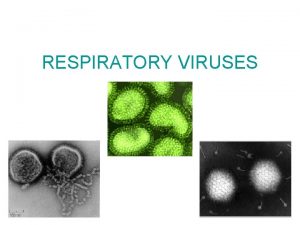
Adenovirus vectors Gene Therapy2008 Virology 1 n Infectivity






















- Slides: 34

Adenovirus vectors Gene Therapy-2008

Virology 1 n Infectivity n n Viral particles n n How do you count them? Quality control n n How do you measure it? Why is it important? Host range n What determines it?

Infectivity Measure by infecting dilutions of the virus stock on permissive (e. g. 293) cells. n Plaque assay (1 -2 weeks) n n n Rapid titer (2 days) n n Measure by immunostaining viral antigens Endpoint dilution n n Foci of infection TCID 50 FACS after immunostaining viral antigens

Infectivity (continued) n Infectivity is variable and dependent upon: Passage number and health of cell substrate n Time of virus adsorption n Volume of virus adsorption n

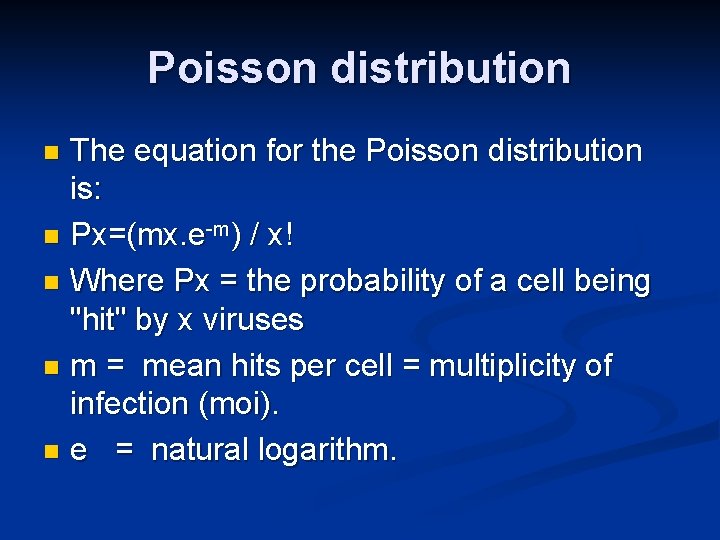
Poisson distribution The equation for the Poisson distribution is: n Px=(mx. e-m) / x! n Where Px = the probability of a cell being "hit" by x viruses n m = mean hits per cell = multiplicity of infection (moi). n e = natural logarithm. n

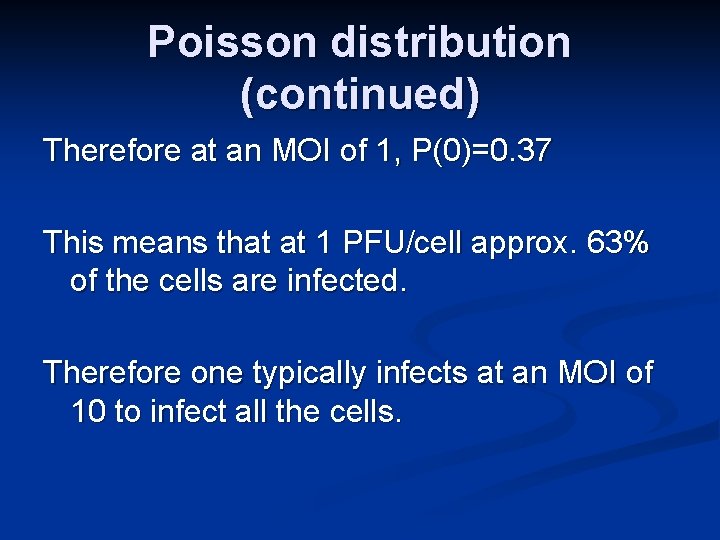
Poisson distribution (continued) Therefore at an MOI of 1, P(0)=0. 37 This means that at 1 PFU/cell approx. 63% of the cells are infected. Therefore one typically infects at an MOI of 10 to infect all the cells.

Virus purification n Ultracentrifugation n Rate zonal n Dependent upon sedimentation velocity (size and shape) of virus particle. Underutilized. n Isopycnic n Dependent on equilibrium density of virus particle. n Cs. Cl typical n Buoyant density of adenovirus is 1. 35 g/ml n Determine by refractometry

Particle number Measured by A 260 n Quantitative PCR n Is invariant and therefore a good number to use when infecting cells n Particle/PFU ratio typically 10 -100 n n Doesn’t necessarily infect amount of “dead” virus in preparation

Quality Control Replication competent adenovirus n Sterility n Titer n Seed lot system n Virus n Cells n

