 80% SA women will have acquired genital HPV • 6. 2 million new genital HPV infections/year in US • 70% Cervical CA worldwide d/t serotypes 16 [54%], 18 [13%] • >90% Genital warts due to serotypes 6, 11 http: //www. cdc. gov/mmwr/preview/mmwrhtml/rr 5602 a 1. htm 38

Cervical Cancer Genital Warts 39

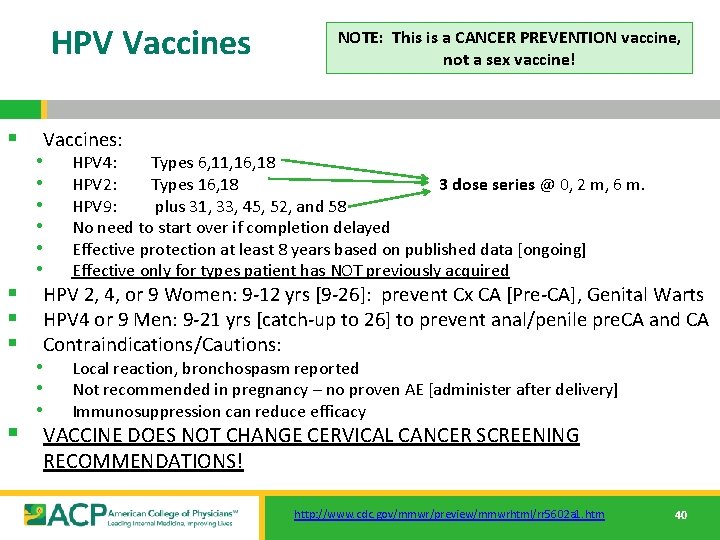
HPV Vaccines NOTE: This is a CANCER PREVENTION vaccine, not a sex vaccine! § Vaccines: • HPV 4: Types 6, 11, 16, 18 • HPV 2: Types 16, 18 3 dose series @ 0, 2 m, 6 m. • HPV 9: plus 31, 33, 45, 52, and 58 • No need to start over if completion delayed • Effective protection at least 8 years based on published data [ongoing] • Effective only for types patient has NOT previously acquired § HPV 2, 4, or 9 Women: 9 -12 yrs [9 -26]: prevent Cx CA [Pre-CA], Genital Warts § HPV 4 or 9 Men: 9 -21 yrs [catch-up to 26] to prevent anal/penile pre. CA and CA § Contraindications/Cautions: • Local reaction, bronchospasm reported • Not recommended in pregnancy – no proven AE [administer after delivery] • Immunosuppression can reduce efficacy § VACCINE DOES NOT CHANGE CERVICAL CANCER SCREENING RECOMMENDATIONS! http: //www. cdc. gov/mmwr/preview/mmwrhtml/rr 5602 a 1. htm 40

Healthcare Workers § Key in implementation of Adult Immunization • Education: Multiple studies show that… • STRONG MD recommendation Increased patient vaccine uptake § HCW need preventive benefits for ‘themselves’ • Potential source for disease transmission • • Patients Other staff Communities Families • Potential for VPD to impair patient care • • Adversely affect efficiency Prevent HCW from working with [their] patients http: //www. cdc. gov/mmwr/preview/mmwrhtml/00050577. htm 41

Healthcare Worker Vaccination § § Annual influenza vaccination Tdap: All should receive 1 adult dose MMR, Varicella: Proof of immunity or 2 doses [each] HBV: 3 dose series • Titer 1 month after series Repeat entire series x 1 if titer < 10 IU • No recommendation to screen/recheck titer otherwise • 42

