Adrienne Elise Inger Cal Poly Pomona Dietetic Intern
























- Slides: 41

Adrienne Elise Inger Cal Poly Pomona Dietetic Intern 2012 -2013 Clinical Case Study

Overview �Patient Background �History of Present Illness �Diagnoses �Medications �Additional Therapies �ADIME �Economic Benefits of MNT �Literature Reviews �My Role & Feedback

Patient Background

Patient Background This information has been removed to protect patient confidentiality

Team Members � RD � MD � RN (blood sample collection, heights, weights) � OB/GYN Nutrition Care Manual recommends that a Social Worker + Psychologist be part of the team to help individuals with: Ø Social barriers to adherence Ø Problem solving around insurance and formula issues Ø Psychological and neuropsychological status �

Patient’s General Health � Sleep Patterns: Patient reports sleeping well (9 -10 hours/night) � Elimination: Regular. Typical BM 1 x/day � Exercise, recreation and activity level: Patient is moderately active; reports walking 2 -4 miles per day and playing with brother at the park 1 -2 x per week � Dental Health: Good dentition � Tobacco use: Denies � Alcohol and other drug use: Denies

History of Present Illness

History of Present Illness � Pt with elevated phe levels since age 3 � (2010) Stopped drinking phe-free, tyrosine enriched formula � (11/2011) Trial of Kuvan failed � (9/2/2012) Patient’s last menstrual period � (10/26/12) Chief complaint: Missed period Pregnancy confirmed in Genetics clinic and pt seen by nutrition services � Patient informed that she is required to come to Genetics Clinic weekly during pregnancy for close monitoring of Maternal PKU � Now: 18 weeks pregnant (2 nd trimester)

Diagnoses �Phenylketonuria (diagnosed at birth) �Maternal Phenylketonuria �Adolescent Pregnancy (high nutritional risk)

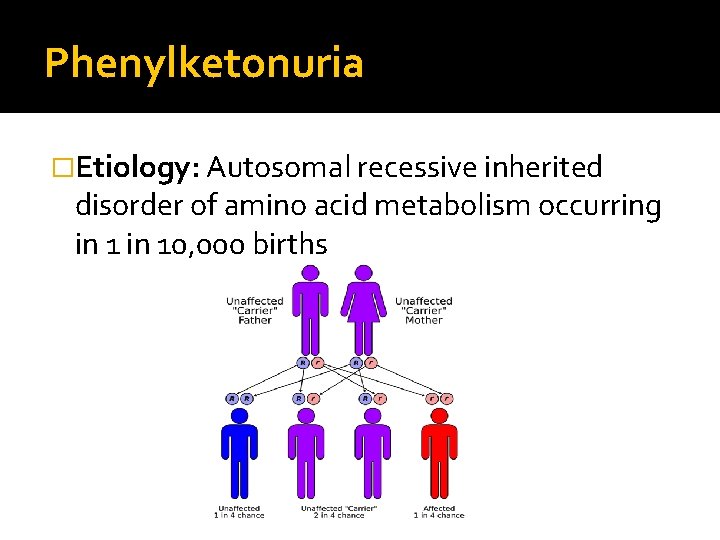
Phenylketonuria �Etiology: Autosomal recessive inherited disorder of amino acid metabolism occurring in 10, 000 births

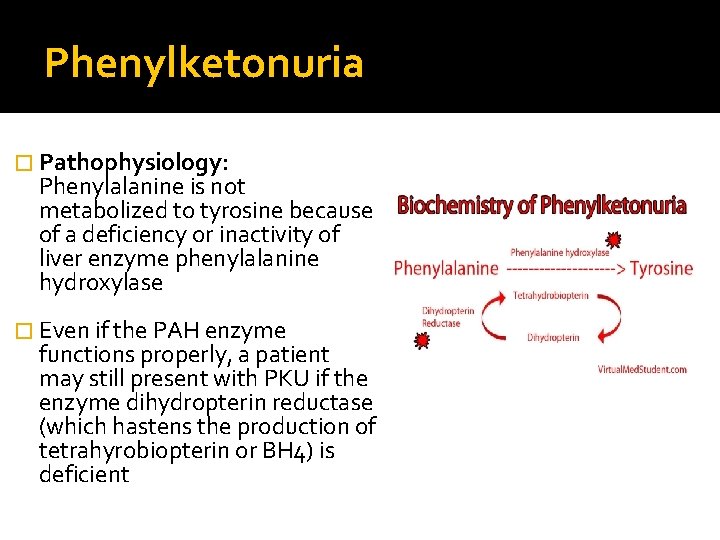
Phenylketonuria � Pathophysiology: Phenylalanine is not metabolized to tyrosine because of a deficiency or inactivity of liver enzyme phenylalanine hydroxylase � Even if the PAH enzyme functions properly, a patient may still present with PKU if the enzyme dihydropterin reductase (which hastens the production of tetrahyrobiopterin or BH 4) is deficient

