ADSORPSI KARBON AKTIF Fungsi Adsorpsi Menurunkan kandungan komponen
ADSORPSI KARBON AKTIF

Fungsi Adsorpsi Menurunkan kandungan komponen organik yang tidak dapat disisihkan dengan proses biologi : � THM precursors, chlorinated organic carbon, pesticides, � Senyawa organik penyebab timbulnya warna dan bau (Merchaptan, asam vulvic, H 2 S dll) � Senyawa – senyawa hidrokarbon lainnya Menurunkan beberapa jenis ion logam

KARAKTERISTIK KARBON AKTIF • High surface area/volume ratio (ca. 1, 000 m 2/g) • Sizes of micropore : ≦100Å • Bentuk fisik karbon aktif : – Powdered – granular activated carbon
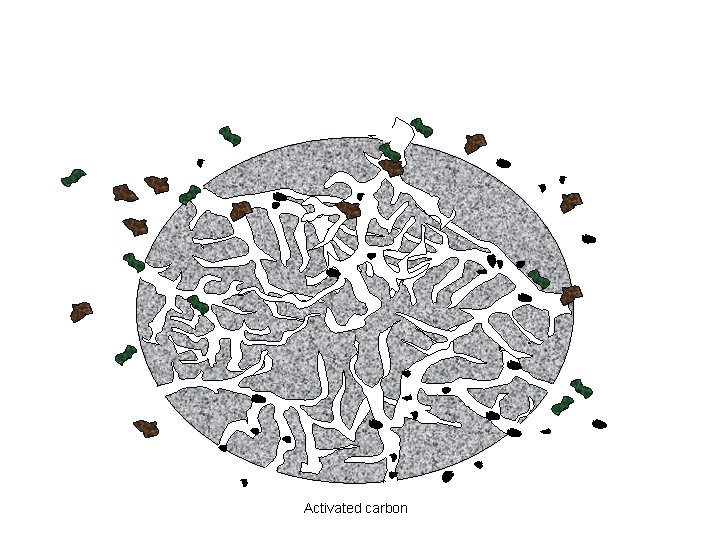
Activated carbon
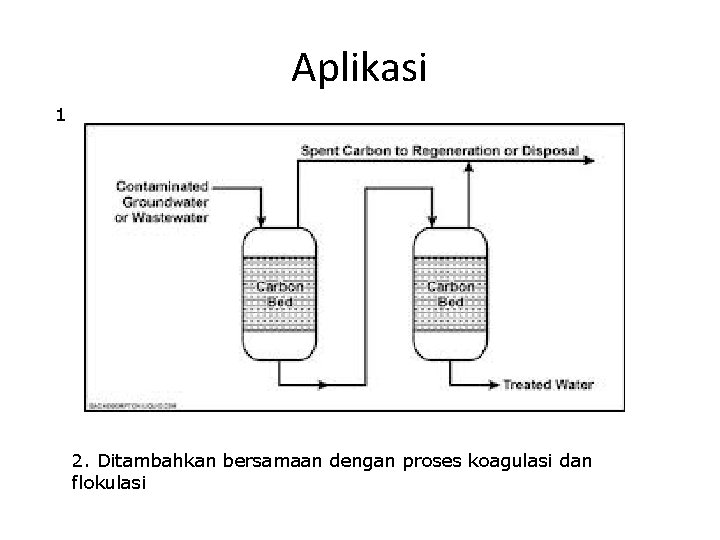
Aplikasi 1 2. Ditambahkan bersamaan dengan proses koagulasi dan flokulasi

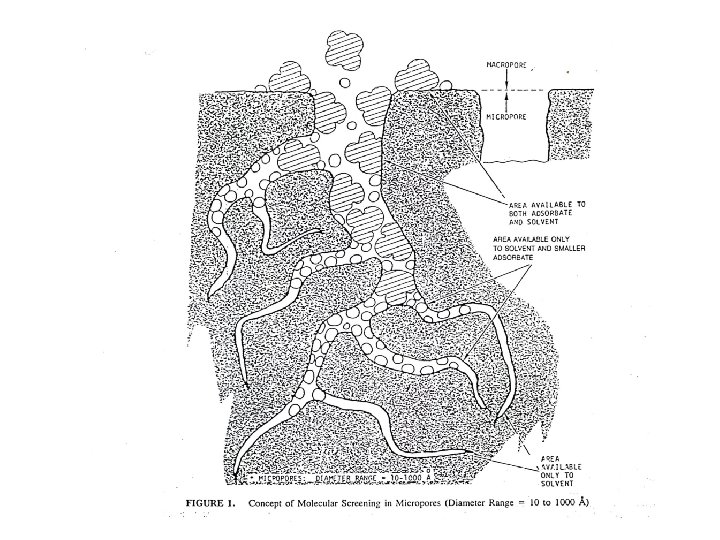
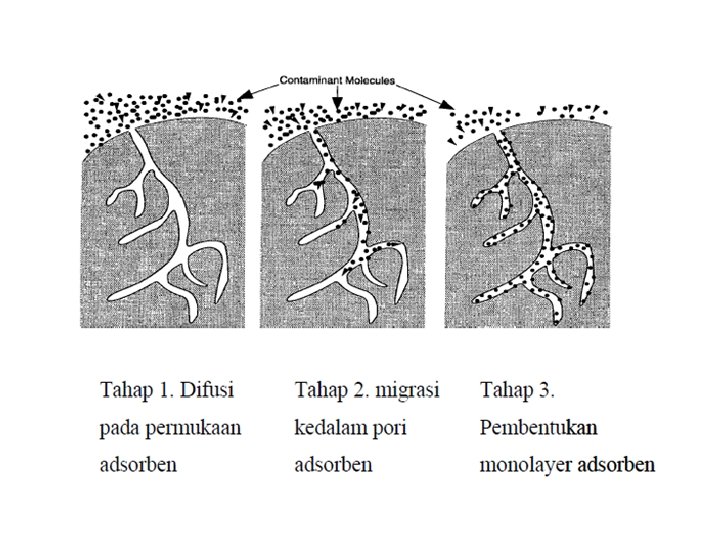
Faktor – faktor berpengaruh pada adorpsi karbon • Karakteristik fisik dan kimiawi karbon (Luas permukaan dan ukuran pori) • Karakteristik fisik dan kimiawi zat yang diserap (adsorbate) Ø (Ukuran molekul, polaritas