Adsorption Diagenesis Lecture 18 Diffusion and heatflow limited








- Slides: 24

Adsorption Diagenesis Lecture 18

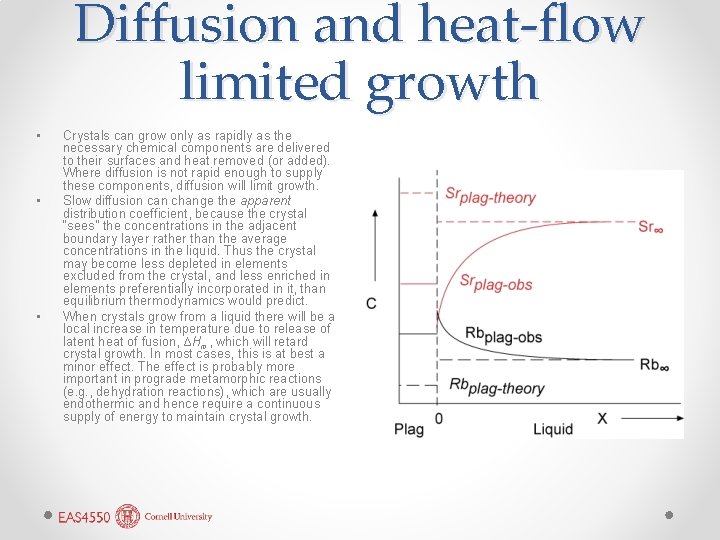
Diffusion and heat-flow limited growth • • • Crystals can grow only as rapidly as the necessary chemical components are delivered to their surfaces and heat removed (or added). Where diffusion is not rapid enough to supply these components, diffusion will limit growth. Slow diffusion can change the apparent distribution coefficient, because the crystal “sees” the concentrations in the adjacent boundary layer rather than the average concentrations in the liquid. Thus the crystal may become less depleted in elements excluded from the crystal, and less enriched in elements preferentially incorporated in it, than equilibrium thermodynamics would predict. When crystals grow from a liquid there will be a local increase in temperature due to release of latent heat of fusion, ∆Hm, which will retard crystal growth. In most cases, this is at best a minor effect. The effect is probably more important in prograde metamorphic reactions (e. g. , dehydration reactions), which are usually endothermic and hence require a continuous supply of energy to maintain crystal growth.

Zoning • Diffusion limits the ability of the interior of a crystal to maintain equilibrium with the magma from which it is precipitating. • This leads to zoning in crystals, apparent under the microscope. • Zoning can record magma history. Zoned pyx and plag in lava.

Adsorption • Physical o attachment of atom or molecule to a surface through van der Waals or intermolecular forces. o Weak: ∆Had ≈ 4 -12 k. J/mol • Chemical o formation of new chemical bond between adsorbed species and atoms on surface o Stronger: ∆Had >40 k. J/mol • Importance of Adsorption o The first step in precipitation is generally the adsorption of an ion or molecule to the crystal surface. o Adsorption/desorption is critically important in supplying soil nutrients to plants. o The seawater concentrations of many trace elements is controlled by adsorption on pre-existing surfaces, not precipitation.

Adsorption and Surface Free Energy • Adsorption changes the nature of the surface and therefore the surface free energy. • We can express the surface free energy change as: • where ni, s is the number of moles of component i adsorbed to the surface, s, and A is the area of the surface. • We define Γi as the Gibbs Absorption Density: moles of i absorbed on the surface per unit area.