Hepatitis B §Formulations/Route: IM § 3 and 4 dose schedules over at least 4 month interval §Standard: time 0, 1 and 6 months later [alt 1: 0, 2, 4 months; alt 2: 0, 1 4 mo; Alt 3: 0, 1, 2, 12 mo (Engerix only)] §If series is delayed, no need to restart. Complete series from prior dose(s) §High dose vaccine, specific schedule for dialysis patients § Risk groups [in addition to HCW, Diabetes] §All adults who want to be protected from hepatitis B (HBV) §>1 sex partner in 6 mo. , Household contacts & sex partners of HBs. Ag + people; IVDU, people seeking STD evaluation or treatment; hemodialysis patients and those awaiting dialysis, MSM; staff working with developmentally disabled; inmates in LT correctional facilities; certain international travelers, persons with chronic liver disease (including HCV, cirrhosis) http: //www. cdc. gov/vaccines/hcp/acip-recs/vacc-specific/hepb. html 43

Case 3 § Christine Pulaski is a 32 year old pregnant woman with no significant past medical history who is presenting for a pre-natal visit in her first trimester. § Which vaccinations should she receive? Which vaccinations are contraindicated? 44
![Vaccine Groups Universals Influenza Pneumococcal PCV 13 PPSV 23 Tdap Vaccine Groups “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-45.jpg)
Vaccine Groups “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap § Zoster “Selectives” § HPV § § § • [HPV 9, HPV 4, HPV 2] MMR Varicella Meningococcal • Quad [MCV 4, MPSV 4] • Men. B Hepatitis A Hepatitis B 45

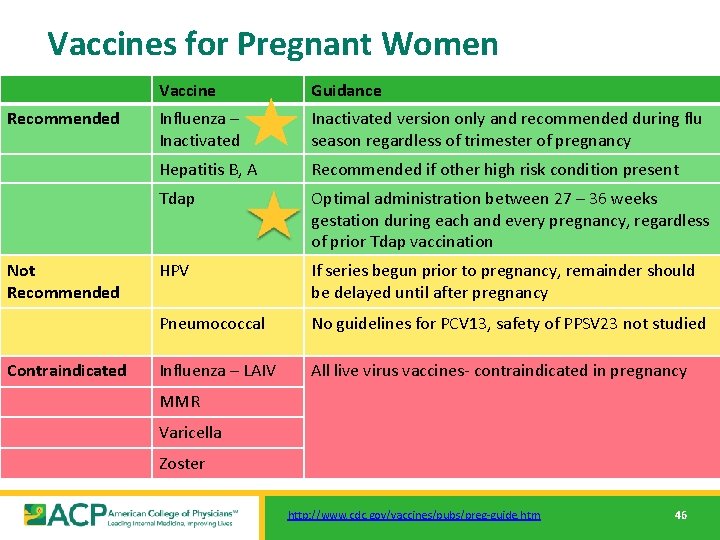
Vaccines for Pregnant Women Recommended Not Recommended Contraindicated Vaccine Guidance Influenza – Inactivated version only and recommended during flu season regardless of trimester of pregnancy Hepatitis B, A Recommended if other high risk condition present Tdap Optimal administration between 27 – 36 weeks gestation during each and every pregnancy, regardless of prior Tdap vaccination HPV If series begun prior to pregnancy, remainder should be delayed until after pregnancy Pneumococcal No guidelines for PCV 13, safety of PPSV 23 not studied Influenza – LAIV All live virus vaccines- contraindicated in pregnancy MMR Varicella Zoster http: //www. cdc. gov/vaccines/pubs/preg-guide. htm 46

Case 4 § Christopher Watkins is a 68 year old man with a prior 10 – week history of a persistent cough presenting for his annual Medicare wellness visit. § Mr. Watkins lives with his daughter and his 2 young grandchildren (ages 6 months and 2 years). Which vaccines should Mr. Watkins receive? 47
![Vaccine Groups Universals Influenza Pneumococcal PCV 13 PPSV 23 Tdap Vaccine Groups… “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-48.jpg)
Vaccine Groups… “Universals” § Influenza § Pneumococcal • [PCV 13, PPSV 23] § Tdap § Zoster “Selectives” § HPV • [HPV 9, HPV 4, HPV 2] § MMR § Varicella § Meningococcal • Quad [MCV 4, MPSV 4] • Men. B § Hepatitis A § Hepatitis B 48