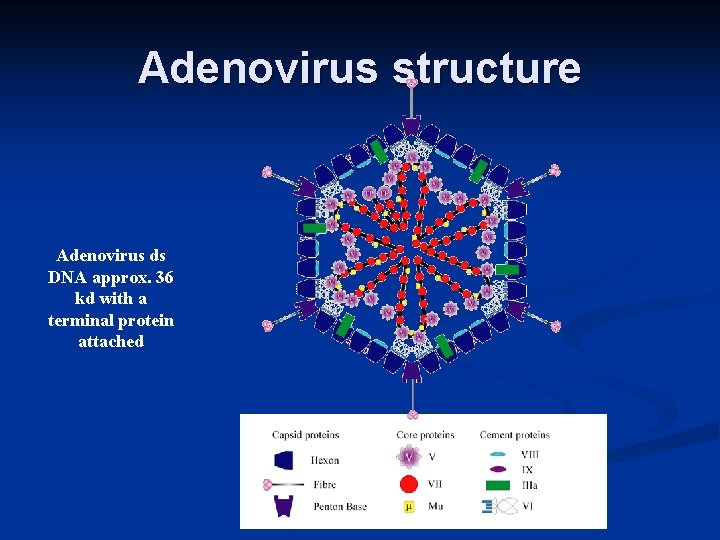
Adenovirus structure Adenovirus ds DNA approx. 36 kd with a terminal protein attached

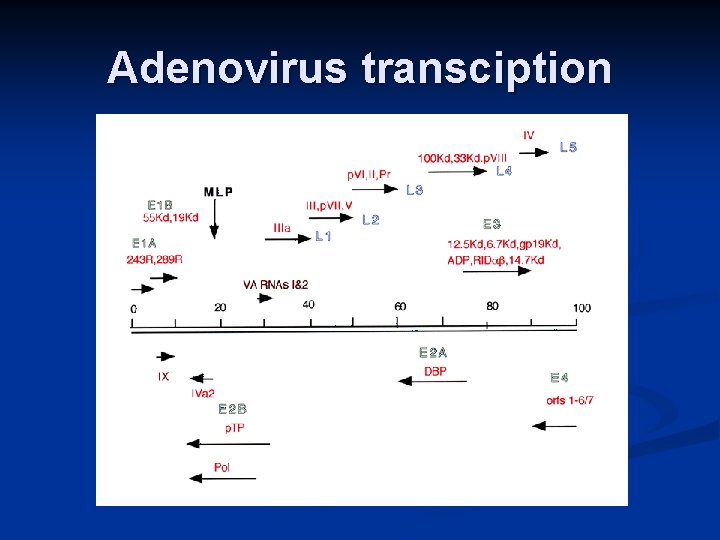
Adenovirus transciption

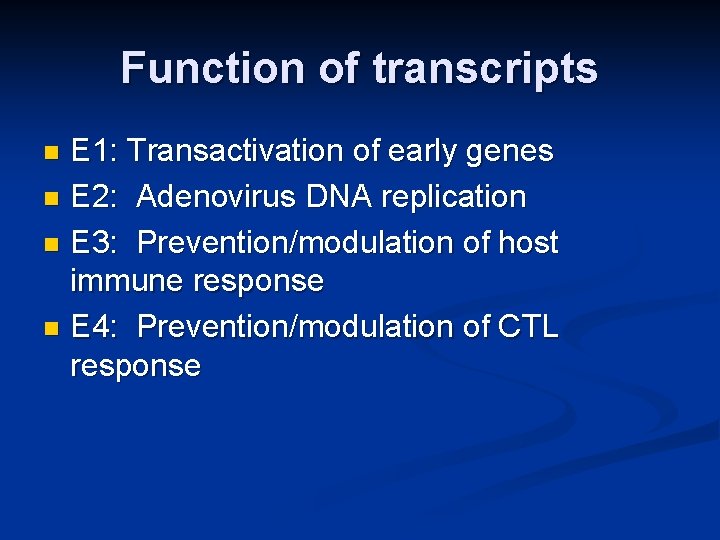
Function of transcripts E 1: Transactivation of early genes n E 2: Adenovirus DNA replication n E 3: Prevention/modulation of host immune response n E 4: Prevention/modulation of CTL response n

Adenovirus receptors n Fiber Spike of the virus n Binds coxsachie-adenovirus receptor (CAR) in sub-group C n Binds CD 46 in subgroup B n n Penton base n Co-receptor-binds RGD (integrin) for internalization of virion

Adenovirus Receptors (continued) n Receptors are not fully understood In vivo distribution doesn’t match receptor distribution n Other serotypes (e. g. type 37, ocular) may have other receptors (e. g. sialic acid) n Certain serotypes bind clotting factors n Small molecules may be involved in adenovirus host range (adamantine). n

Adenovirus Host Range Adenovirus infects humans n No other good animal model n Chimpanzee n Monkeys are non-permissive n Mouse model is poor because most murine cells lack viral receptors n Host range can be enhanced by using lipids containing polyamines (see Davis, Human Gene Therapy, 2005) n