Equilibrium Adsorption • Consider adsorption of species M on surface S: M + S = M S • Let the fraction of surface sites occupied by M be θM. • Fraction of free sites is then 1 - θM. • Assuming elementary reactions, rate of adsorption is: • Rate of desorption is • At equilibrium • and

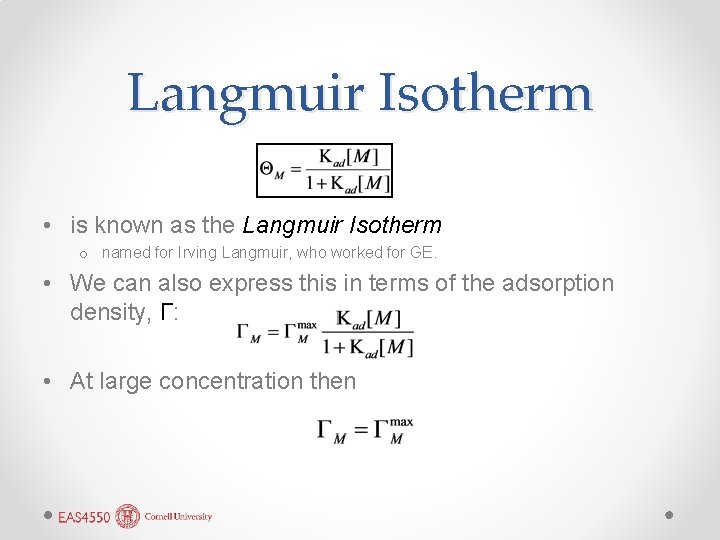
Langmuir Isotherm • is known as the Langmuir Isotherm o named for Irving Langmuir, who worked for GE. • We can also express this in terms of the adsorption density, Γ: • At large concentration then

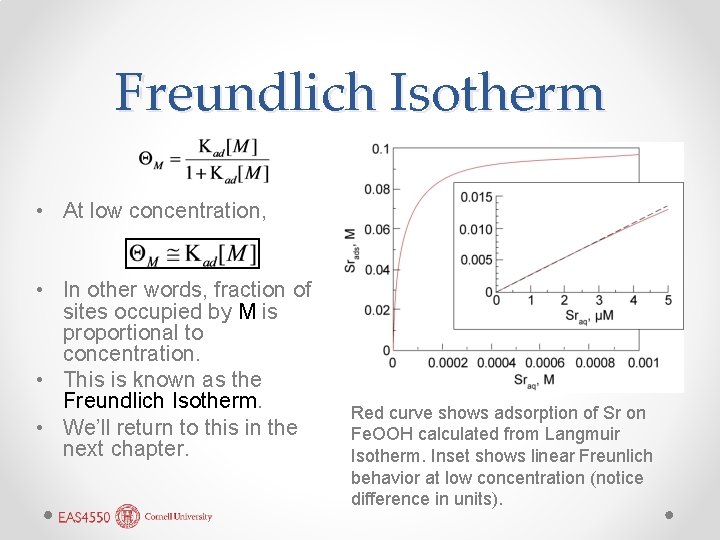
Freundlich Isotherm • At low concentration, • In other words, fraction of sites occupied by M is proportional to concentration. • This is known as the Freundlich Isotherm. • We’ll return to this in the next chapter. Red curve shows adsorption of Sr on Fe. OOH calculated from Langmuir Isotherm. Inset shows linear Freunlich behavior at low concentration (notice difference in units).

Adsorption and Catalysis • • • Catalysis quite frequency involves adsorption of species on solid surfaces. An everyday example is the catalytic converters in exhaust systems of cars. In these, gaseous species are adsorbed to a noble metal surface (e. g. , palladium) to catalyze reactions such as NOx → N 2 + x. O 2 Mineral surfaces are thought to have been important in the initiation of terrestrial life by catalyzing critical organic compounds from inorganic ones such as methane, N 2, etc.

Catalysis • The International Union of Pure and Applied Chemistry (IUPAC) defines catalyst as follows: A catalyst is a substance that increases the rate without modifying the overall standard Gibbs energy change in the reaction; the process is called catalysis, and a reaction in which a catalyst in involved is known as a catalyzed reaction. • Catalysts work by providing an alternative reaction path with lower activation energy. In many cases, the lowering of the activation energy arises when reacting species are adsorbed. The heat liberated by the adsorption (∆Hads) is available to contribute toward the activation energy.