Nutrition Therapy � Consumption of a semisynthetic, phenylalanine-free, tyrosine-supplemented formula � Small amounts of natural foods to provide the required amount of essential amino acid phenylalanine � Exclude high-protein foods and Aspartame

Medical Treatment � Tetrahydrobiopterin (BH 4), a cofactor needed for the proper activity of PAH can be supplemented in patients that have BH 4 -responsive PKU (i. e. Kuvan) � Large neutral amino acid supplementation (i. e. threonine) may help to decrease serum phe levels by competing with phe absorption at the gut-blood barrier. More evidence is available on their work at the blood-brain barrier � Untreated PKU is characterized by severe to profound intellectual disability, seizures, autistic-like behaviors, microcephaly, rashes, hypopigmentation, and a musty body odor (phenylacetic acid)

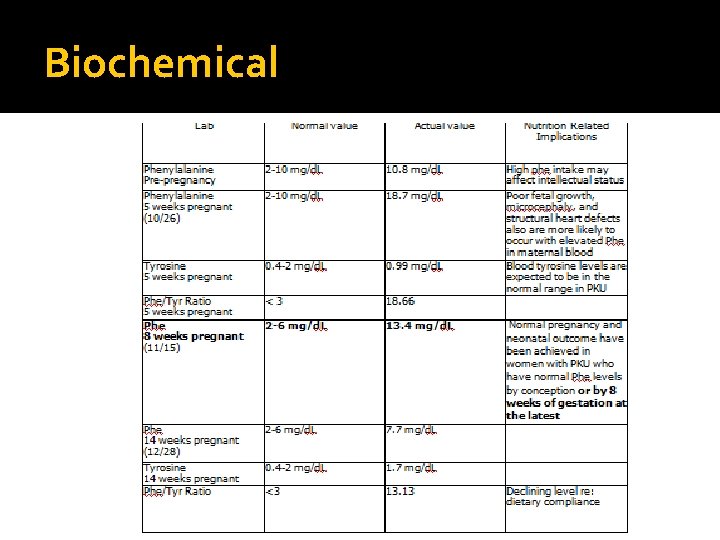
Maternal Phenylketonuria � Pathophysiology: Amplified transport of amino acids across the placenta occurs during pregnancy, thus the fetus is exposed to approximately twice the phe level contained in normal maternal blood � May result in growth retardation, significant psychomotor handicaps, and birth defects in the offspring of unmonitored and untreated pregnancies � Normal pregnancy and neonatal outcome where blood phe concentrations between 120 and 360 mol/L are reached before conception or by 8 weeks of gestation at the latest

Adolescent Pregnancy � Approximately 1 million U. S. adolescents become pregnant every year, accounting for 25% of U. S. pregnancies � Adolescents most likely to get pregnant are those with inadequate nutritional status and unfavorable socio-economic background � Pathophysiology: Competition for nutrients between the growing pregnant adolescent and her fetus � Common complications: Low birth weight, infant anemia, delivery complications, and prematurity � Medical/Nutritional Treatment: Ensure adequate macronutrient and micronutrient intake (Especially Ca, Fe, vitamin A, vitamin C)

