Added value of WHO Prequalification Updates from product













- Slides: 25

Added value of WHO Prequalification. Updates from product streams (medicines, vaccines and diagnostics) IPC 2014, Washington DC Dr Lembit Rägo Head Regulation of Medicines and other Health Technologies Essential Medicines and Health Products World Health Organization Geneva, Switzerland E-mail: ragol@who. int

Content l Reorganization l Added value of PQ l News from product streams l Concluding remarks 2 |

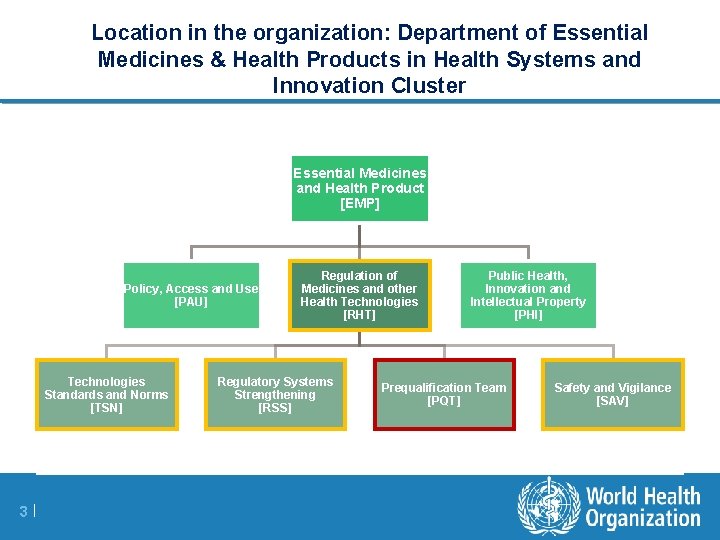
Location in the organization: Department of Essential Medicines & Health Products in Health Systems and Innovation Cluster Essential Medicines and Health Product [EMP] Policy, Access and Use [PAU] Technologies Standards and Norms [TSN] 3 | Regulation of Medicines and other Health Technologies [RHT] Regulatory Systems Strengthening [RSS] Public Health, Innovation and Intellectual Property [PHI] Prequalification Team [PQT] Safety and Vigilance [SAV]

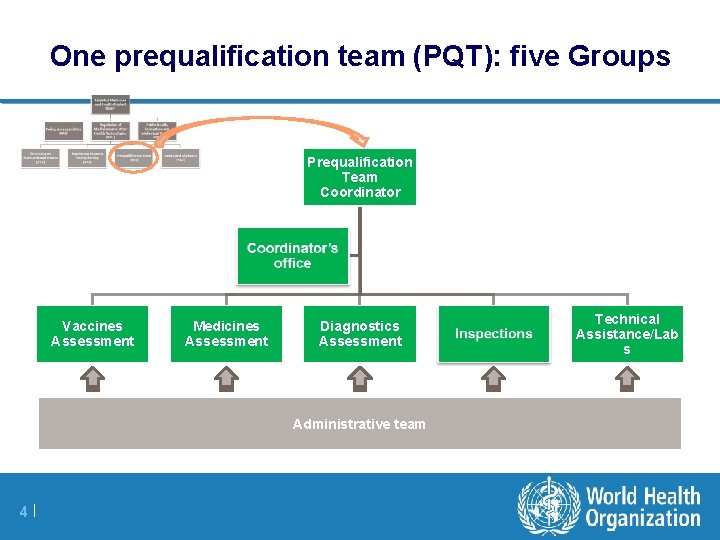
One prequalification team (PQT): five Groups Prequalification Team Coordinator Vaccines Assessment Medicines Assessment Diagnostics Assessment Administrative team 4 | Technical Assistance/Lab s

Prequalification l WHO prequalification (PQ) covers three product types: – in-vitro diagnostics – IVDs (Dx), – medicines (Rx) – and vaccines (Vx). l A cross-cutting intervention that effectively addresses shortcomings relating to development, evaluation, manufacturing, supply chain management and monitoring of essential global health commodities l It is of value to procurers, manufacturers, regulators, laboratories, health care providers and, most importantly, patients 5 |