Td >> Tdap § Pertussis incidence increasing since 1970’s • • 2012: US >42, 000 cases (CDC Passive Surveillance) 2013: US 28, 639 cases (CDC Passive Surveillance) Community outbreaks: Most in fall, winter and in all ages Nosocomial Disease: Academic, Community • • [Med/Surg, OR, L&D, NICU, Oncology] Residential Care § Adults/Adolescents do not have ‘classic’ triphasic disease • Most have persistent Cough: Median 4 months [6 studies] • • • 20 -40 % ‘Whoop’, 40 – 55 % Posttussive emesis 12 -32 % Lymphocytosis ~10% develop complications [Pneumonia most common] http: //www. cdc. gov/vaccines/vpd-vac/pertussis/ http: //www. cdc. gov/vaccines/vpd-vac/combo-vaccines/DTa. P-Td-DT/Tdap. htm 49

Impact of Pertussis Vaccination www. cdc. gov/pertussis/outbreaks. html 50

Pertussis 2013 to 2014 § 46 states report increases since 2011 § >25, 000 cases reported to the CDC with 13 deaths § As of August 24, 2014 www. cdc. gov/pertussis/outbreaks. html 51

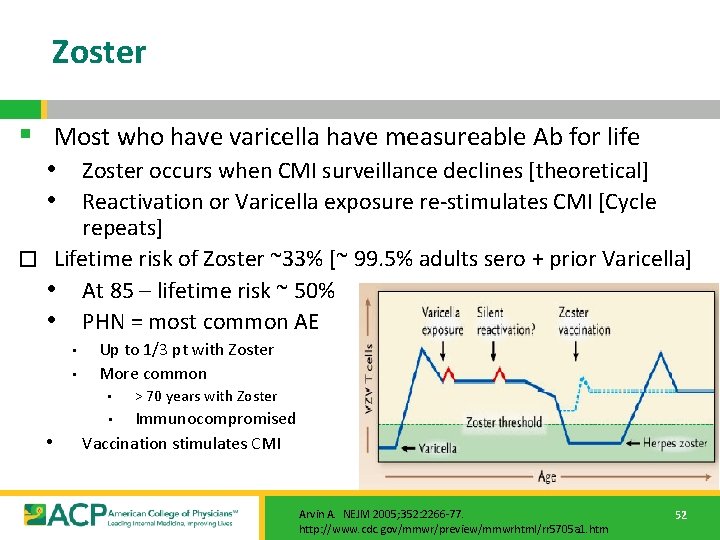
Zoster § Most who have varicella have measureable Ab for life • Zoster occurs when CMI surveillance declines [theoretical] • Reactivation or Varicella exposure re-stimulates CMI [Cycle repeats] � Lifetime risk of Zoster ~33% [~ 99. 5% adults sero + prior Varicella] • At 85 – lifetime risk ~ 50% • PHN = most common AE • • Up to 1/3 pt with Zoster More common • > 70 years with Zoster Immunocompromised Vaccination stimulates CMI • • Arvin A. NEJM 2005; 352: 2266 -77. http: //www. cdc. gov/mmwr/preview/mmwrhtml/rr 5705 a 1. htm 52
![Zoster Vaccinate Healthy 60 adults ACIP Not immunocompromised Zoster § § § Vaccinate Healthy 60+ adults [ACIP: Not immunocompromised] • • •](https://slidetodoc.com/presentation_image_h2/41d1090014f0593fc2f64c1726b012fa/image-53.jpg)
Zoster § § § Vaccinate Healthy 60+ adults [ACIP: Not immunocompromised] • • • FDA approved from age 50 differs from ACIP recommendation Regardless of prior episode(s) of Zoster [opinion: wait 1 year] No need to test and/or vaccinate vs. Varicella first Contraindications • • Pregnancy Anaphylactic Hypersensitivity to Neomycin, Gelatin No need to defer for ‘at risk contacts’ – transmission risk low No need to defer if recent transfusion, Ab containing products Adverse events • Occasional mild varicella – like rash @ vaccine site Frozen vaccine: Administer w/in 60 minutes, 0. 65 ml SQ Deltoid Duration of protection: At least 4 years. No booster. http: //www. cdc. gov/mmwr/preview/mmwrhtml/rr 5705 a 1. htm 53

