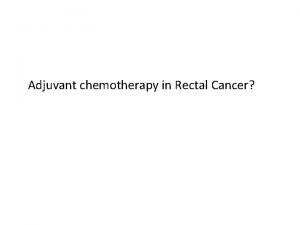Adjuvant chemotherapy in patients with resected NSCLC Current


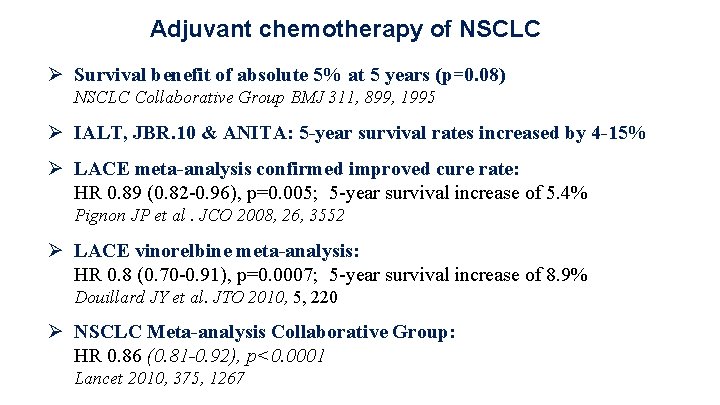






- Slides: 27

Adjuvant chemotherapy in patients with resected NSCLC Current status & perspectives Robert Pirker, MD Vienna, Austria Novel Clinical Strategies in NSCLC Policlinico Umberto I, Rome May 9 -10, 2019

Conflict of Interest Honoraria for Advisory Board/Consulting Astra. Zeneca Boehringer Ingelheim Jansen Oncology Takeda Speaker‘s fee Astra. Zeneca Boehringer Ingelheim Honoraria for Data Safety Monitoring Board Gedeon Richter Genmab Merck Sharp Dohme Regeneron
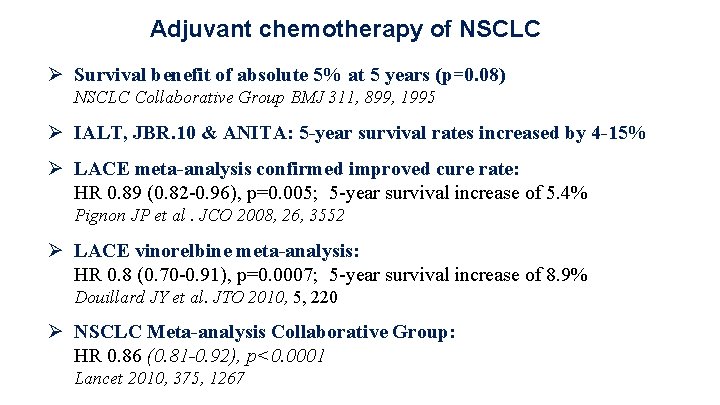
Adjuvant chemotherapy of NSCLC Ø Survival benefit of absolute 5% at 5 years (p=0. 08) NSCLC Collaborative Group BMJ 311, 899, 1995 Ø IALT, JBR. 10 & ANITA: 5 -year survival rates increased by 4 -15% Ø LACE meta-analysis confirmed improved cure rate: HR 0. 89 (0. 82 -0. 96), p=0. 005; 5 -year survival increase of 5. 4% Pignon JP et al. JCO 2008, 26, 3552 Ø LACE vinorelbine meta-analysis: HR 0. 8 (0. 70 -0. 91), p=0. 0007; 5 -year survival increase of 8. 9% Douillard JY et al. JTO 2010, 5, 220 Ø NSCLC Meta-analysis Collaborative Group: HR 0. 86 (0. 81 -0. 92), p<0. 0001 Lancet 2010, 375, 1267

LACE Meta-Analysis Pignon JP et al. JCO 2008, 26, 3552 4584 patients: IALT, JBR. 10, ANITA, ALPI-EORTC & BLT Overall survival Disease-free survival HR 0. 89 (0. 82 -0. 96), p=0. 005 5 -y survival increased by 5. 4% ± 1. 6% HR 0. 8 (0. 78 -0. 9), p<0. 001