Embodiment of public health values l WHO prequalification embodies and continuously demonstrates public health values. l WHO prequalification is an objective, independent process. The standards upon which it is based, have been developed and are updated according to rigorous scientific evidence and via an international consultative process. l WHO prequalification promotes regulatory transparency by making the results of its decisions publicly available, and promotes equity through information sharing and transfer of skills and knowledge. l The WHO Prequalification Team (PQT) strives for excellence: both in performance and by continuously improving its knowledge and expertise. l It is innovative and responsive to new challenges, as demonstrated by its contribution to development paediatric medicines, fast-track assessment of products in emergencies and, works collaboratively and optimally, and supports many others ― regulators, manufacturers, procurers and health care providers 6 |

Value for procurers (1) l The most readily quantifiable impact of prequalification relates to procurement. Hundreds of millions of US dollars’ worth of Dx, Rx and Vx are purchased through international financing or procurement mechanisms ― such as the Global Fund, UNITAID, GAVI, UNICEF, UNFPA, PAHO etc. l WHO PQ, its handling of variations and follow-up monitoring (including testing), investigation product complaints, ensure that these products continue to meet standards for quality, safety and efficacy. Agencies procuring these products save considerable time and resources that would otherwise have to be dedicated to similar duplicative activities. l PQ increases fair competition among quality products, contributing to market sustainability and lower prices. 7 |

Value for procurers (2) l According to Mc. Kinsey & Company, in 2012, WHO PQ enabled procurement of around ~US$ 2. 4 billion (~US$ 1, 550 million for Vx, ~ US$ 730 million for Rx and ~US$ 95 million for Dx), and potentially an additional ~US$ 300− 600 million of national and private markets, of quality-assured products. l Mc. Kinsey has also calculated that WHO PQ enabled Rx procurers to save around US$ 1 billion in 2012, equivalent to a return on investment (ROI) of 75: 1 l Additional PQT efforts to meet supply needs include its hosting of the Expert Review Panel (ERP) for medicines and the Expert Review Panel for Diagnostics (ERPD). l ERP and ERPD play an important role for international procurers who must urgently procure products for which no stringently-approved or prequalified version yet exists. l Most recently, PQT has established its Emergency Quality Assurance Mechanism to assess Dx for Ebola, similar processes will be set up for Dx and Rx. 8 |

Value for manufacturers (1) l Additional "seal" of quality and increased trust from buyers and regulators l New manufactures coming of board - appreciation and understanding of the benefits that PQ brings to manufacturers continue to grow. l Attaining PQ is the only means whereby Vx manufacturers can access GAVIfunded and UNICEF/PAHO procurement of vaccines. PQ grants manufacturers of HIV rapid diagnostic tests (RDTs) greater access to international donor-funded markets. l For Rx manufacturers, PQ is the primary route to donor-funded procurement of TB and malaria Rx, and a key route to donor-funded procurement of HIV/AIDS Rx. l It can facilitate bids for national tenders and requests for national registrations l Innovative regulatory approaches, unique additional guidance not given by any other regulator 9 |

Value for manufacturers (2) l Prequalification of active pharmaceutical ingredients (APIs) represents an increasingly valued PQT service – easy switch to new API source l WHO approves API and FPP variations very promptly, which can enable manufacturers to generate savings and satisfy supply demands l The collaborative procedure for accelerating national registration of FPPs and vaccines helps manufacturers gain speedier access to markets while also substantially accelerating access to urgently-needed medical products. Development of a similar procedure is now being considered for Dx. l PQ offers regulatory advice and technical assistance in product development phase l PQT offers manufacturers technical assistance and training aimed at improvement of product dossier and manufacturing quality, not just in relation to the product to be submitted to WHO for assessment 10 |