molekular, komposisi kimiawi) Ø Materi dengan Berat molekul semakin besar semakin mudah diserap Ø Diameter Molekul Adsorbate • • Konsentrasi adsorbate pada phase liquid, p. H, temperatur Waktu Kontak Tingkat kelarutan solute dalam cairan pembawa. I, semakin mudah larut maka kemampuan serapan

Adsorption Isotherm • Plot of contaminant adsorbed per unit mass of carbon (X/M) vs. equilibrium contaminant concentration in bulk fluid • Mathematical forms – Langmuir: X/M = (a. Ce)/(1+b. Ce) – Freundlich: X/M = k. Ce 1/n
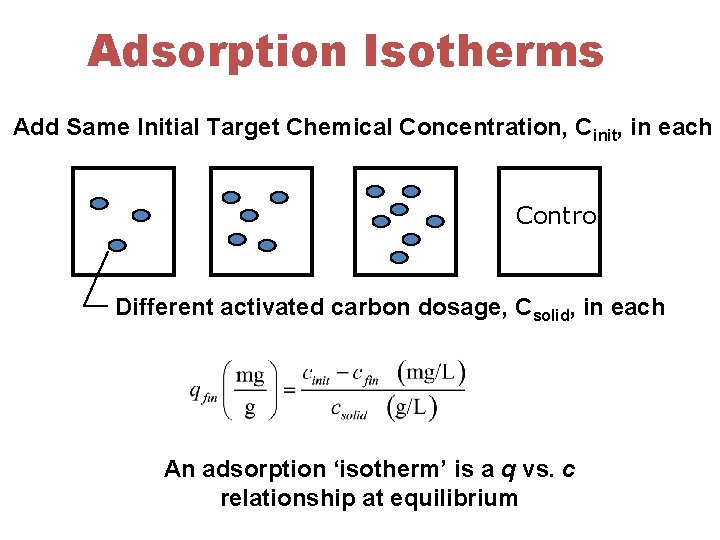
Adsorption Isotherms Add Same Initial Target Chemical Concentration, Cinit, in each Control Different activated carbon dosage, Csolid, in each An adsorption ‘isotherm’ is a q vs. c relationship at equilibrium
Determination of appropriate model: To determine which model to use to describe the adsorption for a particular adsorbent/adsorbate isotherms experiments are usually run. Data from these isotherm experiments are then analyzed using the following methods that are based on linearization of the models.