Adjuvant Chemotherapy in resected NSCLC Recommendations for daily practice • Patients with - stage II & III, selected IB (6 th TNM classification) - good performance status - adequate organ functions - rapid postoperative recovery - informed consent • Cisplatin-based chemotherapy - preferably cisplatin/vinorelbine, based on phase III trials - 4 cycles - begin 4 -8 weeks after surgery • Advice & support for smoking cessation

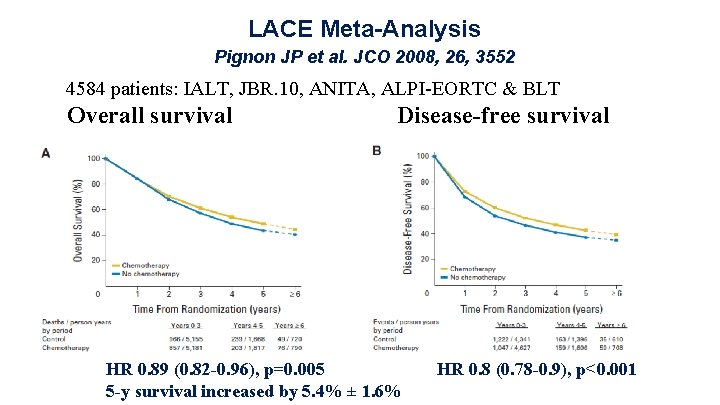
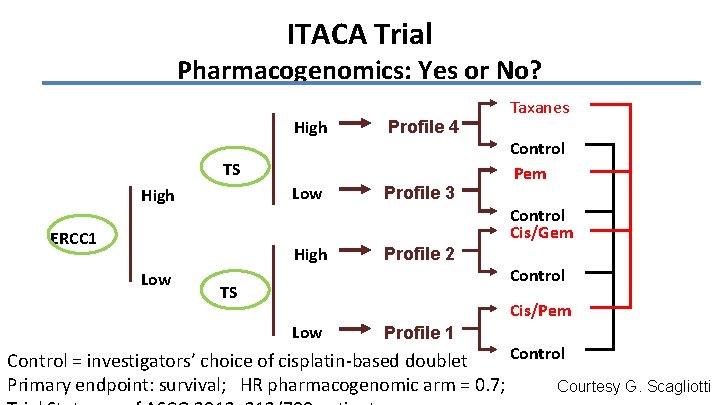
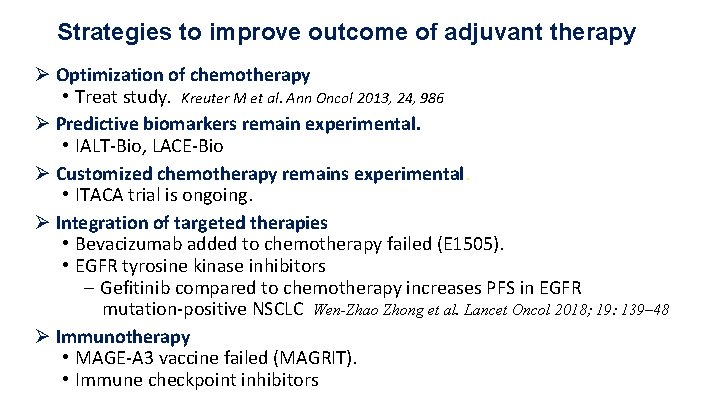
Strategies to improve outcome of adjuvant therapy Ø Optimization of chemotherapy • Treat study. Kreuter M et al. Ann Oncol 2013, 24, 986 Ø Predictive biomarkers remain experimental. • IALT-Bio, LACE-Bio Ø Customized chemotherapy remains experimental. • ITACA trial is ongoing.

ITACA Trial Pharmacogenomics: Yes or No? High Profile 4 Low Profile 3 TS High ERCC 1 High Low Taxanes Control Pem Control Cis/Gem Profile 2 TS Control Cis/Pem Low Profile 1 Control = investigators’ choice of cisplatin-based doublet Primary endpoint: survival; HR pharmacogenomic arm = 0. 7; Courtesy G. Scagliotti

Strategies to improve outcome of adjuvant therapy Ø Optimization of chemotherapy • Treat study. Kreuter M et al. Ann Oncol 2013, 24, 986 Ø Predictive biomarkers remain experimental. • IALT-Bio, LACE-Bio Ø Customized chemotherapy remains experimental. • ITACA trial is ongoing. Ø Integration of targeted therapies • Bevacizumab added to chemotherapy failed (E 1505). • EGFR tyrosine kinase inhibitors