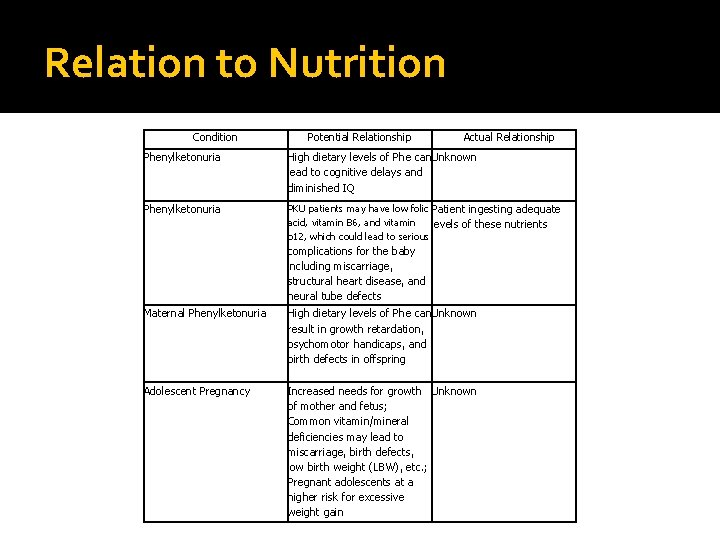
Relation to Nutrition Condition Potential Relationship Actual Relationship Phenylketonuria High dietary levels of Phe can Unknown lead to cognitive delays and diminished IQ Phenylketonuria PKU patients may have low folic Patient ingesting adequate acid, vitamin B 6, and vitamin levels of these nutrients b 12, which could lead to serious complications for the baby including miscarriage, structural heart disease, and neural tube defects Maternal Phenylketonuria High dietary levels of Phe can Unknown result in growth retardation, psychomotor handicaps, and birth defects in offspring Adolescent Pregnancy Increased needs for growth Unknown of mother and fetus; Common vitamin/mineral deficiencies may lead to miscarriage, birth defects, low birth weight (LBW), etc. ; Pregnant adolescents at a higher risk for excessive weight gain