Subgroups, serotypes, and disease Adenovirus serotypes and clinical disease

Adenovirus in gene therapy

Initial safety and immunogenicity Vaccine (1992) studies of an oral recombinant adenohepatitis B vaccine Carol 0. Tacket*i, Genevieve Losonsky*, Michael D. Lubeck? , Alan R. Davis? , Satoshi Mizutani+, Gary Horwith+, Paul Hung+, Robert Edelman* and Myron M. Levine* Orally administered adenovirus may be a useful vaccine carrier of cloned antigens of other pathogens. A recombinant adenohepatitis vaccine Wy-Ad 7 HZ 6 -I, which expressed hepatitis B surface antigen and contained a large deletion in early region 3 (E 3), was constructed and studied in humans. Volunteers received Wy-Ad 7 HZ 6 -I (n = 3), adenovirus type 7 vaccine (n = 3) or placebo (n = 3). Recipients of Wy-Ad 7 HZ 6 -1 shed less vaccine virus in the stool for a shorter period and had a lower titre of anti-adenovirus type 7 antibodies than recipients of the adenovirus 7 vaccine. None of the three Wy-Ad 7 HZ 6 -I vaccinees developed antibody to hepatitis B surface antigen after this one dose primary immunization regimen. The E 3 region may be required for optimal enteric growth of adenovirus-vectored vaccines. *Center for Vaccine Development, Department of Medicine, University of Maryland School of Medicine, Baltimore, MD 21201, USA. +Wyeth-Ayerst Research, Philadelphia, PA 19101, USA. *To whom correspondence should be addressed. (Received 10 December 1991; revised 7 February 1992; accepted 10 February 1992)

Adenovirus-HIV vaccine Title: Recombinant adenovirus vaccines Document Type and Number: United States Patent 20040170647 Kind Code: A 1 AR Davis, MD Lubeck, RJ Natuk, PK Chanda, Abstract: This invention provides a method of protecting a primate from an infectious organism by stimulating the production of antibodies or cell mediated immunity to the infectious organism which comprises administering to said primate intranasally, intramuscularly, or subcutaneously, live recombinant adenoviruses in which the virion structural protein is unchanged from that in the native adenovirus from which the recombinant adenovirus is produced, and which contain the gene coding for the antigen corresponding to said antibodies or inducing said cell mediated immunity. Preferably, the infectious organism is HIV and the primate is a human.

June 1997 Volume 3 Number 6 p 651 Nature Medicine Long-term protection of chimpanzees against highdose HIV-1 challenge induced by immunization Michael D. Lubeck 1, Robert Natuk 1, Maria Myagkikh 2, Narender Kalyan 1, Kristine Aldrich 2, Faruk Sinangil 3, Shabnam Alipanah 2, Shri C. S. Murthy 1, Pranab K. Chanda 1, Stephen M. Nigida, Jr. 4, Phillip D. Markham 5, Susan Zolla-Pazner 6, Kathy Steimer 3, Mark Wade 1, Marvin S. Reitz, Jr. 2, Larry O. Arthur 4, Satoshi Mizutani 1, Alan Davis 1, Paul P. Hung 1, Robert C. Gallo 2, Jorg Eichberg 1, Marjorie Robert-Guroff 2 A combination AIDS vaccine approach consisting of priming with adenovirus-HIV-1 MN gp 160 recombinants followed by boosting with HIV-1 SF 2 gp 120 was evaluated in chimpanzees. Long-lasting protection, requiring only three immunizations, was achieved against a lowdose challenge with the SF 2 strain of HIV-1 and a subsequent high-dose SF 2 challenge administered 1 year later without an intervening boost. Notably, neutralizing antibody responses against both clinical and laboratory isolates developed in three chimpanzees and persisted until the time of high-dose challenge. The possibility that cytotoxic T-lymphocytes contribute to low-dose protection of a chimpanzee lacking neutralizing antibodies is suggested. Our results validate the live vector priming/subunit booster approach and should stimulate interest in assessing this combination vaccine approach in humans. 1 Wyeth-Ayerst Research, 145 King of Prussia Road, Radnor, PA 19087, USA, 2 Laboratory of Tumor Cell Biology, National Cancer Institute, Building 37, Room 6 B 03, National Institutes of Health, Bethesda, MD 20892 -4255, USA, 3 Chiron Corporation, 4560 Horton Street, Emeryville, CA 94608 -2916, USA, 4 Program Resources, Inc. , Frederick Cancer Research and Development Center, National Cancer Institute, P. O. Box B, Frederick, MD 21702, USA, 5 Advanced Bio. Science Laboratories, 5510 Nicholson Lane, Kensington, MD 20895 -1078, USA, 6 Veterans Affairs Medical Centers, New York, NY 10010, USA, M. D. L. present address: Wyeth-Lederle Vaccines & Pediatrics, P. O. Box 304, Marietta, PA 17547, USA, R. N. , N. K. , S. M. present address: Wyeth-Lederle Vaccines, 401 North Middleton Road, Pearl River, NY 10965 USA, A. D. present address: Institute for Gene Therapy, University of Pennsylvania, 601 Maloney Building, 36 th & Spruce Streets, Philadelphia, PA 19104, USA, P. P. H. present address: 506 Ramblewood, Bryn Mawr, PA 19010, USA, M. S. R. , R. C. G. present address: Institute of Human Virology, Medical Biotechnology Center, University of Maryland at Baltimore, 725 West Lombard Street, Baltimore, MD 21201, USA, J. E. present address: Dutch Primate Centre, Biomedical Primate Research Centre, Lange Kleiweg 151, 2280 HV Rijswijk, the Netherlands