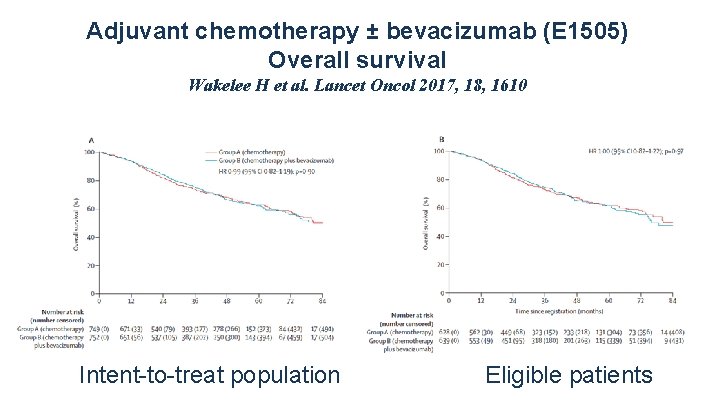
Adjuvant chemotherapy ± bevacizumab (E 1505) Overall survival Wakelee H et al. Lancet Oncol 2017, 18, 1610 Intent-to-treat population Eligible patients

EGFR TKIs as adjuvant therapy in resected NSCLC Ø Gefitinib in unselected patients (NCIC CTG BR 19 Study) Goss GD et al. JCO 2013, 31, 3320 Ø Erlotinib in patients with EGFR-positive tumors (RADIANT) Kelly K et al. JCO 2015, 33, 400 Ø Patients with EGFR mutation-positive tumors • Subgroup analyses (NCIC CTG BR 19; RADIANT) • Icotinib studies • SELECT (Phase 2) Pennell NA et al. ASCO 2014 • Phase 3 trials ADJUVANT (CTONG 1104; NCT 01405079) WJOG 6410 L ALCHEMIST ADAURA

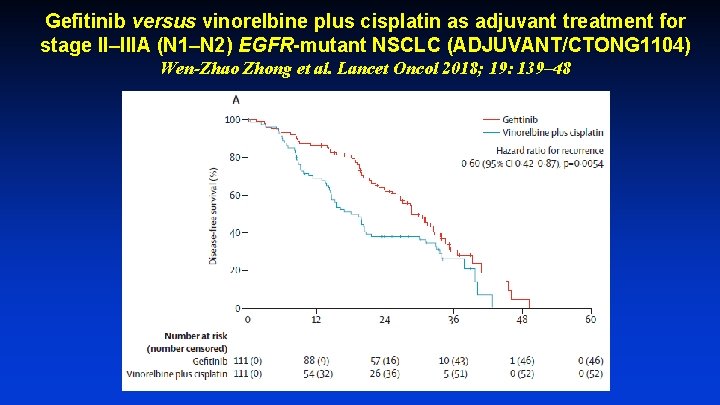
Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II–IIIA (N 1–N 2) EGFR-mutant NSCLC (ADJUVANT/CTONG 1104) Wen-Zhao Zhong et al. Lancet Oncol 2018; 19: 139– 48

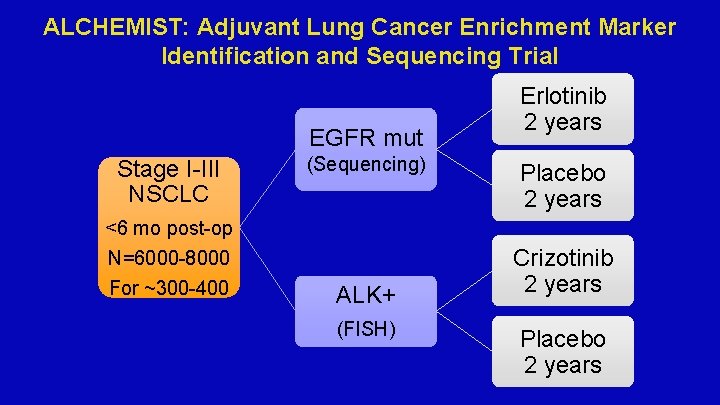
ALCHEMIST: Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trial EGFR mut Stage I-III NSCLC <6 mo post-op N=6000 -8000 For ~300 -400 (Sequencing) ALK+ (FISH) Erlotinib 2 years Placebo 2 years Crizotinib 2 years Placebo 2 years