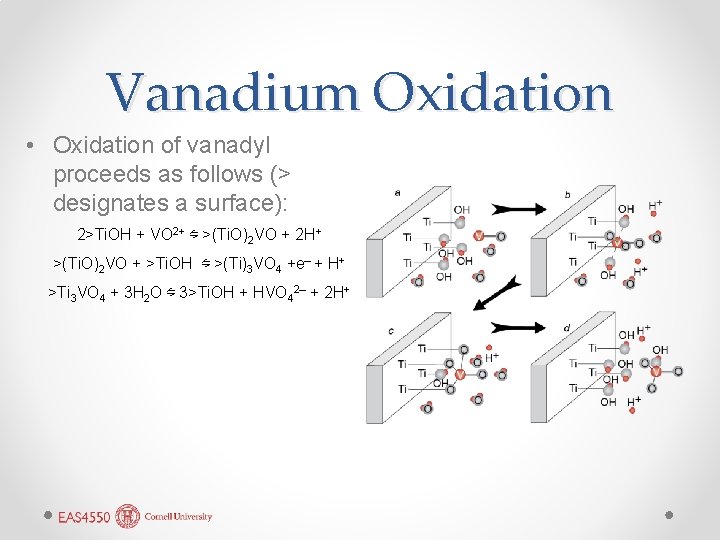
Example: oxidation of 5+ to V by Ti. O 2 4+ V • Surfaces of many metal oxides and sulfides can catalyze oxidation-reduction reactions by conducting electrons (i. e. , serving as an electrode). • As we’ll see, a metal oxide immersed in water would adsorb H+ and OH– to the surface (> designates a surface): >Ti+ + H 2 O ⇋ >Ti. OH +

Vanadium Oxidation • Oxidation of vanadyl proceeds as follows (> designates a surface): 2>Ti. OH + VO 2+ ⇋ >(Ti. O)2 VO + 2 H+ >(Ti. O)2 VO + >Ti. OH ⇋ >(Ti)3 VO 4 +e– + H+ >Ti 3 VO 4 + 3 H 2 O ⇋ 3>Ti. OH + HVO 42– + 2 H+

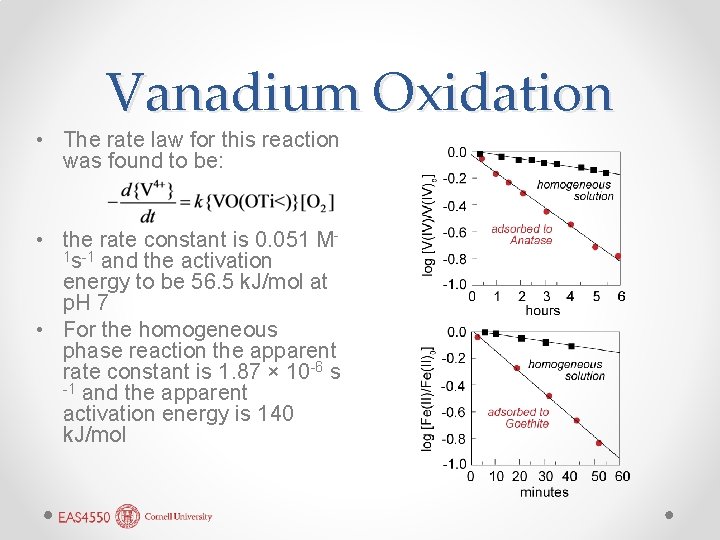
Vanadium Oxidation • The rate law for this reaction was found to be: • the rate constant is 0. 051 M 1 s-1 and the activation energy to be 56. 5 k. J/mol at p. H 7 • For the homogeneous phase reaction the apparent rate constant is 1. 87 × 10 -6 s -1 and the apparent activation energy is 140 k. J/mol

Diagenesis is the transformation of a sediment into a sedimentary rock. Physical processes involve compaction and expulsion of water as the sediment is buried. We’ll briefly consider some of the chemical processes that accompany this.