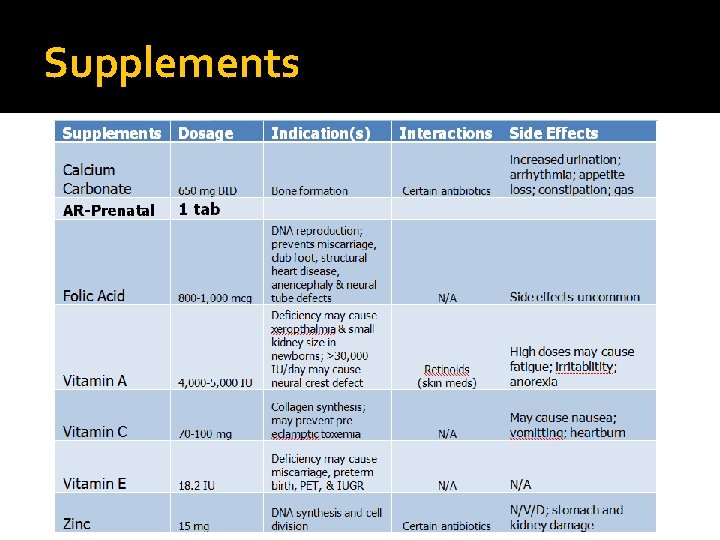
Supplements

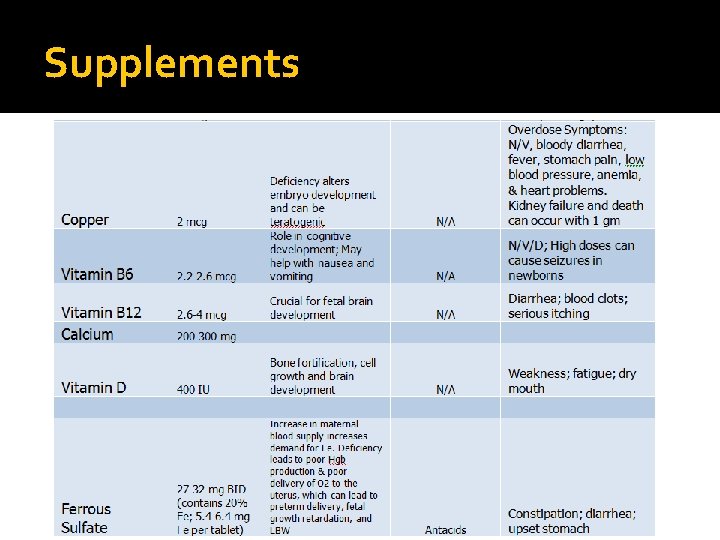
Supplements

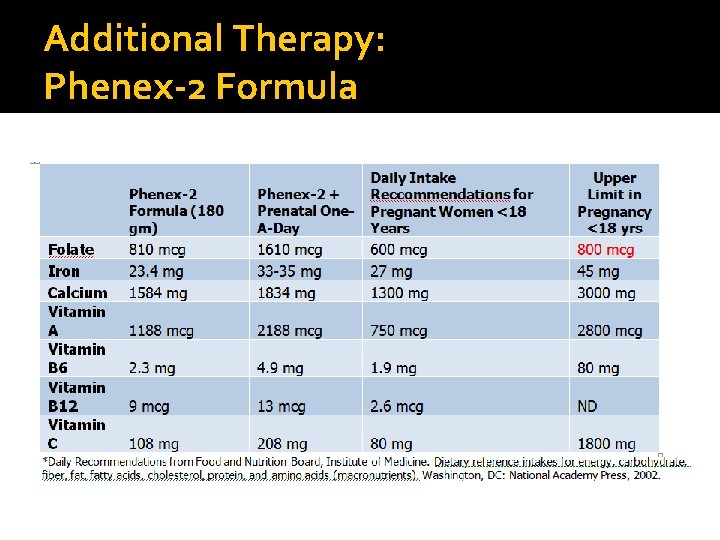
Additional Therapy: Phenex-2 Formula

ADIME

Anthropometrics �Height: 161 cm; 5’ 3” �Current wt: 61. 3 kg (Mechanical Beam Physician Scale) �Admit weight: 63 kg; outlier �Usual weight: 58 kg (pre-pregnancy) �Pre-pregnancy IBW: 52. 3 kg, 110% of IBW �Pre-pregnancy BMI: 22. 6 (within normal limits)

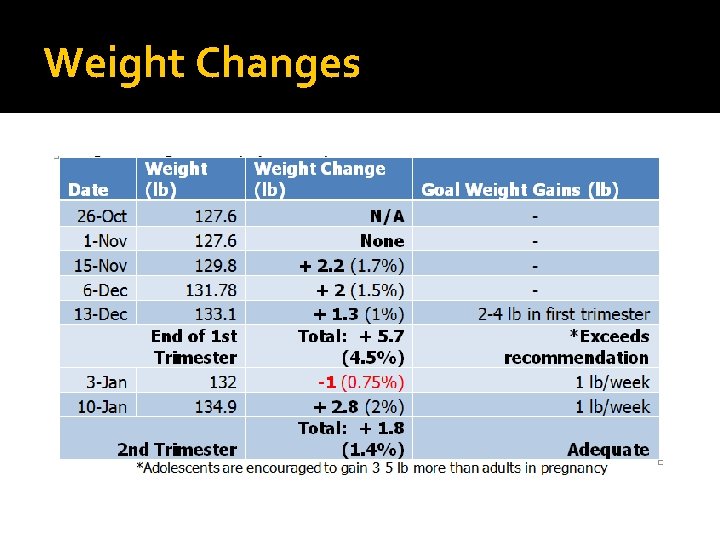
Weight Changes

Biochemical

Clinical �Possible Physical Conditions: Hypopigmentation, musty odor and eczema �Patient has no present physical conditions �Neuropsychological Conditions: Poor memory, decreased attention span, and impaired reasoning �Increased focus and memory noted in past month

Dietary Recall (10/12 -11/15) Before Intervention Breakfast: Pasta (1 cup); 416 mg Phe Strawberries (1 cup); 32 mg Phe Lunch: Chili Cheese Fries – Carl’s Jr. ; 307 mg Phe Or Chilaquiles; 160 mg Phe Dinner: White Rice (1 cup); 188 mg Phe Broccoli (1 cup); 45 mg Phe Snacks: Oreos (2 cookies); 52 mg Phe Potato Chips – Lay’s Original; 93 mg Phe Mc. Donald’s Fries (medium); 152 mg Phe Totals: 1, 819 calories, 47 gm protein, 75 gm fat, 1, 445 mg phe � Average intake of Phe calculated from diet recalls = 800 -1, 217 mg/day � Desired phe intake = 200 -600 mg/day