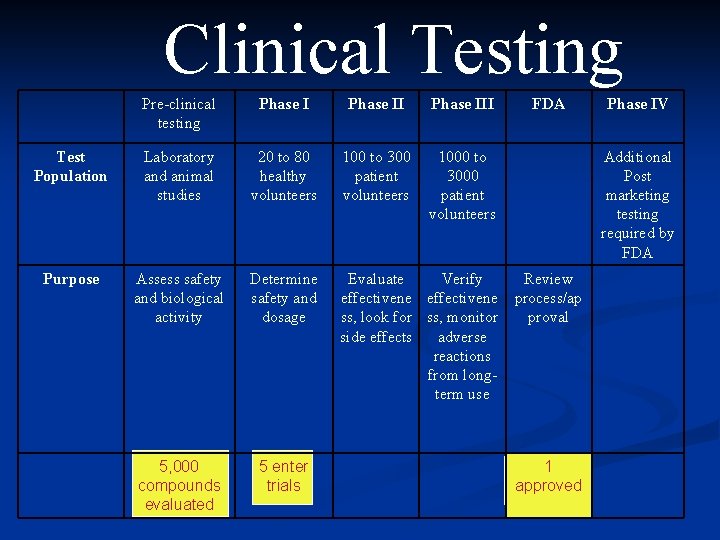
Clinical Testing Pre-clinical testing Phase III FDA Test Population Laboratory and animal studies 20 to 80 healthy volunteers 100 to 300 patient volunteers 1000 to 3000 patient volunteers Purpose Assess safety and biological activity Determine safety and dosage Evaluate Verify Review effectivene process/ap ss, look for ss, monitor proval side effects adverse reactions from longterm use 5, 000 compounds evaluated 5 enter trials Phase IV Additional Post marketing testing required by FDA 1 approved

Current Gene Therapy Clinical Studies Using Adenovirus Vectors 1. Recruiting. Adenovirus and Fungal Load in Pediatric Stem Cell Transplant Patients Conditions: Adenovirus; Other Mycoses 2. Recruiting Virus-Specific Cytotoxic T-Lymphocytes (CTLs) for Adenovirus Infection Following an Allogeneic Stem Cell Transplant 2. Recruiting. Virus-Specific Cytotoxic T-Lymphocytes (CTLs) for Adenovirus Infection Following an Allogeneic Stem Cell Transplant Condition: Adenoviridae Infections 3. Recruiting Safety and Immunogenicity of Recombinant DNA and Adenovirus Expressing L 523 S Protein in Early Stage Non-Small 3. Recruiting. Safety and Immunogenicity of Recombinant DNA and Adenovirus Expressing L 523 S Protein in Early Stage Non-Small Cell Lung Cancer Condition: Non-Small Cell Lung Cancer 4. Recruiting Safety of and Immune Response to a DNA HIV Vaccine Followed by an Adenoviral Vaccine Boost Given 3 Different 4. Recruiting. Safety of and Immune Response to a DNA HIV Vaccine Followed by an Adenoviral Vaccine Boost Given 3 Different Ways to HIV Uninfected Adults Condition: HIV Infections 5. Recruiting. Adenovirus Vaccine for Malaria 5. Condition: Malaria 6. Recruiting Safety and Efficacy of a Three-Dose Regimen of an Adenoviral HIV Vaccine (MRKAd 5 HIV-1 Gag/Pol/Nef) in HIV 6. Recruiting. Safety and Efficacy of a Three-Dose Regimen of an Adenoviral HIV Vaccine (MRKAd 5 HIV-1 Gag/Pol/Nef) in HIV Uninfected South African Adults Condition: HIV Infections 7. Recruiting Administration of Autologous DCs Infected with an Adenovirus Expressing Her-2 7. Recruiting. Administration of Autologous DCs Infected with an Adenovirus Expressing Her-2 Conditions: Inclusion criteria: Patients with metastatic breast cancer who are HER 2/neu positive (3+ by immunohistochemistry or FISH positive); and either, ; . . . 8. Recruiting Adenovirus Encoding Rat HER-2 in Patients With Metastatic Breast Cancer (Ad. HER 2. 1) 8. Recruiting. Adenovirus Encoding Rat HER-2 in Patients With Metastatic Breast Cancer (Ad. HER 2. 1) Conditions: Metastatic Breast Cancer; Recurrent Breast Cancer 9. . Recruiting Wild Type p 53 Adenovirus for Oral Premalignancies 9. . Recruiting. Wild Type p 53 Adenovirus for Oral Premalignancies Conditions: Mouth Cancer; Dysplasia/Carcinoma in Situ (CIS) of the Oral Cavity; Dysplasia/Carcinoma in Situ (CIS) of the Oral Pharynx 10. Recruiting. Study to Evaluate the Safety and Efficacy of Adeno-IFN Gamma in Cutaneous B-Cell Lymphoma 10. Condition: Lymphoma, B-Cell 11. Recruiting NMRC-M 3 V-Ad-Pf. CA Vaccine - Clinical Trial 1 11. Recruiting. NMRC-M 3 V-Ad-Pf. CA Vaccine - Clinical Trial 1 Condition: Plasmodium Falciparum 12. Recruiting Chemotherapy Followed By Vaccine Therapy in Treating Patients With Extensive-Stage Small Cell Lung Cancer 12. Recruiting. Chemotherapy Followed By Vaccine Therapy in Treating Patients With Extensive-Stage Small Cell Lung Cancer Condition: Lung Cancer 13. Recruiting Vaccine Therapy With Either Neoadjuvant or Adjuvant Chemotherapy and Adjuvant Radiation Therapy in Treating 13. Recruiting. Vaccine Therapy With Either Neoadjuvant or Adjuvant Chemotherapy and Adjuvant Radiation Therapy in Treating Women With p 53 -Overexpressing Stage II or Stage III Breast Cancer Condition: Breast Cancer 14. Not yet recruiting. Safety of and Immune Response to an HIV DNA Plasmid Vaccine Followed by HIV Adenoviral Vector Vaccine in Healthy African Adults Condition: HIV Infections