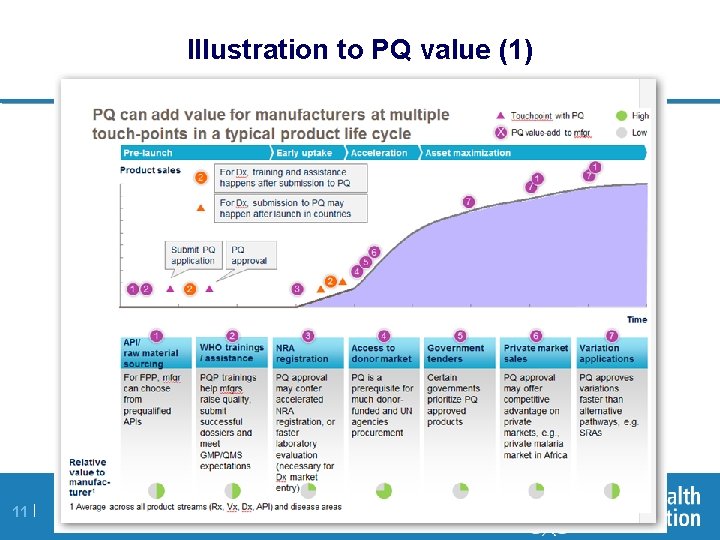
Illustration to PQ value (1) 11 |

Value for regulators (1) l Regulatory development and enhanced regulatory capacity are key PQT outputs. In Africa this has facilitated overall regulatory systems strengthening such as increased technical capacity and in more general – harmonization and convergence. l Lists of prequalified products and laboratories, public assessment and public inspections reports, guidance and training materials, quality and safety alerts, and the reports of investigations into product and/or quality issues, are consulted frequently by Rx regulators, especially those working in lowincome countries. l PQ enables national regulatory authorities (NRAs) in recipient countries to save human and financial resources; the collaborative procedure for Rx and Vx is good example (starting increasingly to deliver) l PQT offers hands-on training to regulators ― through participation in dossier assessment sessions (for Rx), observation of inspections (for all three product streams), as well as the opportunity to participate in training workshops or be a three months rotational fellow in PQ 12 |

Value for strengthening quality control laboratories l PQT provides advice and support to QCLs, in the form of inventory audits, consultancy and training, to help them build up their operational capacity sufficiently to meet international and PQ standards. l PQT also organizes training for national QCLs and laboratories that provide testing services to their government. Prequalified QC labs can actively contribute to monitoring the quality of Rx procured nationally, or of Rx procured on its country's behalf by international organizations. l National laboratories also benefit from support to improve their capacity to test Vx. If, when assessing NRA functionality, PQT ascertains that a country’s capacity to test and monitor Vx quality is insufficient, it will work with the relevant NRA to incorporate improvement of that capacity in its institutional development plan. l In parallel, improved performance and reliability of quality control testing laboratories is integral to strengthened national regulatory systems. 13 |

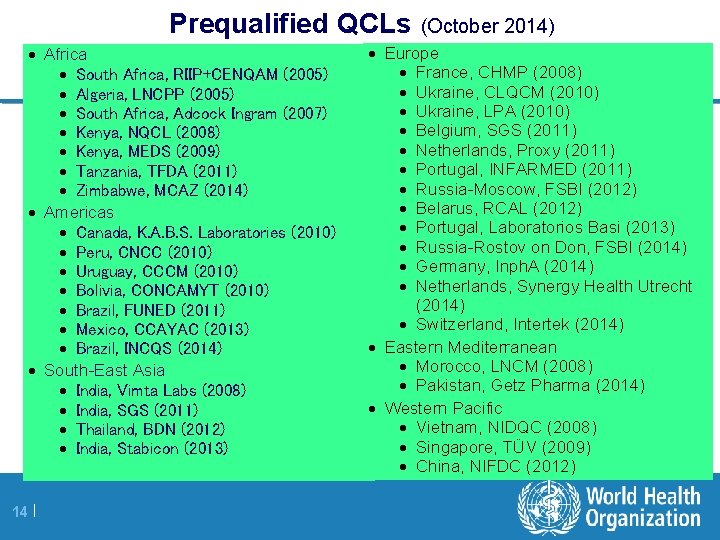
Prequalified QCLs (October 2014) · Africa · South Africa, RIIP+CENQAM (2005) · Algeria, LNCPP (2005) · South Africa, Adcock Ingram (2007) · Kenya, NQCL (2008) · Kenya, MEDS (2009) · Tanzania, TFDA (2011) · Zimbabwe, MCAZ (2014) · Americas · Canada, K. A. B. S. Laboratories (2010) · Peru, CNCC (2010) · Uruguay, CCCM (2010) · Bolivia, CONCAMYT (2010) · Brazil, FUNED (2011) · Mexico, CCAYAC (2013) · Brazil, INCQS (2014) · South-East Asia · India, Vimta Labs (2008) · India, SGS (2011) · Thailand, BDN (2012) · India, Stabicon (2013) 14 | · Europe · France, CHMP (2008) · Ukraine, CLQCM (2010) · Ukraine, LPA (2010) · Belgium, SGS (2011) · Netherlands, Proxy (2011) · Portugal, INFARMED (2011) · Russia-Moscow, FSBI (2012) · Belarus, RCAL (2012) · Portugal, Laboratorios Basi (2013) · Russia-Rostov on Don, FSBI (2014) · Germany, Inph. A (2014) · Netherlands, Synergy Health Utrecht (2014) · Switzerland, Intertek (2014) · Eastern Mediterranean · Morocco, LNCM (2008) · Pakistan, Getz Pharma (2014) · Western Pacific · Vietnam, NIDQC (2008) · Singapore, TÜV (2009) · China, NIFDC (2012)