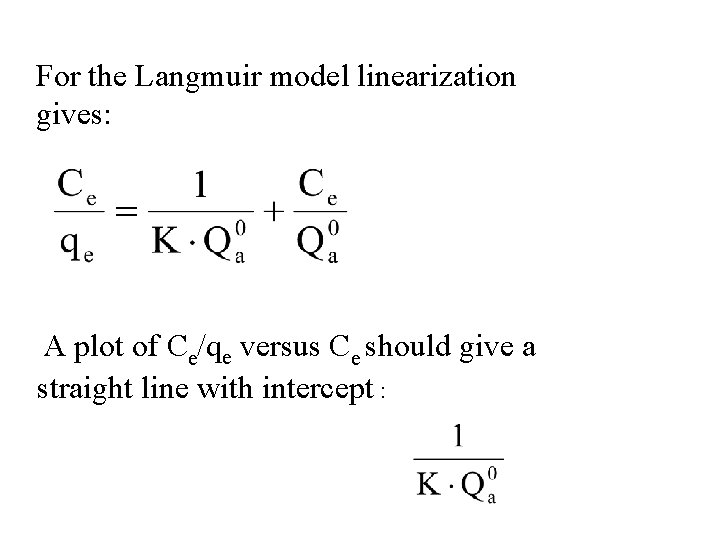
For the Langmuir model linearization gives: A plot of Ce/qe versus Ce should give a straight line with intercept :
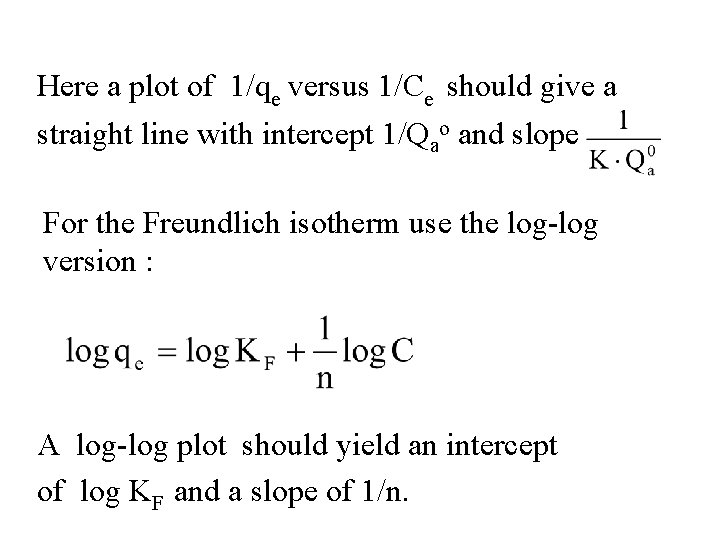
Here a plot of 1/qe versus 1/Ce should give a straight line with intercept 1/Qao and slope For the Freundlich isotherm use the log-log version : A log-log plot should yield an intercept of log KF and a slope of 1/n.