15. Recruiting. Safety of and Immune Response to a DNA HIV Vaccine Followed By an Adenoviral Vector HIV Vaccine in Healthy Adults Condition: HIV Infections 16. Recruiting. Interleukin-12 Gene in Treating Patients With Liver Metastases Secondary to Colorectal Cancer Conditions: Colorectal Cancer; Metastatic Cancer 17. Recruiting. Biological Therapy in Treating Women With Breast Cancer That Has Spread to the Liver Conditions: Breast Cancer; Metastatic Cancer 18. Recruiting. Phase I Study of Vaccination Schedule of Experimental HIV Vaccines Condition: HIV Infections 19. Recruiting. Phase I Study Combining Suicide Gene Therapy With Neoadjuvant Chemoradiotherapy in the Treatment of Potentially Resectable Pancreatic Adenocarcinoma Condition: Pancreatic Cancer 20. Recruiting. Gene Therapy for Prostate Cancer That Returns After Radiation Therapy Conditions: Prostatic Neoplasms; Neoplasm Recurrence, Local 21. Not yet recruiting. Safety Study on the Transfer of the CD 40 Ligand Gene (Adcu. CD 40 L) to Patients With Esophageal Carcinoma Condition: Esophageal Neoplasms 22. Recruiting. Neoadjuvant Capecitabine and Radiation Therapy With or Without TNFerade Followed By Surgical Resection in Treating Patients With Stage II, Stage III, or Locally Recurrent Rectal Cancer Condition: Colorectal Cancer 23. Recruiting. Effect of Adh. AQP 1 on Salivary Flow in Patients Treated With Radiation for Head and Neck Cancer Condition: Parotid Salivary Dysfunction 24. Recruiting. Gene Therapy for Pleural Malignancies Conditions: Pleural Mesothelioma; Metastatic Pleural Effusions 25. Recruiting. Safety and Immune Response to a Prime-Boost Vaccination Schedule in HIV-Infected Patients Condition: HIV 26. Recruiting. A Clinical Trial to Evaluate the Safety, Efficacy, and Immunogenicity of DR-5001 Condition: Respiratory Tract Diseases 27. Recruiting. Vector Delivery of the IL-12 Gene in Men With Prostate Cancer Conditions: Prostatic Neoplasms; Prostate Cancer 28. Recruiting. Vaccine Trial for Clear Cell Sarcoma, Pediatric Renal Cell Carcinoma, Alveolar Soft Part Sarcoma and Children With Stage IV Melanoma Conditions: Sarcoma, Clear Cell; Sarcoma, Alveolar Soft Part; Renal Cell Carcinoma; Secrete Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF Melanoma 29. Recruiting. Vaccination With Autologous Breast Cancer Cells Engineered to ) in Metastatic Breast Cancer Patients Condition: Breast

