Additive solutions for RBC storage AS1 AS3 AS5

Additive solutions for RBC storage: AS-1, AS-3, AS-5 & AS-7 Stephanie N. David, MD 8. 19. 15
Outline • • • History & introduction of additive solutions Comparing additive solutions used today AS-7 Additive solution use in neonates Current VUMC practice of selecting different AS for pediatric vs. adults

History • 1970 s: Saline-adenine-glucose (SAG) • 1981: addition of mannitol SAGM • No new RBC additive solutions have been licensed for use for over 20 years…until now! Blood Transfus 2012; 10 Suppl 2: s 7 -11

Importance of Additive Solutions • During storage, RBCs undergo complex and progressive accumulation of changes—RBC storage lesion

RBC storage lesions • Morphological changes • Slowed metabolism with decrease in concentration of ATP • Acidosis with decrease in concentration of 2, 3 diphosphoglycerate (2, 3 -DPG) increased affinity of hemoglobin for oxygen decreased capacity of rbcs to release oxygen Blood Transfus 2010; 8: 82 -8.

RBC storage lesions continued • Loss of function of cation pumps • Oxidative damage with changes to structure of RBCs • Apoptotic changes • Loss of parts of the membranes through vesiculation Blood Transfus 2010; 8: 82 -8.

Potential consequences of RBC storage lesions • Compromise safety and efficacy of RBCs • Reduce capacity to carry and release O 2 • Promote release of potentially toxic intermediates – Free hemoglobin can act as source of reactive oxygen species • Increased capacity of RBCs to adhere to endothelium • Enhanced thrombogenic or pro-inflammatory potential Blood Transfus 2010; 8: 82 -8.

Complex inter-relationships RBC biochemistry RBC cytoskeletal structure difficult to predict how RBCs respond to different storage conditions Blood Transfus 2012: 10 Suppl 2: s 7 -11
Standard requirements for patenting new AS in USA • Level of hemolysis: below threshold of 0. 8% at the end of the storage period • Survival rate of transfused cells: >75% at 24 hours after transfusion Blood Transfus 2010; 8: 82 -8.

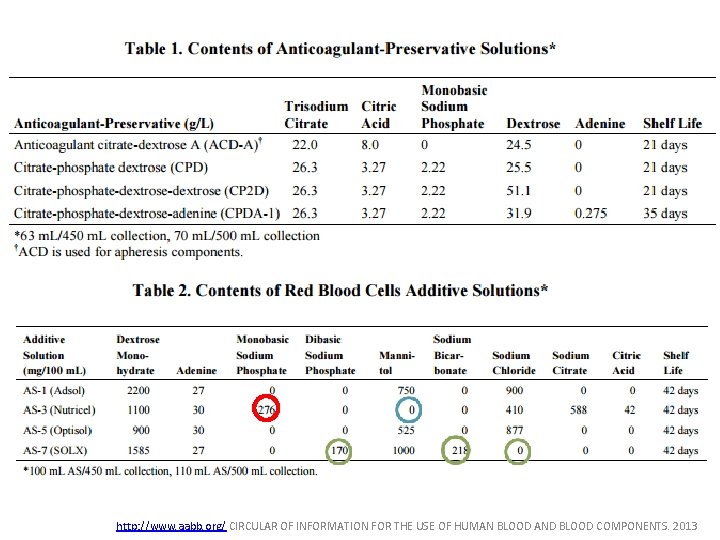
Anticoagulant/Preservative solutions • Acid Citrate Dextrose (ACD): 21 day storage • Citrate-phosphate-dextrose (CPD) & Citratephosphate-dextrose (CP 2 D): 21 day storage • Citrate-phosphate-dextrose-adenine (CPDA-1): 35 day storage – Similar to CPD but + 17. 3 mg adenine