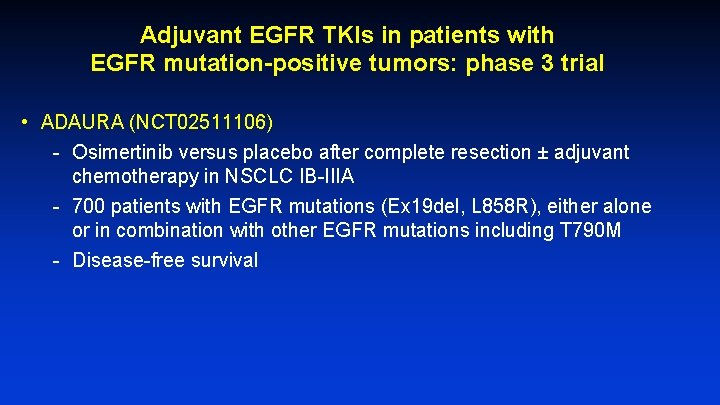
Adjuvant EGFR TKIs in patients with EGFR mutation-positive tumors: phase 3 trial • ADAURA (NCT 02511106) - Osimertinib versus placebo after complete resection ± adjuvant chemotherapy in NSCLC IB-IIIA - 700 patients with EGFR mutations (Ex 19 del, L 858 R), either alone or in combination with other EGFR mutations including T 790 M - Disease-free survival

Strategies to improve outcome of adjuvant therapy Ø Optimization of chemotherapy • Treat study. Kreuter M et al. Ann Oncol 2013, 24, 986 Ø Predictive biomarkers remain experimental. • IALT-Bio, LACE-Bio Ø Customized chemotherapy remains experimental. • ITACA trial is ongoing. Ø Integration of targeted therapies • Bevacizumab added to chemotherapy failed (E 1505). • EGFR tyrosine kinase inhibitors - Gefitinib compared to chemotherapy increases PFS in EGFR mutation-positive NSCLC Wen-Zhao Zhong et al. Lancet Oncol 2018; 19: 139– 48 Ø Immunotherapy • MAGE-A 3 vaccine failed (MAGRIT). • Immune checkpoint inhibitors

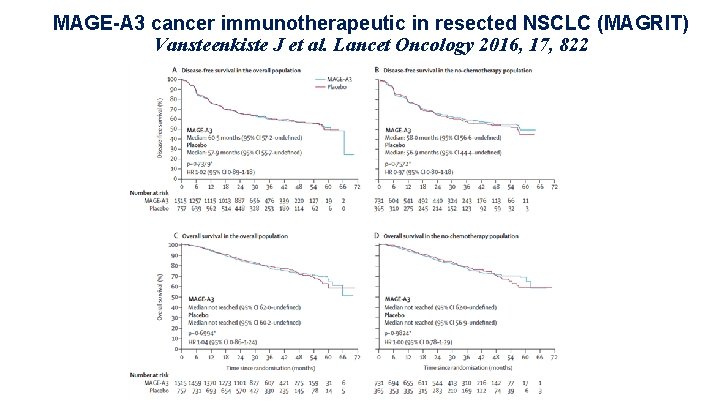
MAGE-A 3 cancer immunotherapeutic in resected NSCLC (MAGRIT) Vansteenkiste J et al. Lancet Oncology 2016, 17, 822

Lessons learnt from MAGRIT Ø Large phase 3 trials can be done in the adjuvant setting. Ø It is difficult to improve outcome of patients with resected NSCLC. Ø Phase 2 trials often do not predict phase 3 results. Ø The development of the MAGE-A 3 cancer immunotherapeutic was discontinued. Ø Cisplatin-based doublets remain standard of care.

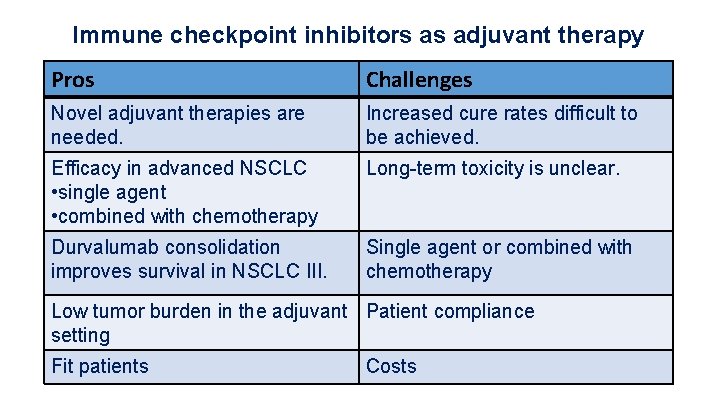
Immune checkpoint inhibitors as adjuvant therapy Pros Challenges Novel adjuvant therapies are needed. Increased cure rates difficult to be achieved. Efficacy in advanced NSCLC • single agent • combined with chemotherapy Long-term toxicity is unclear. Durvalumab consolidation improves survival in NSCLC III. Single agent or combined with chemotherapy Low tumor burden in the adjuvant Patient compliance setting Fit patients Costs