Cancer 30. Recruiting. Her-2/Neu in Patients With Metastatic Breast Cancer (Ad. HERe) Conditions: Metastatic Breast Cancer; Locally Recurrent Breast Cancer 31. Recruiting. Study of Treatment and Metabolism in Patients With Urea Cycle Disorders Condition: Amino Acid Metabolism, Inborn Errors 32. Recruiting. Safety of and Immune Response to an HIV-1 Vaccine (VRC-HIVDNA 016 -00 -VP) and a Vaccine Booster (VRC-HIVADV 014 -00 -VP) in HIV Uninfected East African Adults Condition: HIV Infections 33. Recruiting. Dose-Escalation Study of CG 0070 for Bladder Cancer After BCG (Bacillus Calmette-Guerin) Failure Conditions: Carcinoma, Transitional Cell; Bladder Neoplasms 34. Recruiting. Safety and Efficacy Study of INGN 241 Gene Therapy in Patients With In Transit Melanoma Conditions: Malignant Melanoma; Neoplasm Metastasis 35. Recruiting. Experimental Vaccine for Prevention of Ebola Virus Infection Conditions: Ebola Hemorrhagic Fever; Ebola Virus Disease; Ebola Virus Vaccines; Envelope Glycoprotein, Ebola Virus; Filovirus 36. Recruiting. Gene Therapy in Preventing Cancer in Patients With Premalignant Carcinoma of the Oral Cavity or Pharynx Condition: Head and Neck Cancer 37. Recruiting. Study to Compare the Overall Survival of Patients Receiving INGN 201 (Study Drug) With Patients Receiving Methotrexate Condition: Carcinoma, Squamous Cell 38. Recruiting. Effectiveness and Safety of INGN 201 in Combination With Chemotherapy Versus Chemotherapy Alone Condition: Carcinoma, Squamous Cell 39. Recruiting. Phase I - Pre-Radical Prostatectomy RTVP-1 Gene Therapy for Prostate Cancer Condition: Prostatic Neoplasms 40. Recruiting. A Study of TNFerade™ Biologic With 5 -FU and Radiation Therapy for First-Line Treatment of Unresectable Locally Advanced Pancreatic Cancer Condition: Pancreatic Cancer

Safety and Efficacy of a Three-Dose Regimen of an Adenoviral HIV Vaccine (MRKAd 5 HIV-1 Gag/Pol/Nef) in HIV Uninfected South African Adults This study is currently recruiting patients. Verified by National Institute of Allergy and Infectious Diseases (NIAID) December 2006 Sponsors and Collaborators: National Institute of Allergy and Infectious Diseases (NIAID) HIV Vaccine Trials Network Merck Information provided National Institute of Allergy and by: Infectious Diseases (NIAID) Clinical. Trials. gov NCT 00413725 Identifier:

Safety and Efficacy of a Three-Dose Regimen of an Adenoviral HIV Vaccine (MRKAd 5 HIV-1 Gag/Pol/Nef) in HIV Uninfected South African Adults This study has been suspended. ( Based on an interim data review, the DSMB concluded that the vaccine cannot be shown in this trial to prevent HIV infection or affect the course of the disease. )


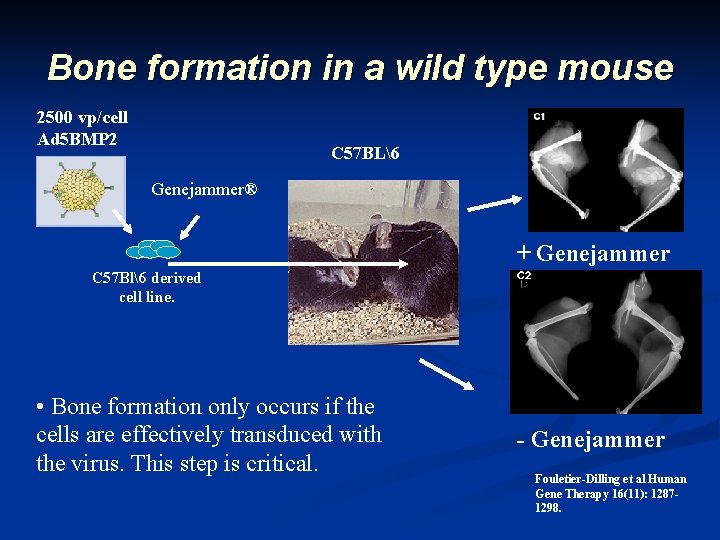
Bone formation in a wild type mouse 2500 vp/cell Ad 5 BMP 2 C 57 BL6 Genejammer® + Genejammer C 57 Bl6 derived cell line. • Bone formation only occurs if the cells are effectively transduced with the virus. This step is critical. - Genejammer Fouletier-Dilling et al Human Gene Therapy 16(11): 12871298.

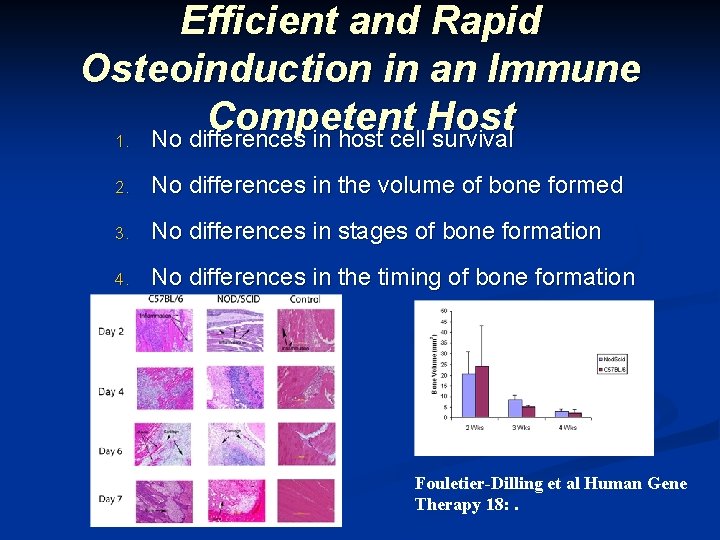
Efficient and Rapid Osteoinduction in an Immune Competent Host 1. No differences in host cell survival 2. No differences in the volume of bone formed 3. No differences in stages of bone formation 4. No differences in the timing of bone formation Fouletier-Dilling et al Human Gene Therapy 18: .