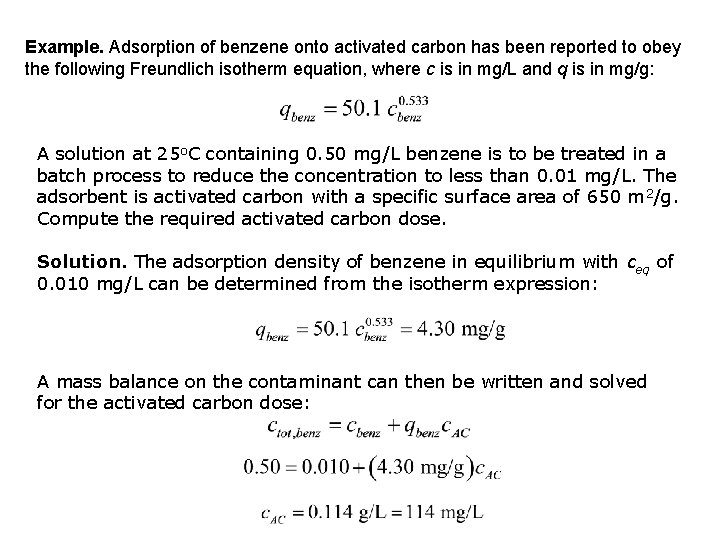
Example. Adsorption of benzene onto activated carbon has been reported to obey the following Freundlich isotherm equation, where c is in mg/L and q is in mg/g: A solution at 25 o. C containing 0. 50 mg/L benzene is to be treated in a batch process to reduce the concentration to less than 0. 01 mg/L. The adsorbent is activated carbon with a specific surface area of 650 m 2/g. Compute the required activated carbon dose. Solution. The adsorption density of benzene in equilibrium with ceq of 0. 010 mg/L can be determined from the isotherm expression: A mass balance on the contaminant can then be written and solved for the activated carbon dose:
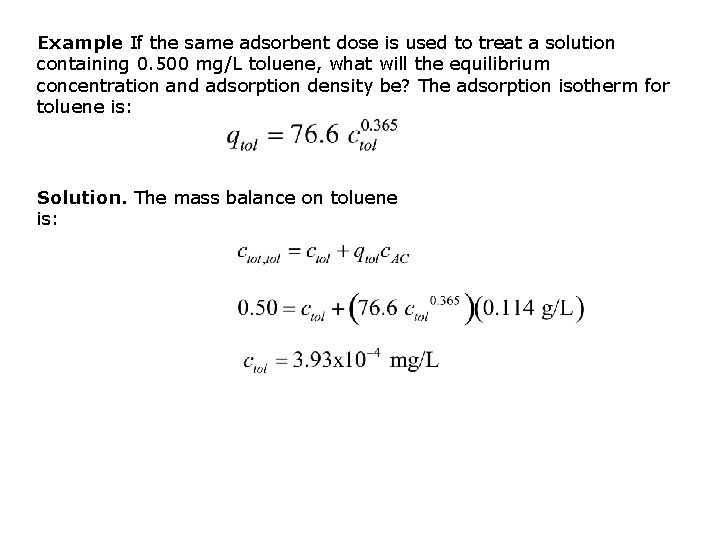