Additive Solutions • • • shelf life: 42 days AS-1: Adsol AS-3: Nutricel AS-5: Optisol AS-7: SOLX
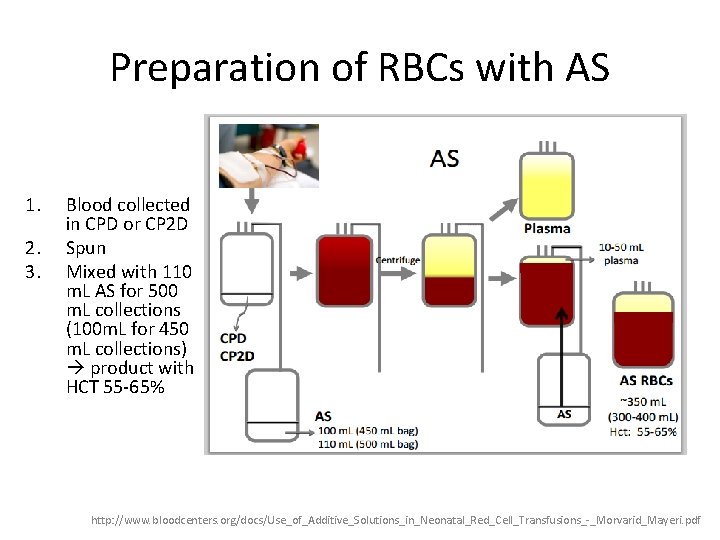
Preparation of RBCs with AS 1. 2. 3. Blood collected in CPD or CP 2 D Spun Mixed with 110 m. L AS for 500 m. L collections (100 m. L for 450 m. L collections) product with HCT 55 -65% http: //www. bloodcenters. org/docs/Use_of_Additive_Solutions_in_Neonatal_Red_Cell_Transfusions_-_Morvarid_Mayeri. pdf
http: //www. aabb. org/ CIRCULAR OF INFORMATION FOR THE USE OF HUMAN BLOOD AND BLOOD COMPONENTS. 2013

AS-7 (SOLX) • Alkaline • Previously called EAS-81 (Experimental AS-81) • Designed to improve RBC metabolism during storage by increasing the range and capacity of p. H buffering by adding phosphate and bicarbonate • April 19, 2013 - First AS approved by FDA in US in >20 years! • Approved for RBC storage at 1 -6 C for up to 42 days after collection Transfusion. 2015 March; 55: 491 -498 http: //www. fda. gov/biologicsbloodvaccines/bloodproducts/approvedproducts/newdrugapplicationsndas/ucm 352625. htm