Estimated Needs � Estimated needs from the ROSS Metabolics Nutrition Support Protocols, 4 th Edition: Second Trimester (<19 years old): Kilocalories: 2, 000 -3, 500 kcals/day Protein: ≥ 75 gm/day Phe: 200 -900 mg/day Tyrosine: 5. 75 -7. 5 gm/day � Estimated needs from the RDA for Normal Pregnancy 25 -30 kcal/kg (BMI wnl) Patient weighs 58 kg= 1450 -1740 kcal/day Increased Needs for 2 nd Trimester: 340 -360 kcal/day Kilocalories: 1, 810 -2, 100 kcals Protein: 1. 1 gm/kg = 64 gm

Estimated Needs Based on References and Clinical Judgement: �Kilocalories: 2350 kcals (40 kcal/kg) �Protein: ≥ 75 gm/day �Phe: < 300 mg/day

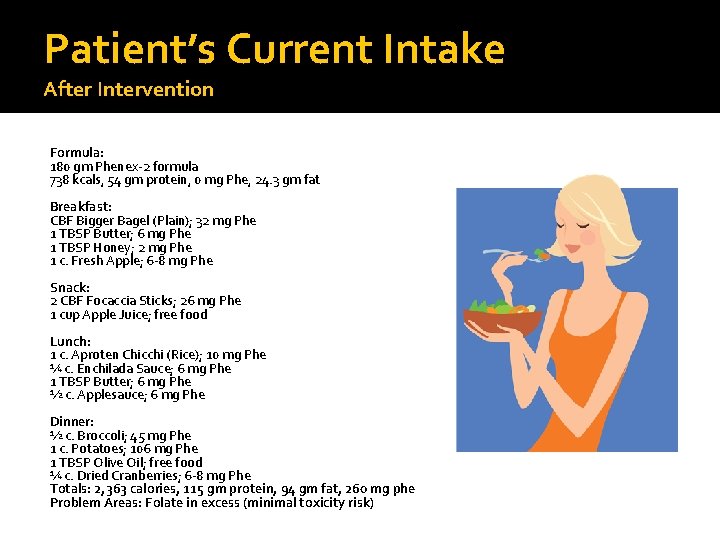
Patient’s Current Intake After Intervention Formula: 180 gm Phenex-2 formula 738 kcals, 54 gm protein, 0 mg Phe, 24. 3 gm fat Breakfast: CBF Bigger Bagel (Plain); 32 mg Phe 1 TBSP Butter; 6 mg Phe 1 TBSP Honey; 2 mg Phe 1 c. Fresh Apple; 6 -8 mg Phe Snack: 2 CBF Focaccia Sticks; 26 mg Phe 1 cup Apple Juice; free food Lunch: 1 c. Aproten Chicchi (Rice); 10 mg Phe ¼ c. Enchilada Sauce; 6 mg Phe 1 TBSP Butter; 6 mg Phe ½ c. Applesauce; 6 mg Phe Dinner: ½ c. Broccoli; 45 mg Phe 1 c. Potatoes; 106 mg Phe 1 TBSP Olive Oil; free food ¼ c. Dried Cranberries; 6 -8 mg Phe Totals: 2, 363 calories, 115 gm protein, 94 gm fat, 260 mg phe Problem Areas: Folate in excess (minimal toxicity risk)

Dietary Assessment of nutritional status �Sub-optimal �Phe levels that remain above 2 -6 mg/d. L �Weight gains that exceed recommended levels � One incidence of unintended weight loss �Patient is achieving optimal levels of macronutrient and micronutrient intakes, and thus is not at risk for deficiencies �Intake of folic acid may exceed tolerable Upper Limit (UL) – minimal toxicity risk