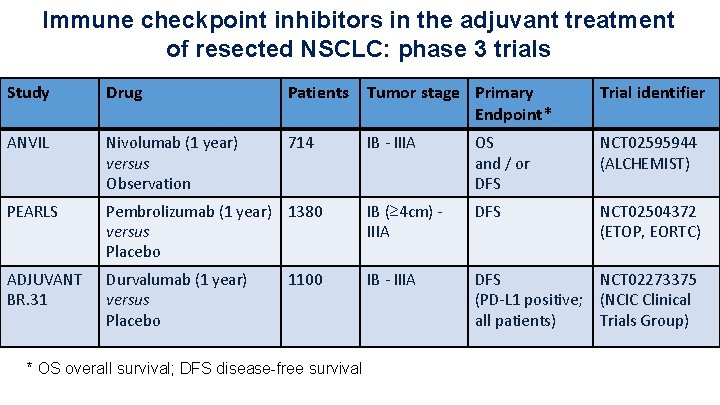
Immune checkpoint inhibitors in the adjuvant treatment of resected NSCLC: phase 3 trials Study Drug Patients Tumor stage Primary Endpoint* Trial identifier ANVIL Nivolumab (1 year) versus Observation 714 IB - IIIA OS and / or DFS NCT 02595944 (ALCHEMIST) PEARLS Pembrolizumab (1 year) 1380 versus Placebo IB (≥ 4 cm) IIIA DFS NCT 02504372 (ETOP, EORTC) ADJUVANT BR. 31 Durvalumab (1 year) versus Placebo IB - IIIA DFS NCT 02273375 (PD-L 1 positive; (NCIC Clinical all patients) Trials Group) 1100 * OS overall survival; DFS disease-free survival

Challenges of adjuvant therapy trials in resected NSCLC Ø Large sample sizes are required because of moderate benefits. Ø Long trial duration and high costs Ø Selection of drugs is based on efficacy in the advanced setting. Ø Most appropriate endpoint: Overall survival, disease-free survival (progression-free survival), time-to-disease progression Ø Novel trial designs • Focus on patients with high risk of recurrence • Surrogate endpoints for survival • Residual disease assessed by circulating tumor DNA * * Dasari A et al. JCO 2018, 36, 3437

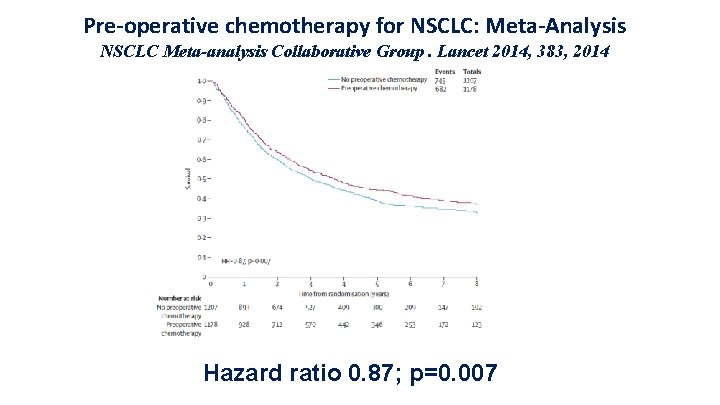
Pre-operative chemotherapy for NSCLC: Meta-Analysis NSCLC Meta-analysis Collaborative Group. Lancet 2014, 383, 2014 Hazard ratio 0. 87; p=0. 007