Transfusion. 2015 March; 55: 491 -498.
Study design & Methods • Storage quality measured in prospective, randomized and three-center trial • Subjects randomly assigned to have whole blood collected and processed into RBC and plasma components stored in one of 3 ways: – Control: processed within 8 hours and stored in AS-1 at 1 -6°C for 42 days (n=60) – Test: processed within 2 hours and stored in AS-7 at 16°C for up to 56 days (n=60) – Test: Processed within 8 hours and stored in AS-7 at 16°C for up to 56 days (n=60) Transfusion. 2015 March; 55: 491 -498.
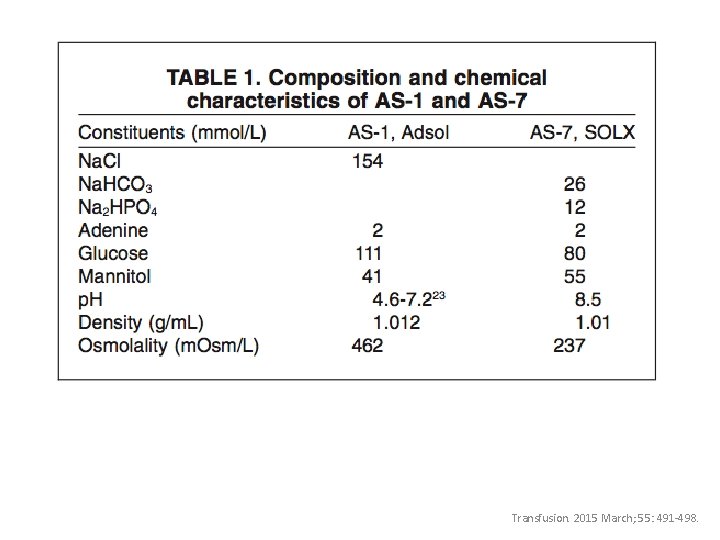
Transfusion. 2015 March; 55: 491 -498.
ATP Intracellular RBC ATP concentration μmol/g Hb AS-1 Day 42 AS-7 Day 56 4. 4 ± 0. 7 3. 6 ± 0. 8 4. 3 ± 0. 7 3. 9 ± 0. 7 4. 3 ± 0. 7 3. 1 ± 0. 8 • Both day 42 & 56 AS-7—within the levels considered sufficient to maintain RBC viability ATP recovery (%) At day 42 AS-1 <8 hr storage AS-7 <2 hr storage AS-7 6 -8 hr storage 82. 5 ± 14. 6 93. 7 ± 17. 9 89. 5 ± 13. 7 • ATP recoveries (assessed as % of ATP at prestorage level) were significantly higher in AS-7 than AS-1 Transfusion. 2015 March; 55: 491 -498.
Morphology index Day 42 AS-1 <8 hr storage AS-7 <2 hr storage AS-7 6 -8 hr storage 69 ± 8 81 ± 8 80 ± 6. 6 79 ± 7 77 ± 7 16. 8 ± 11. 3 At day 56 Morphology index Day 56 Shed microvesicle protein (mg /d. L) 28. 9 ± 18. 2 At day 42 -By day 56, AS-7 maintained 10% higher morphology indices than day 42 AS-1 -Day 56 AS-7 RBCs contained 40% less shed microvesicle protein than day 42 AS-1 RBCs Transfusion. 2015 March; 55: 491 -498.
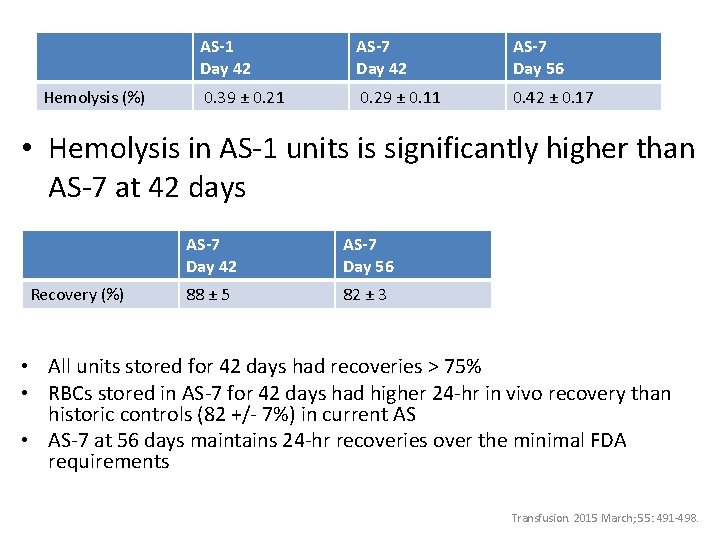
Hemolysis (%) AS-1 Day 42 AS-7 Day 56 0. 39 ± 0. 21 0. 29 ± 0. 11 0. 42 ± 0. 17 • Hemolysis in AS-1 units is significantly higher than AS-7 at 42 days Recovery (%) AS-7 Day 42 AS-7 Day 56 88 ± 5 82 ± 3 • All units stored for 42 days had recoveries > 75% • RBCs stored in AS-7 for 42 days had higher 24 -hr in vivo recovery than historic controls (82 +/- 7%) in current AS • AS-7 at 56 days maintains 24 -hr recoveries over the minimal FDA requirements Transfusion. 2015 March; 55: 491 -498.
Summary of AS-1 vs AS-7 • • Improved biochemical status Decreased vesicle formation Reduced hemolysis Increased in vivo recovery at conventional and prolonged period of storage Transfusion. 2015 March; 55: 491 -498.