Value for populations and patients l PQT creates significant value for populations and patients. By ensuring accurate diagnosis, prevention and treatment, prequalified Dx, Rx and Vx save and improve the quality of lives. l Prequalified Vx are used in 134 countries and approximately 64% of the global birth cohort is immunized with prequalified Vx. l Of the 8 million people receiving treatment for HIV in 2012, 6. 5 million were receiving WHO-prequalified antiretrovirals l Majority of antimalarials and antituberculosis medicines procured by GF and UNITAID funding, increasing share of RH products procured (PQ + ERP) l In 2013, 80% of HIV RDTs procured worldwide by major international procurement agencies were prequalified. 15 |

Regulatory cooperation l PQ facilitates collaboration between regulators and promotes avoiding duplications l Collaboration in scientific assessment – PQ – done collectively by regulators, for regulators – High involvement of developing country regulators – Participates pro-actively in International Generic Drug Regulators Pilot (GDRP) – Participates in Developing Country Vaccines Regulatory Network (DCVRN) l Collaboration in the area of inspections – developed and developing country inspectorates l Collaboration in the are of QCs 16 |

Annual reports 17 |

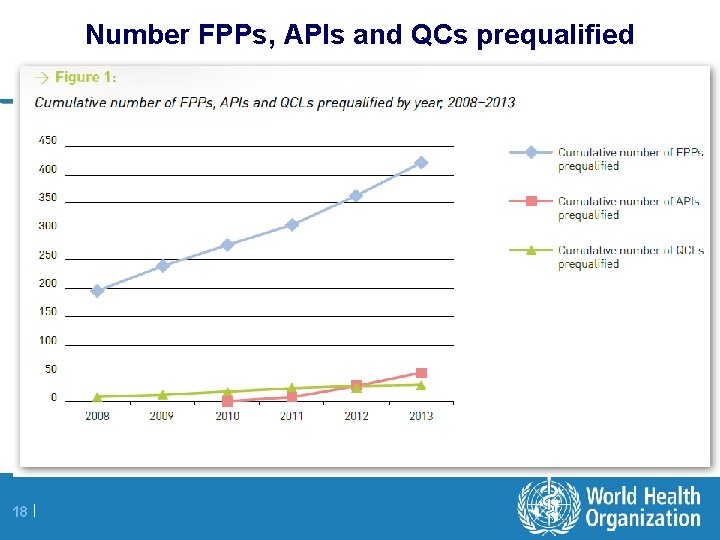
Number FPPs, APIs and QCs prequalified 18 |