Example If the same adsorbent dose is used to treat a solution containing 0. 500 mg/L toluene, what will the equilibrium concentration and adsorption density be? The adsorption isotherm for toluene is: Solution. The mass balance on toluene is:
General Process Design Features • Contactors provide large surface area • Types of contactors – Continuous flow, slurry reactors – Batch slurry reactors (infrequently) – Continuous flow, packed bed reactors • Product water concentration may be – Steady state or – Unsteady state
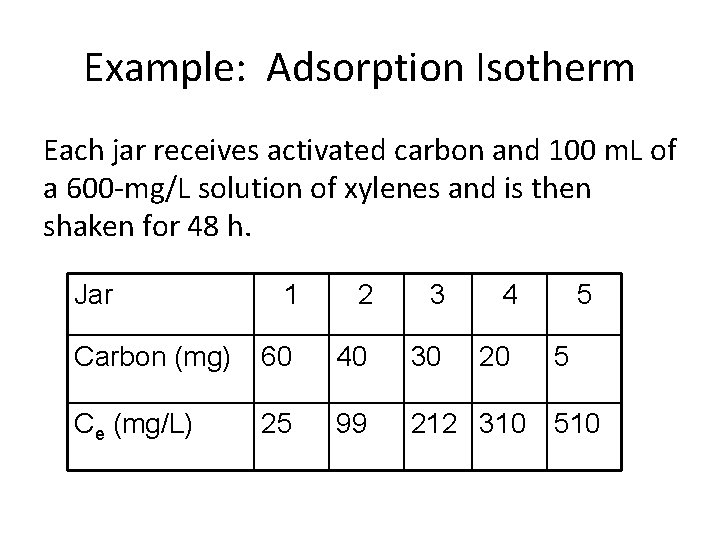
Example: Adsorption Isotherm Each jar receives activated carbon and 100 m. L of a 600 -mg/L solution of xylenes and is then shaken for 48 h. Jar 1 2 3 4 Carbon (mg) 60 40 30 20 Ce (mg/L) 25 99 212 310 5 5 510
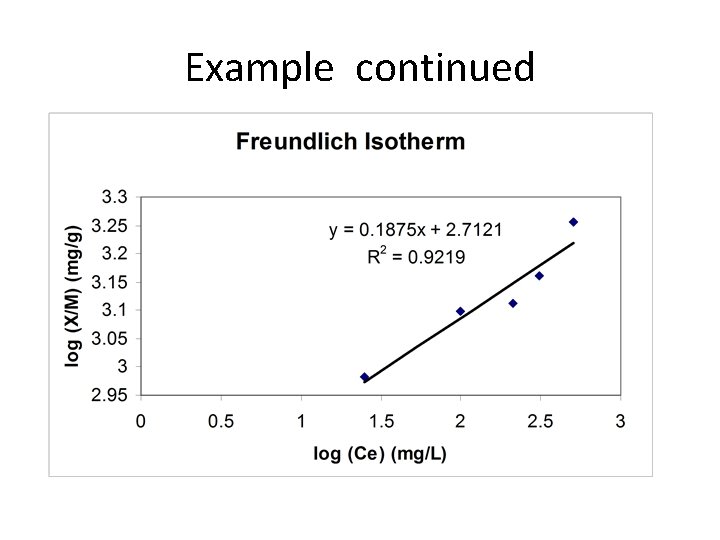