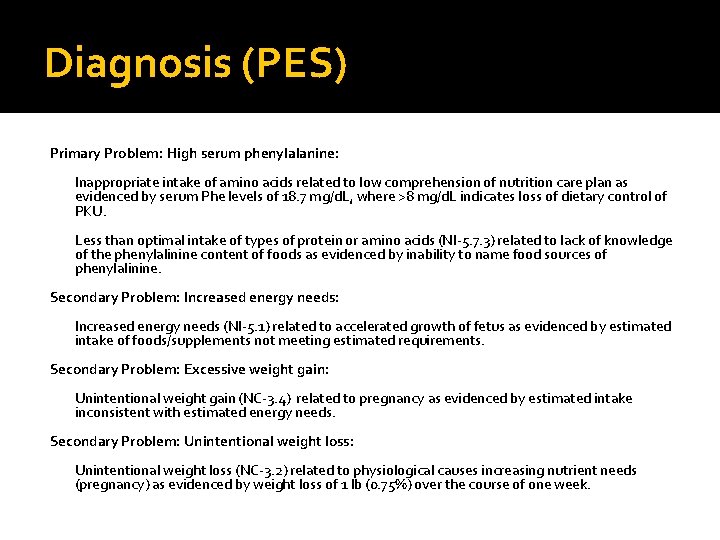
Diagnosis (PES) Primary Problem: High serum phenylalanine: Inappropriate intake of amino acids related to low comprehension of nutrition care plan as evidenced by serum Phe levels of 18. 7 mg/d. L, where >8 mg/d. L indicates loss of dietary control of PKU. Less than optimal intake of types of protein or amino acids (NI-5. 7. 3) related to lack of knowledge of the phenylalinine content of foods as evidenced by inability to name food sources of phenylalinine. Secondary Problem: Increased energy needs (NI-5. 1) related to accelerated growth of fetus as evidenced by estimated intake of foods/supplements not meeting estimated requirements. Secondary Problem: Excessive weight gain: Unintentional weight gain (NC-3. 4) related to pregnancy as evidenced by estimated intake inconsistent with estimated energy needs. Secondary Problem: Unintentional weight loss (NC-3. 2) related to physiological causes increasing nutrient needs (pregnancy) as evidenced by weight loss of 1 lb (0. 75%) over the course of one week.

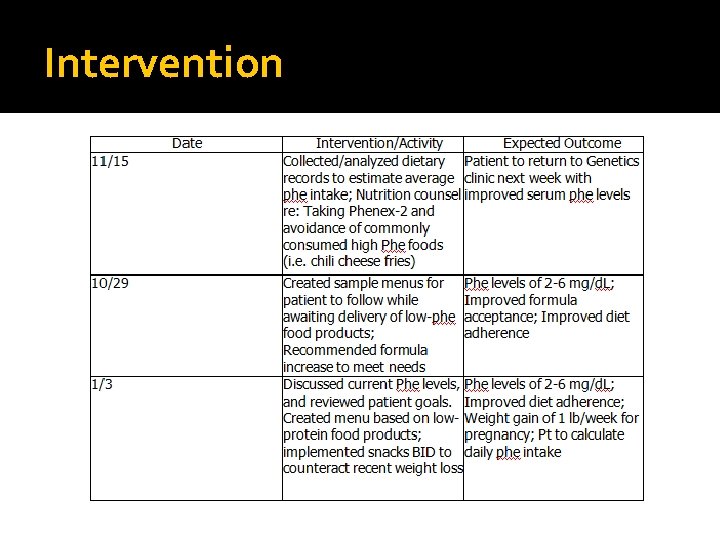
Intervention �Due to the potential teratogenic effects of patient’s elevated serum phe levels, primary intervention includes nutrition education of phenylalanine-containing foods and the potential consequences of poorly managed PKU on offspring �Dietary prescription of phe-free and low-phe containing foods �Menu planning to ensure adequate energy intake/avoid excessive intakes

Intervention Nutrition Goals �Phe levels of 2 -6 mg/d. L �Improved formula acceptance �Improved diet adherence �Weight gain of 2 -4 lb in first trimester �Weight gain of approximately 1 lb/week in second trimester �Patient to calculate daily phe intake