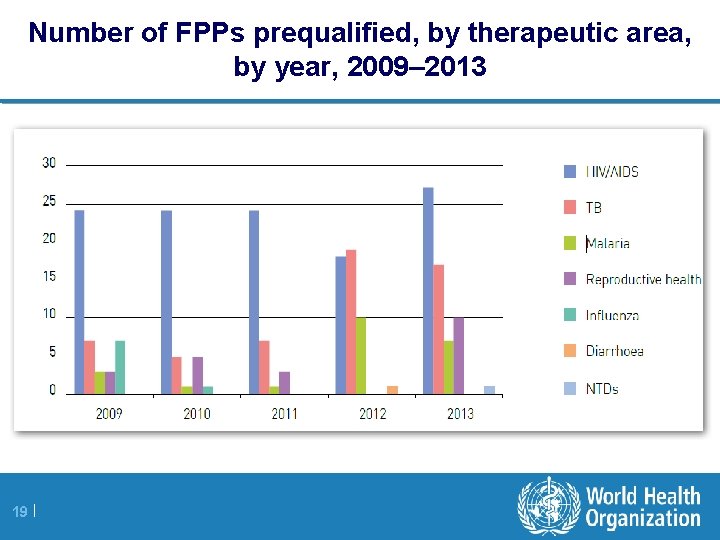
Number of FPPs prequalified, by therapeutic area, by year, 2009– 2013 19 |

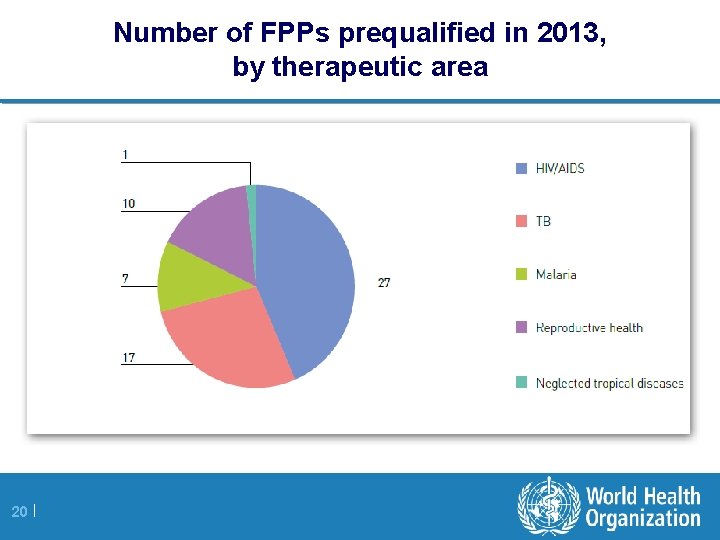
Number of FPPs prequalified in 2013, by therapeutic area 20 |

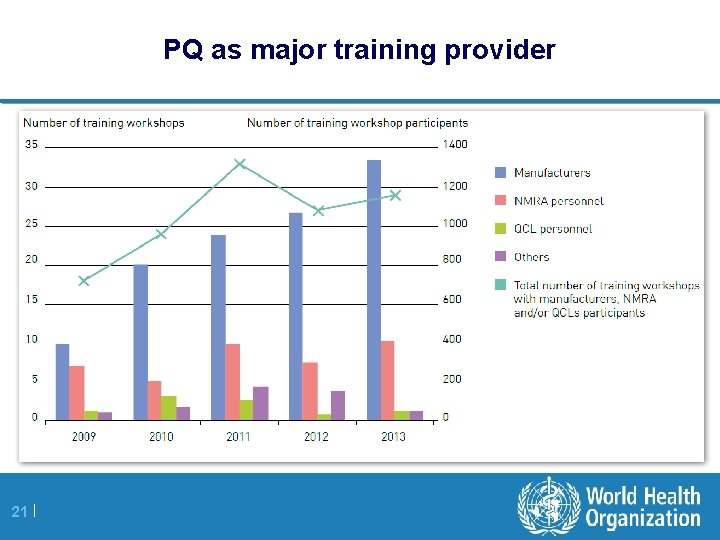
PQ as major training provider 21 |

PQT is involved in innovative science - examples (produces annually 2 -5 scientific articles, reviews and book chapters) 22 |