Red cell transfusions in neonates • Traditionally, used fresh CPDA-1 units (<7 days) • Initially concern for using AS due to mannitol (AS-1, AS-5) & adenine additives • Also concern about hyperkalemia, acidosis and decreased function of older red cells Transfusion 1996; 36: 873 -878 Transfusion and Apheresis Science 24 (2001): 111 -115
AS use in neonates • Strauss RG, Burmeister LF, Johnson K, et al. AS-1 red cells for neonatal transfusions: a randomized trial assessing donor exposure and safety. Transfusion. 1996; 36(10): 873 -878 – Red cells stored in AS-1 <42 days from single donor vs red cells stored in CPDA-1 stored <7 days – No significant differences in: • Pre-transfusion and post-transfusion blood chemistries • Renal and hepatic chemistries – Minimized donor exposure – Similar conclusions for AS-3 study (2000) • Jain R, Jarosz C. Safety and efficacy of AS-1 red blood cell use in neonates. Transfusion and Apheresis Science. 2001; 24(2): 111– 115. – Compared to storage in CPDA-1, found no clinical or laboratory evidence that AS-1 RBCs had any deleterious effects J Pediatr 2000. 136: 215– 219
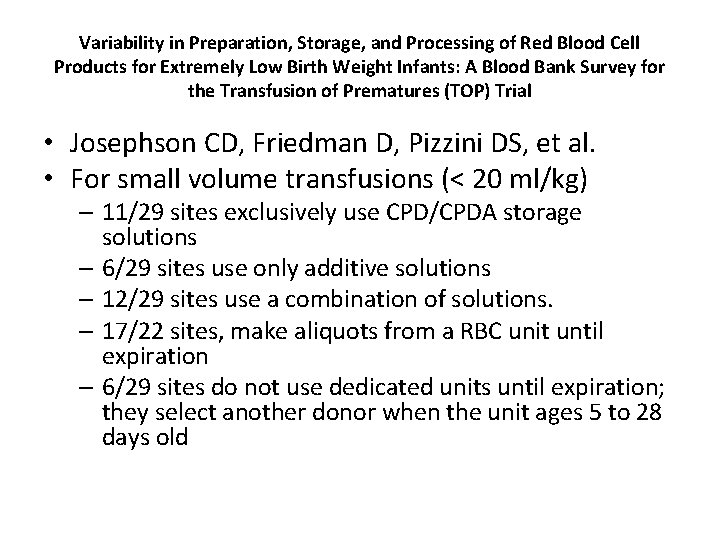
Variability in Preparation, Storage, and Processing of Red Blood Cell Products for Extremely Low Birth Weight Infants: A Blood Bank Survey for the Transfusion of Prematures (TOP) Trial • Josephson CD, Friedman D, Pizzini DS, et al. • For small volume transfusions (< 20 ml/kg) – 11/29 sites exclusively use CPD/CPDA storage solutions – 6/29 sites use only additive solutions – 12/29 sites use a combination of solutions. – 17/22 sites, make aliquots from a RBC unit until expiration – 6/29 sites do not use dedicated units until expiration; they select another donor when the unit ages 5 to 28 days old

RBC transfusion in neonates • For small-volume transfusions: – Aliquots of same parent bag in AS to minimize donor exposure – AS-3 over AS-1 if concern about mannitol – AS-5?
Large volume transfusions in neonates • Evidence for use of AS is not well established • No randomized controlled trials • Abstract from Eder et al. at CHOP – “Comparison of CPDA vs. AS Red Cell Transfusion to Infants on ECMO” – AS-1 & AS-3 tolerated as well as CPDA-1 units – Comparable post-transfusion lab values (Hct, Na, K, glc, Ca)

Current VUMC practice of selecting a different AS for pediatric versus adults?
Questions?
- Slides: 28