• Consider a sedimentary layer ‘A’ being buried as sediment accumulates above it. • We can chose a reference frame fixed to the layer, or to the sediment-water interface. • In the latter case, the layer will appear to move downward with time. • Change in concentration with time at some fixed depth x is then: • First term on the right is any changes in concentration within layer, second term is the concentration gradient times the burial rate, ω. • We can use this equation to change reference frames.

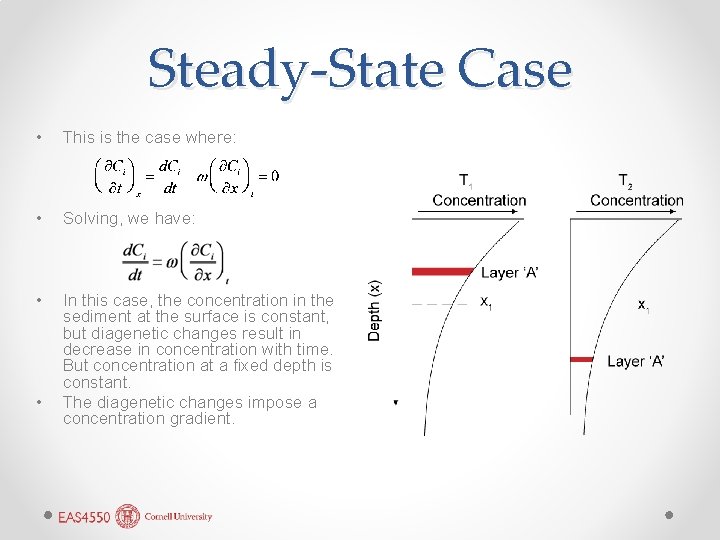
Steady-State Case • This is the case where: • Solving, we have: • In this case, the concentration in the sediment at the surface is constant, but diagenetic changes result in decrease in concentration with time. But concentration at a fixed depth is constant. The diagenetic changes impose a concentration gradient. •

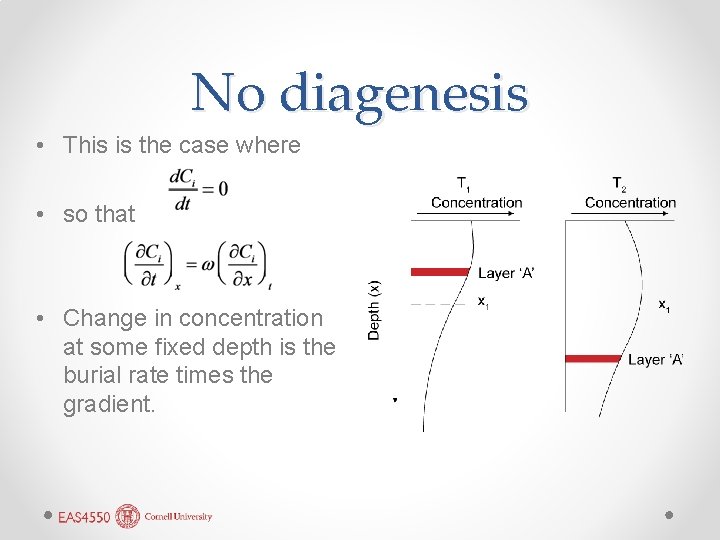
No diagenesis • This is the case where • so that • Change in concentration at some fixed depth is the burial rate times the gradient.

Diagenetic Process • Sediment consists of particles plus ‘pore water’. If porosity is �� then fraction of particles is 1 - ��. • As weight of sediment accumulating above increases, water tends to be expelled - resulting in upward velocity of pore fluid. • In addition, dissolution and cementation (precipitation) can change porosity. • Due to compaction, rate of burial will not be equal to sedimentation rate.