Summary § Current vaccination rates are well below goal § Vaccines can prevent morbidity and mortality associated with vaccine preventable disease § Adult immunization is complex, rapidly changing § Physician recommendation is key to patient uptake! Next steps: § [Add PROGRAM SPECIFIC INFO] § Module 2 will be [add date] • Strategies to increase adult immunization in practice! 54

Support § This program is supported by the American College of Physicians, and by an educational grant from Merck & Co. , Inc. and Sanofi Pasteur. 55
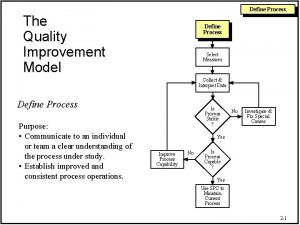
 Process of nursing audit
Process of nursing audit Compliance vs quality
Compliance vs quality Quality improvement vs research
Quality improvement vs research Qsen definition
Qsen definition Quality improvement paradigm
Quality improvement paradigm Define continuous quality improvement
Define continuous quality improvement Pocqi ppt
Pocqi ppt Define continuous quality improvement
Define continuous quality improvement Efmd quality improvement system
Efmd quality improvement system Indiana perinatal quality improvement collaborative
Indiana perinatal quality improvement collaborative Cqi action plan template
Cqi action plan template Tea quality improvement
Tea quality improvement Quality improvement
Quality improvement Sustainability in quality improvement
Sustainability in quality improvement Quality improvement nurse
Quality improvement nurse Quality improvement
Quality improvement Juran 10 steps to quality improvement
Juran 10 steps to quality improvement Swot analysis quality improvement
Swot analysis quality improvement Mqii
Mqii Quality improvement
Quality improvement Data driven quality
Data driven quality Rapid cycle quality improvement
Rapid cycle quality improvement Quality is free
Quality is free Uw neurological surgery
Uw neurological surgery Umass nurse residency program
Umass nurse residency program Vanderbilt nurse residency interview questions
Vanderbilt nurse residency interview questions Https://ncc-efm.org/game/efmgame.cfm
Https://ncc-efm.org/game/efmgame.cfm Uihc internal medicine residents
Uihc internal medicine residents Tufts anesthesiology residency
Tufts anesthesiology residency Unmc obgyn
Unmc obgyn Qualifying shift paro
Qualifying shift paro Post campaign analysis presentation
Post campaign analysis presentation Unm internal medicine
Unm internal medicine Uthscsa family medicine residency
Uthscsa family medicine residency Karl suur
Karl suur Internal tourism includes
Internal tourism includes How can an na best help residents with eating?
How can an na best help residents with eating? Chapter 13 personal care skills
Chapter 13 personal care skills Famous people from yonkers
Famous people from yonkers Slu family medicine residency
Slu family medicine residency Uf neurology residency
Uf neurology residency C device module module 1
C device module module 1 Quality control and quality assurance
Quality control and quality assurance Basic quality concepts
Basic quality concepts Georgia registry for immunizations
Georgia registry for immunizations Global alliance for vaccines and immunization
Global alliance for vaccines and immunization Quality assurance module
Quality assurance module Quality management pmp
Quality management pmp Pmp gold plating
Pmp gold plating Tqm guru
Tqm guru Old quality vs new quality
Old quality vs new quality Kontinuitetshantering i praktiken
Kontinuitetshantering i praktiken Typiska novell drag
Typiska novell drag Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Ekologiskt fotavtryck
Ekologiskt fotavtryck Varför kallas perioden 1918-1939 för mellankrigstiden?
Varför kallas perioden 1918-1939 för mellankrigstiden?