Intervention

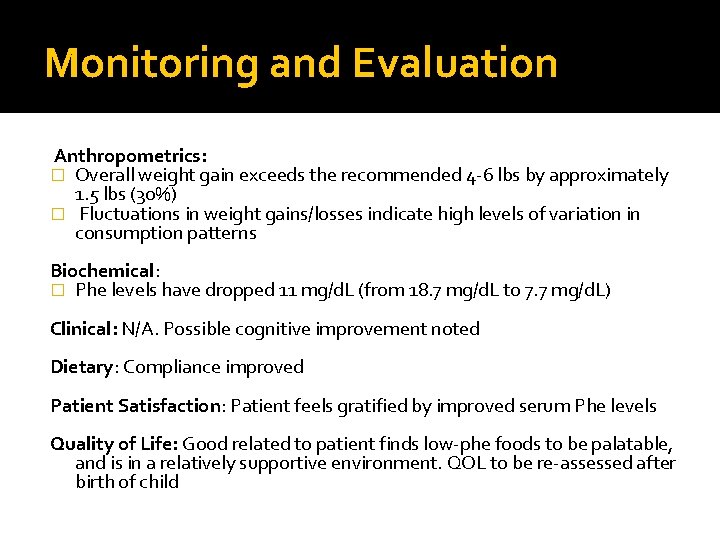
Monitoring and Evaluation Anthropometrics: � Overall weight gain exceeds the recommended 4 -6 lbs by approximately 1. 5 lbs (30%) � Fluctuations in weight gains/losses indicate high levels of variation in consumption patterns Biochemical: � Phe levels have dropped 11 mg/d. L (from 18. 7 mg/d. L to 7. 7 mg/d. L) Clinical: N/A. Possible cognitive improvement noted Dietary: Compliance improved Patient Satisfaction: Patient feels gratified by improved serum Phe levels Quality of Life: Good related to patient finds low-phe foods to be palatable, and is in a relatively supportive environment. QOL to be re-assessed after birth of child

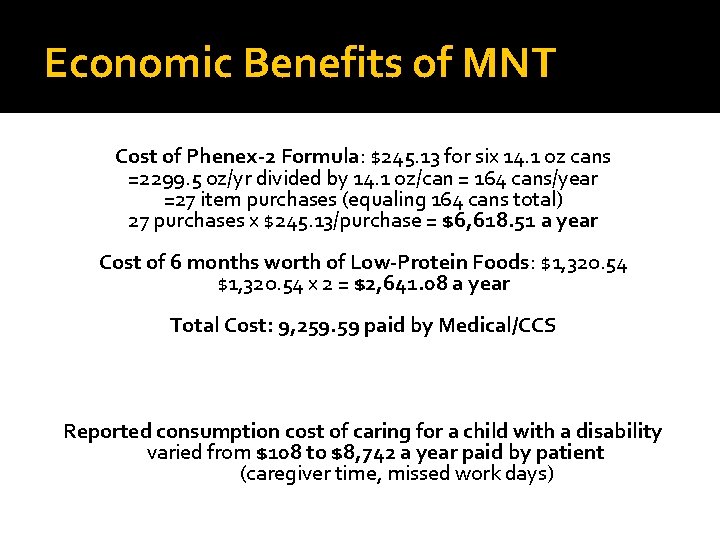
Economic Benefits of MNT Cost of Phenex-2 Formula: $245. 13 for six 14. 1 oz cans =2299. 5 oz/yr divided by 14. 1 oz/can = 164 cans/year =27 item purchases (equaling 164 cans total) 27 purchases x $245. 13/purchase = $6, 618. 51 a year Cost of 6 months worth of Low-Protein Foods: $1, 320. 54 x 2 = $2, 641. 08 a year Total Cost: 9, 259. 59 paid by Medical/CCS Reported consumption cost of caring for a child with a disability varied from $108 to $8, 742 a year paid by patient (caregiver time, missed work days)

Literature Reviews Spronsen, F. Large neutral amino acids in the treatment of PKU: from theory to practice. J Inherit Metab Dis. 2010 December; 33(6): 671– 676. Discussed the use of Large Neutral Amino Acid (LNAA) supplementation to compete with phenylalanine absorption at the blood-brain barrier and gut-blood barrier. Methods discussed here were used to understand patient’s medication history. � Saal, H. Maternal Phenylketonuria. J. of the American Academy of Pediatrics. 2008; 122: 445 -449. Outlined the teratogenic effects of poorly managed maternal phenylketonuria; discussed newborn screening of phenylketonuria in the United States; provided reference serum phenylalanine levels for normal neonatal outcome. Information obtained from this article was used in patient intervention. � Koch, R. Psychosocial issues and outcomes in maternal pku. J. of Molecular Genetics and Metabolism, 2010; 99: 568 -574. Discussed the factors affecting dietary adherence including poor access to medical care, practical difficulties implementing the diet, financial constraints, demographics, and psychosocial issues. Used to gain perspective on patient’s situation, and to counsel patient in a culturally sensitive manner. �

My Role & Feedback

My Role � Obtained consent from physician and team to assume responsibility for the nutritional care of the patient � Overviewed patient condition and intervention strategies with team � Researched and reported target phe levels, phe-containing foods, and teratogenic effects of poorly managed PKU to hospital RD’s � Analyzed patient dietary recalls and calculated average phe intakes � Created low-phenylalanine meal plans � Advocated for lab draws to MD � Charted patient note into HUCLA database

Changes I Would Have Made With the opportunity to re-do this assignment, I would: �Find my patient sooner �Choose an inpatient for the sake of data acquisition

Questions?

Sources Acosta, P. B. (2001). Disorders of amino acid metabolism. In The Ross Metabolic Formula System Nutrition Support Protocols (4 th ed. ). Columbus, Ohio: Abbott Laboratories. Anderson, D. , & Dumont, S. (2007). The personal costs of caring for a child with a disability: A review of the literature. Public Health Rep, 122, 3 -16. Retrieved from http: //www. ncbi. nlm. nih. gov/pmc/articles/PMC 1802121/ (2007). Autosomal recessive inheritance. (2007). [Web Graphic]. Retrieved from http: //www. actionbioscience. org/genomic/siegal. html (201). Biochemistry of phenylketonuria. (201). [Print Photo]. Retrieved from http: //www. virtualmedstudent. com/links/metabolism/phenylketonuria. html Escott-Stump, S. (2012). Nutrition and diagnosis-related care. (7 th ed. , pp. 204 -207). Baltimore, MD: Lippincott Williams & Wilkins. Mahan, K. , Escott-Stump, S. , & Raymond, J. L. (2012). Nutrition in pregnancy and lactation. (13 th ed. , pp. 353 -367). Elsevier Inc. Mahan, K. , Escott-Stump, S. , & Raymond, J. L. (2012). Medical nutrition therapy for inherited metabolic disorders. (13 th ed. , pp. 353 -367). Elsevier Inc.
 Intern
Intern Cal poly pomona database
Cal poly pomona database Cal poly pomona software engineering
Cal poly pomona software engineering Cal poly dashboard
Cal poly dashboard Cal poly pomona registrar office
Cal poly pomona registrar office Cal poly quarter or semester
Cal poly quarter or semester Beth chance
Beth chance Cal poly triathlon
Cal poly triathlon Cal poly bike lockers
Cal poly bike lockers Frank owen cal poly
Frank owen cal poly Ortak portal
Ortak portal Quantitative analysis cal poly
Quantitative analysis cal poly Cal poly database
Cal poly database Construction management cal poly
Construction management cal poly Evd deloitte
Evd deloitte Cal poly academic personnel
Cal poly academic personnel Beth chance
Beth chance Cal poly bike lockers
Cal poly bike lockers Cal and cal
Cal and cal Broncoskopi
Broncoskopi Gruntvig
Gruntvig Rávezető inger
Rávezető inger Szignáldetekciós elmélet
Szignáldetekciós elmélet Kétpontküszöb térkép
Kétpontküszöb térkép Preposisjonsuttrykk
Preposisjonsuttrykk Inger nordby grønn
Inger nordby grønn Inger kraav
Inger kraav Stlan
Stlan 1 es típusú diabetes
1 es típusú diabetes Inger merete terp
Inger merete terp Inger james
Inger james Inger langseth
Inger langseth Så tenner vi et lys i kveld tekst hagerup
Så tenner vi et lys i kveld tekst hagerup Inger lise kaldhol
Inger lise kaldhol Pomona pd news
Pomona pd news Pomona workday
Pomona workday Jared gelb
Jared gelb Struct in assembly
Struct in assembly Dietetic internships in georgia
Dietetic internships in georgia Dietetic support worker
Dietetic support worker Keiser university distance dietetic internship
Keiser university distance dietetic internship Texas student dietetic association
Texas student dietetic association





























