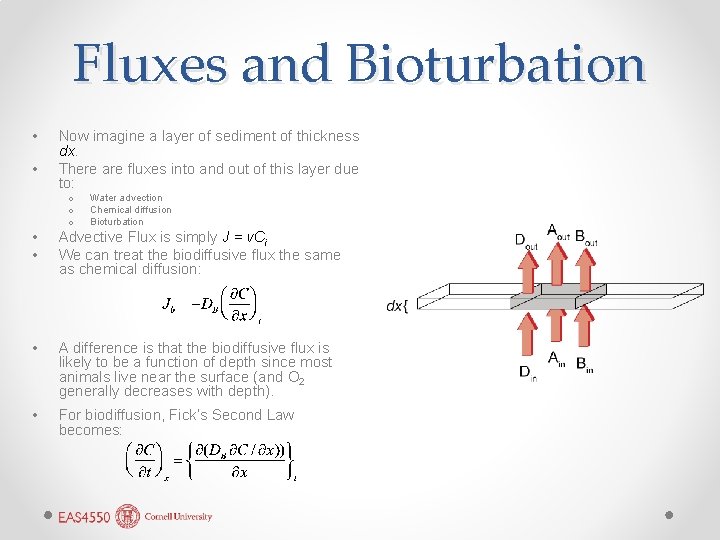
Fluxes and Bioturbation • • Now imagine a layer of sediment of thickness dx. There are fluxes into and out of this layer due to: o o o Water advection Chemical diffusion Bioturbation • • Advective Flux is simply J = v. Ci We can treat the biodiffusive flux the same as chemical diffusion: • A difference is that the biodiffusive flux is likely to be a function of depth since most animals live near the surface (and O 2 generally decreases with depth). • For biodiffusion, Fick’s Second Law becomes:

Diffusion and Porosity • Diffusion is so much faster through the pore water than through solids, the latter can be effectively ignored. • Thus we can view the ‘molecular flux’ - the chemical one we have already discussed, as reduced to that occurring through pores: • Fick’s Second Law becomes

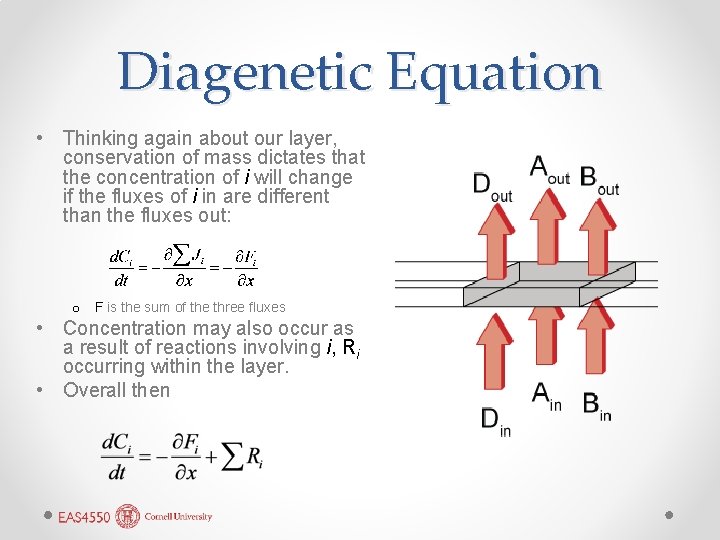
Diagenetic Equation • Thinking again about our layer, conservation of mass dictates that the concentration of i will change if the fluxes of i in are different than the fluxes out: o F is the sum of the three fluxes • Concentration may also occur as a result of reactions involving i, Ri occurring within the layer. • Overall then

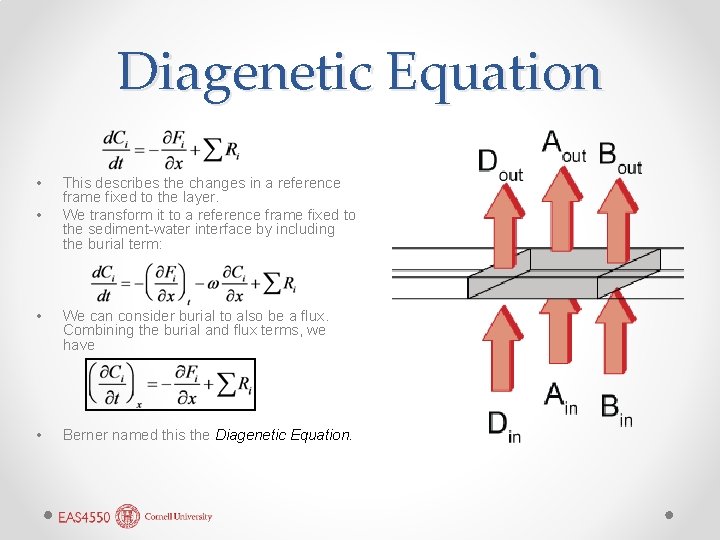
Diagenetic Equation • • This describes the changes in a reference frame fixed to the layer. We transform it to a reference frame fixed to the sediment-water interface by including the burial term: • We can consider burial to also be a flux. Combining the burial and flux terms, we have • Berner named this the Diagenetic Equation.