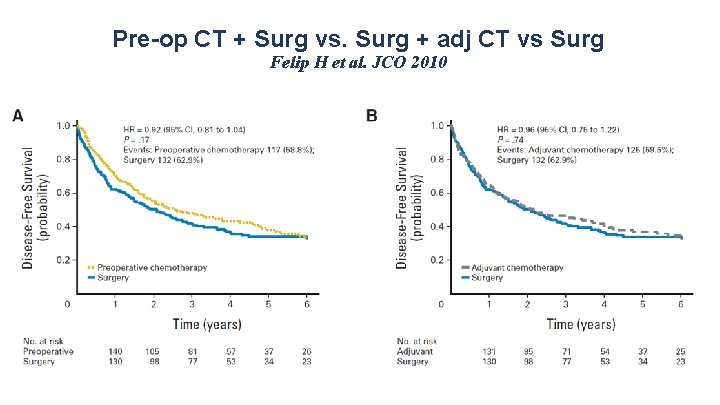
Pre-op CT + Surg vs. Surg + adj CT vs Surg Felip H et al. JCO 2010

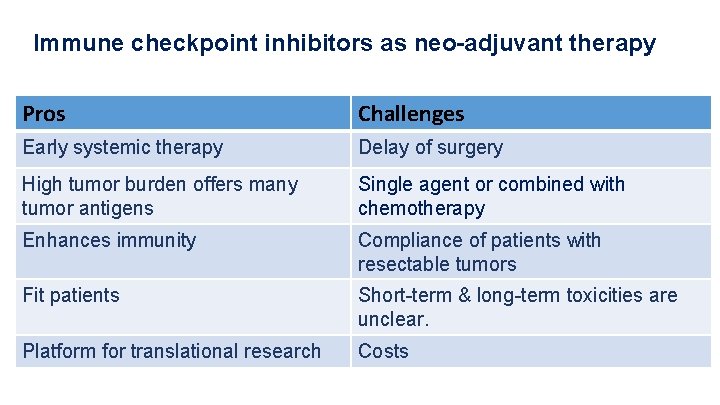
Immune checkpoint inhibitors as neo-adjuvant therapy Pros Challenges Early systemic therapy Delay of surgery High tumor burden offers many tumor antigens Single agent or combined with chemotherapy Enhances immunity Compliance of patients with resectable tumors Fit patients Short-term & long-term toxicities are unclear. Platform for translational research Costs

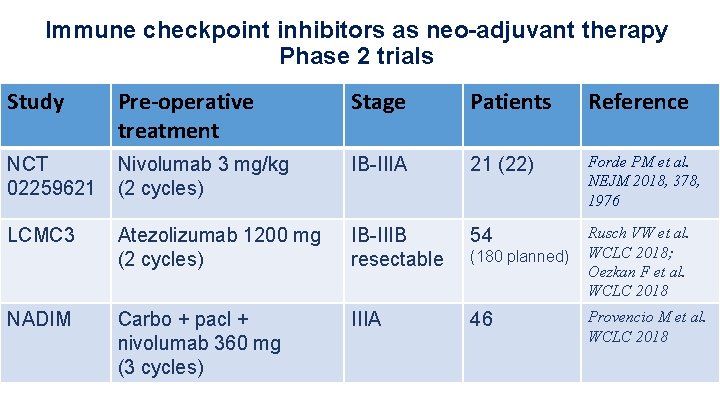
Immune checkpoint inhibitors as neo-adjuvant therapy Phase 2 trials Study Pre-operative treatment Stage Patients Reference NCT 02259621 Nivolumab 3 mg/kg (2 cycles) IB-IIIA 21 (22) Forde PM et al. NEJM 2018, 378, 1976 LCMC 3 Atezolizumab 1200 mg (2 cycles) IB-IIIB resectable 54 (180 planned) Rusch VW et al. WCLC 2018; Oezkan F et al. WCLC 2018 Carbo + pacl + nivolumab 360 mg (3 cycles) IIIA 46 Provencio M et al. WCLC 2018 NADIM