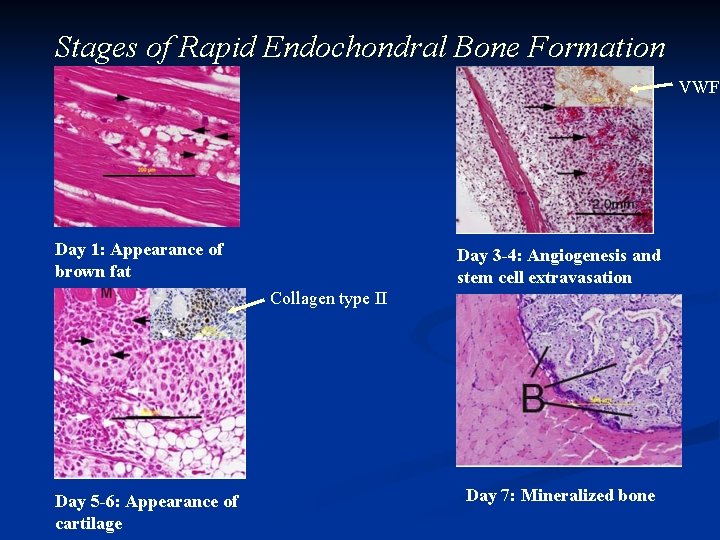
Stages of Rapid Endochondral Bone Formation VWF Day 1: Appearance of brown fat Day 3 -4: Angiogenesis and stem cell extravasation Collagen type II Day 5 -6: Appearance of cartilage Day 7: Mineralized bone

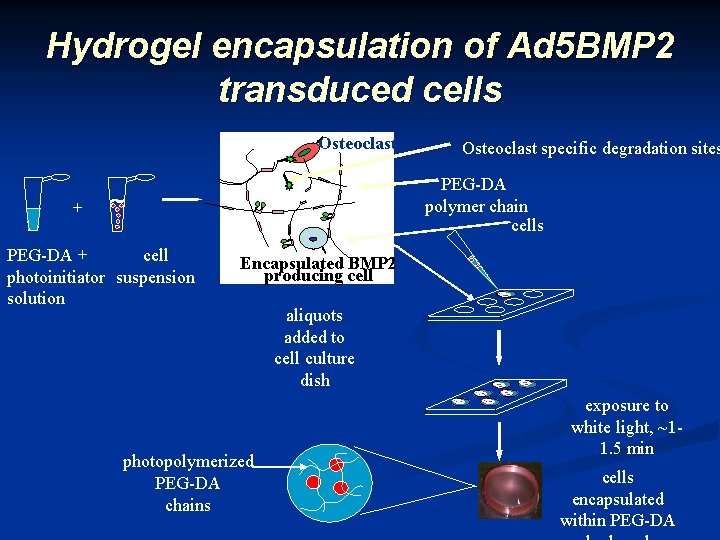
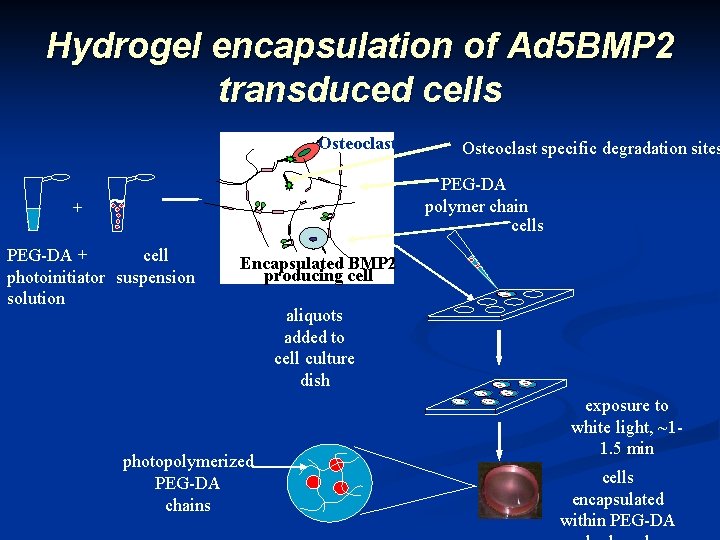
Hydrogel encapsulation of Ad 5 BMP 2 transduced cells Osteoclast specific degradation sites PEG-DA polymer chain cells + PEG-DA + cell photoinitiator suspension solution Encapsulated BMP 2 producing cell photopolymerized PEG-DA chains aliquots added to cell culture dish exposure to white light, ~11. 5 min cells encapsulated within PEG-DA