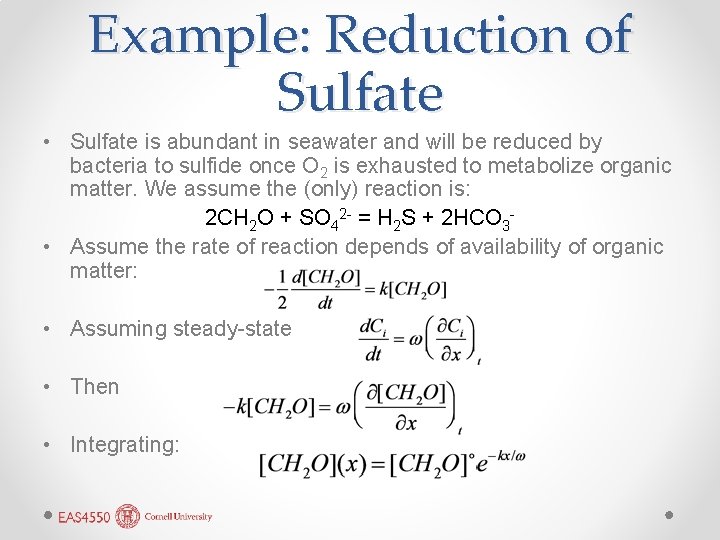
Example: Reduction of Sulfate • Sulfate is abundant in seawater and will be reduced by bacteria to sulfide once O 2 is exhausted to metabolize organic matter. We assume the (only) reaction is: 2 CH 2 O + SO 42 - = H 2 S + 2 HCO 3 • Assume the rate of reaction depends of availability of organic matter: • Assuming steady-state • Then • Integrating:

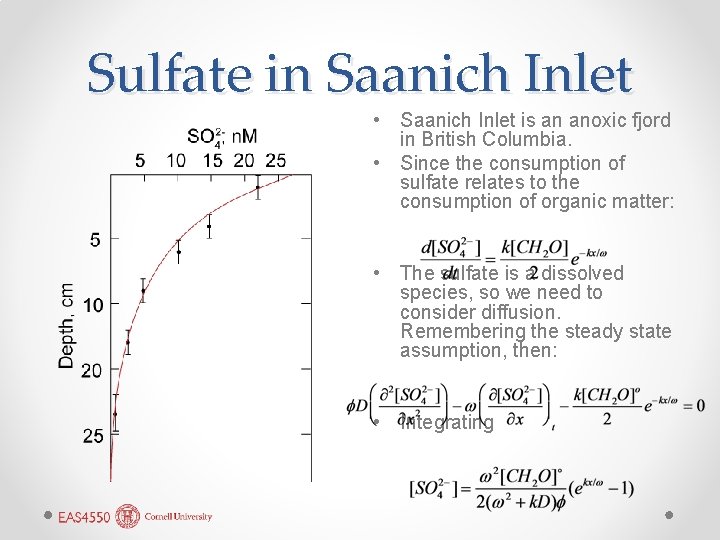
Sulfate in Saanich Inlet • Saanich Inlet is an anoxic fjord in British Columbia. • Since the consumption of sulfate relates to the consumption of organic matter: • The sulfate is a dissolved species, so we need to consider diffusion. Remembering the steady state assumption, then: • Integrating