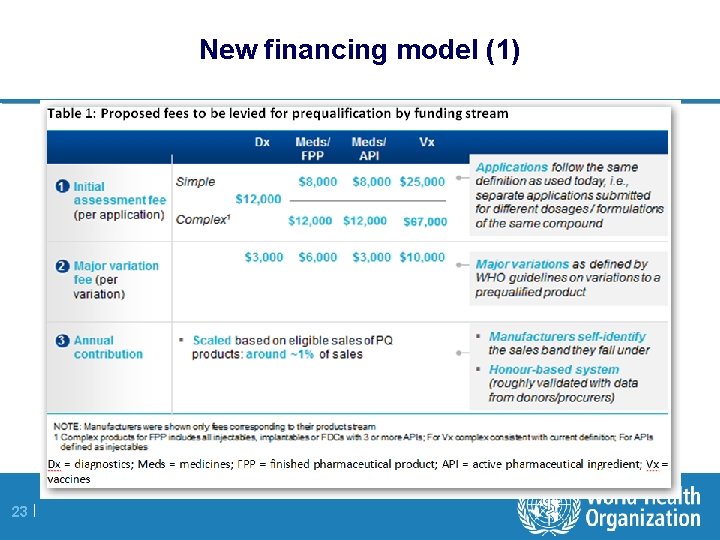
New financing model (1) 23 |

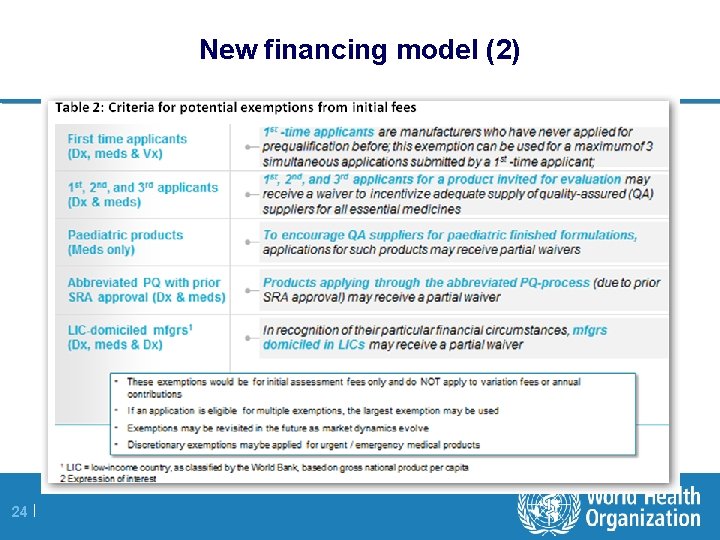
New financing model (2) 24 |

Concluding remarks l PQ has had significant positive documented impact in increasing access to safe quality health products – likely one of the best ever UN initiatives l PQ after reorganization is more logical, builds on synergies and is better positioned to stand future challenges l Financing PQ in a sustainable manner is a must as in near future (5 -10 yrs) need for its multiple services will only increase and its role cannot be transferred to other parties during this time frame l … 25 |
 Indot contractor prequalification
Indot contractor prequalification Value added tax meaning
Value added tax meaning National income formula
National income formula Value added refers to education
Value added refers to education Value added approach
Value added approach Value added course brochure
Value added course brochure Calculate residual income
Calculate residual income Value added approach formula
Value added approach formula Value added approach formula
Value added approach formula Brandz brand equity model
Brandz brand equity model Wwt reseller
Wwt reseller Value-added agriculture
Value-added agriculture Elements of inspiring
Elements of inspiring Importance of added value
Importance of added value Bentuk usaha untuk menciptakan nilai l
Bentuk usaha untuk menciptakan nilai l Value added tea
Value added tea Eva finance
Eva finance Value added products of cauliflower
Value added products of cauliflower Advanced cost and management accounting ppt
Advanced cost and management accounting ppt Brand is the added value endowed to products and services
Brand is the added value endowed to products and services Brand exploratory questions
Brand exploratory questions Tawag sa pagsukat ng gnp
Tawag sa pagsukat ng gnp What oo
What oo Value added
Value added Contoh value creation adalah
Contoh value creation adalah Upstu
Upstu