Example continued
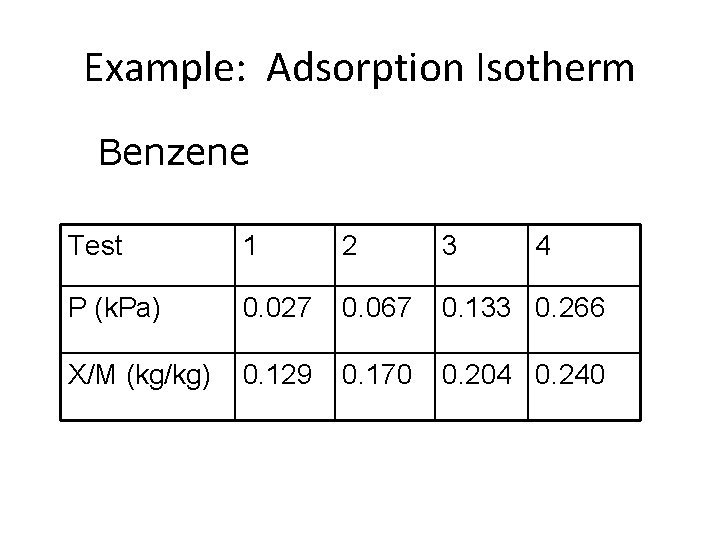
Example: Adsorption Isotherm Benzene Test 1 2 3 4 P (k. Pa) 0. 027 0. 067 0. 133 0. 266 X/M (kg/kg) 0. 129 0. 170 0. 204 0. 240
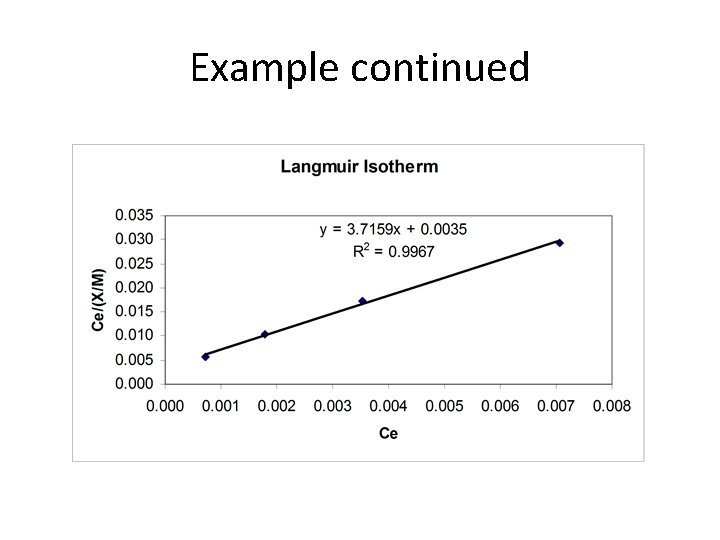
Example continued
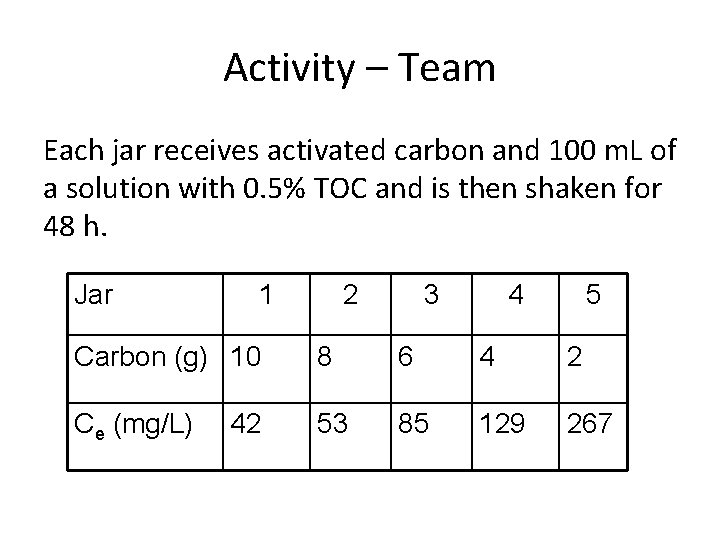
Activity – Team Each jar receives activated carbon and 100 m. L of a solution with 0. 5% TOC and is then shaken for 48 h. Jar 1 2 3 4 5 Carbon (g) 10 8 6 4 2 Ce (mg/L) 53 85 129 267 42
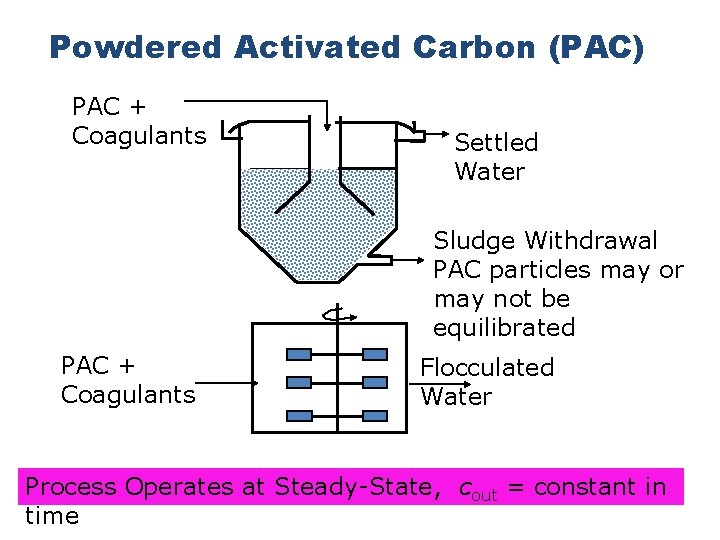
Powdered Activated Carbon (PAC) PAC + Coagulants Settled Water Sludge Withdrawal PAC particles may or may not be equilibrated PAC + Coagulants Flocculated Water Process Operates at Steady-State, cout = constant in time
- Slides: 22