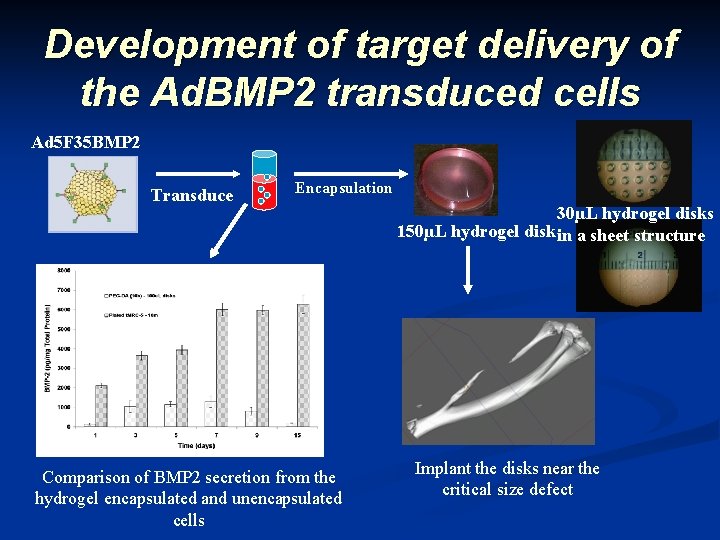
Development of target delivery of the Ad. BMP 2 transduced cells Ad 5 F 35 BMP 2 Transduce Encapsulation Comparison of BMP 2 secretion from the hydrogel encapsulated and unencapsulated cells 30µL hydrogel disks 150µL hydrogel disk in a sheet structure Implant the disks near the critical size defect

Hydrogel encapsulation of Ad 5 BMP 2 transduced cells Osteoclast specific degradation sites PEG-DA polymer chain cells + PEG-DA + cell photoinitiator suspension solution Encapsulated BMP 2 producing cell photopolymerized PEG-DA chains aliquots added to cell culture dish exposure to white light, ~11. 5 min cells encapsulated within PEG-DA

Other Slides of this lecture available on our website http: //vector. bcm. tmc. edu/ n References (will be fair game on exam) n n Biology of adenovirus and its use as a vector for gene therapy. Hum Gene Ther. 2004 Nov; 15(11): 1022 -33. Mc. Connell MJ, Imperiale MJ n Adenovirus: from foe to friend. Rev Med Virol. 2006 May-Jun; 16(3): 167 -86 Goncalves MA, de Vries AA.
 Factors that affect disease transmission
Factors that affect disease transmission Window of infectivity
Window of infectivity Growth curve of virus
Growth curve of virus Virology
Virology Fields virology
Fields virology Introduction to medical virology
Introduction to medical virology Fields virology
Fields virology Koz grippi
Koz grippi Life cycle of adenovirus
Life cycle of adenovirus Protein power point
Protein power point Gene by gene test results
Gene by gene test results 8-7 vectors
8-7 vectors Cloning vector
Cloning vector Law of cosines vectors
Law of cosines vectors Graphical vector addition
Graphical vector addition Physics vectors
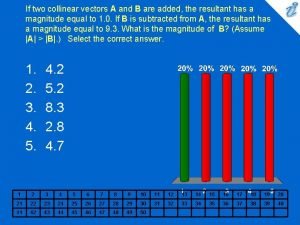
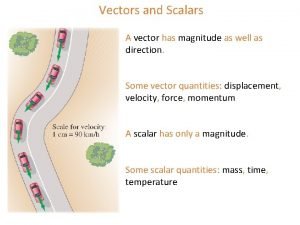
Physics vectors If two collinear vectors a and b are added
If two collinear vectors a and b are added Madas vectors
Madas vectors Rhmd: evasion-resilient hardware malware detectors
Rhmd: evasion-resilient hardware malware detectors Graphical addition of vectors
Graphical addition of vectors Kinematics 2d formulas
Kinematics 2d formulas Condition for linearly independent vectors
Condition for linearly independent vectors Doc frost maths
Doc frost maths Carl yaztremski
Carl yaztremski Simulink matrix multiplication
Simulink matrix multiplication Contoh soal diferensial vektor
Contoh soal diferensial vektor Define instantaneous acceleration
Define instantaneous acceleration Vectors in 2 dimensions
Vectors in 2 dimensions Vector resolution examples
Vector resolution examples Intro to vectors
Intro to vectors Basic concepts of matrices
Basic concepts of matrices Chapter 12 vectors and the geometry of space
Chapter 12 vectors and the geometry of space Least square solution
Least square solution Tell something about the multimodal text big ed mona
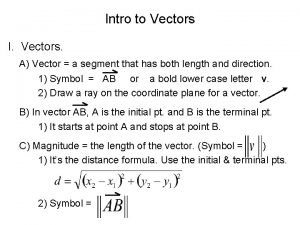
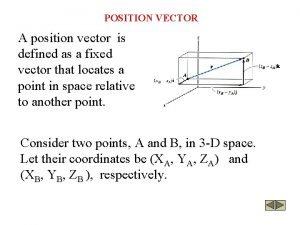
Tell something about the multimodal text big ed mona A position vector is
A position vector is