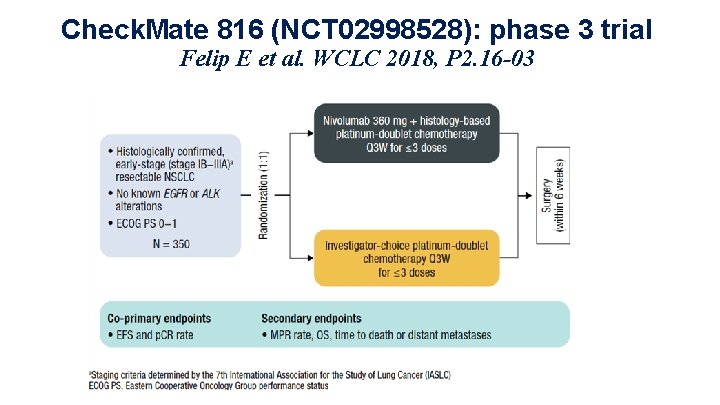
Check. Mate 816 (NCT 02998528): phase 3 trial Felip E et al. WCLC 2018, P 2. 16 -03

Adjuvant versus pre-operative chemotherapy of NSCLC Ø Adjuvant chemotherapy is preferred. No delay in curative surgery Patients want cancers to be removed as quickly as possible. More tumor material for histological & molecular analyses More accurate tumor stage Ø Pre-operative chemotherapy is an option in selected patients. Patients with marginally resectable tumors Patients with large tumors Patients with more advanced tumor stage Waiting list for surgery

Adjuvant therapy of resected NSCLC Summary • Adjuvant chemotherapy with cisplatin-based doublet is established as standard. • Predictive biomarkers & customized chemotherapy remain experimental. • Several major trials failed to improve outcome. • E 1505 (bevacizumab), BR 19 & RADIANT (EGFR TKIs), MAGRIT (MAGE-A 3 vaccine) • Among EGFR mutation-positive patients, gefinitib improved disease-free survival and further trials are ongoing. • Immune checkpoint inhibitors are evaluated within phase 3 trials. • ANVIL, PEARLS, CCTG ADJUVANT • Induction (neo-adjuvant) therapy is an option for selected patients.

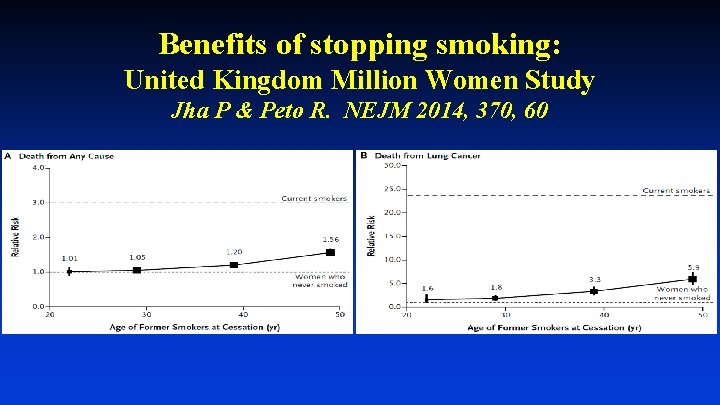
Benefits of stopping smoking: United Kingdom Million Women Study Jha P & Peto R. NEJM 2014, 370, 60
 Adjuvant nsclc
Adjuvant nsclc Adjuvant nsclc
Adjuvant nsclc Nsclc
Nsclc Gondor adjuvant
Gondor adjuvant Adjuvant neoadjuvant palliative
Adjuvant neoadjuvant palliative Chemotherapy
Chemotherapy Icd 9 code for oral thrush
Icd 9 code for oral thrush Chemotherapy
Chemotherapy Principles of chemotherapy
Principles of chemotherapy Common assessment framework
Common assessment framework General principles of chemotherapy
General principles of chemotherapy 4ac 4t chemotherapy
4ac 4t chemotherapy Bsa calculation formula for chemotherapy
Bsa calculation formula for chemotherapy Slideplayer
Slideplayer A balanced delta connected load having an impedance 20-j15
A balanced delta connected load having an impedance 20-j15 Kcl mesh analysis
Kcl mesh analysis Drift current and diffusion current
Drift current and diffusion current Diffusion current formula
Diffusion current formula Energy band diagram of pn junction diode
Energy band diagram of pn junction diode Line current and phase current
Line current and phase current The value of vgs that makes id approximately zero is the
The value of vgs that makes id approximately zero is the Why must the electrode holder be correctly sized?
Why must the electrode holder be correctly sized? Lesson 4 three-phase motors
Lesson 4 three-phase motors Balanced delta delta connection
Balanced delta delta connection Hazard based safety engineering
Hazard based safety engineering Drift current density unit
Drift current density unit Management of patients with neurologic trauma
Management of patients with neurologic trauma Malaysian safety goals
Malaysian safety goals