Adaptive Designs that Prospectively Learn vs Test Biomarker

Adaptive Designs that Prospectively Learn vs. Test Biomarker Sensitive Patients Sue-Jane Wang, Ph. D. Associate Director Adaptive Design and Pharmacogenomics Office of Biostatistics, Office of Translational Sciences Center for Drug Evaluation and Research, U. S. FDA Presented at “Graybill Conference VII”, Fort Collin, Colorado, June 12, 2008
Acknowledgments H. M. James Hung Robert T. O’Neill Thanks are due to Dr. Robert Temple and Dr. Norman Stockbridge of FDA for bringing the interesting problem to our attention The research work was supported by the RSR funds #02 -06, #04 -06, #05 -2, #05 -14, #08 -48 awarded by the Center for Drug Evaluation and Research, U. S. Food and Drug Administration *The research view presented are those of the author’s professional views and not necessarily those of the US FDA Wang SJ, Graybill 06. 12. 2008 2

Outline · (Genomic) Biomarker as a Classifier · AD in Preliminary Biomarker Exploratory Studies · AD in A&WC Setting · Examples · Mechanics of Sample Size Formula · Concluding Remarks Wang SJ, Graybill 06. 12. 2008 3
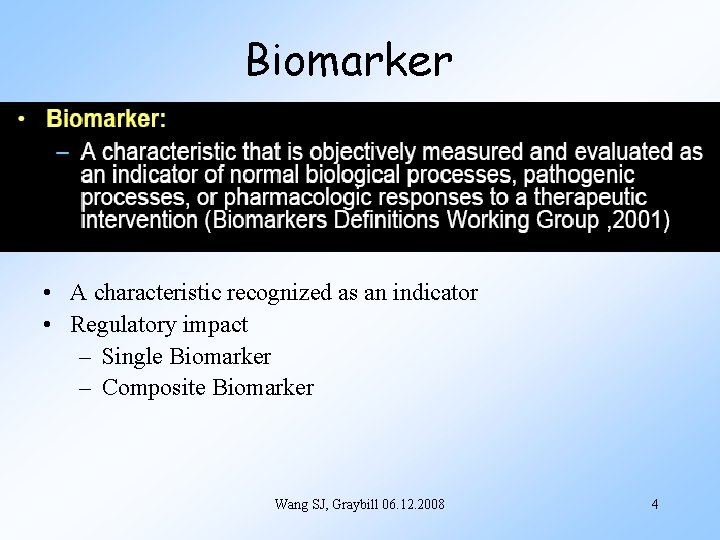
Biomarker • A characteristic recognized as an indicator • Regulatory impact – Single Biomarker – Composite Biomarker Wang SJ, Graybill 06. 12. 2008 4
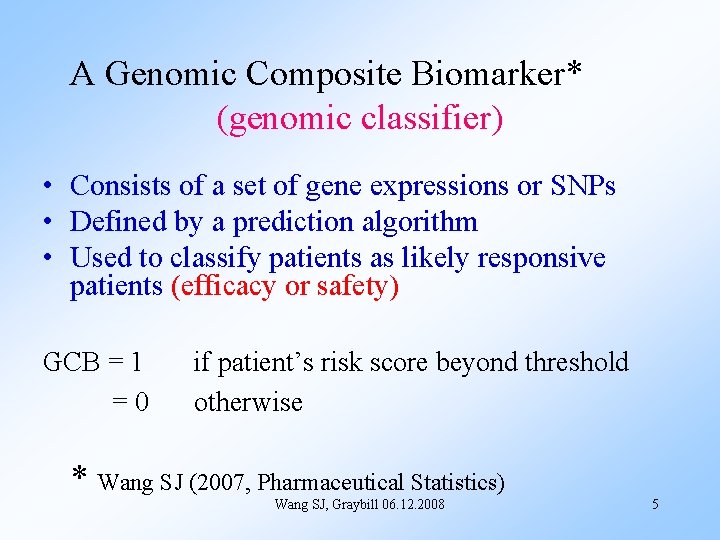
A Genomic Composite Biomarker* (genomic classifier) • Consists of a set of gene expressions or SNPs • Defined by a prediction algorithm • Used to classify patients as likely responsive patients (efficacy or safety) GCB = 1 =0 if patient’s risk score beyond threshold otherwise * Wang SJ (2007, Pharmaceutical Statistics) Wang SJ, Graybill 06. 12. 2008 5
Genomic Composite Biomarker Developed from Microarray, Whole Genome Scan, Other Technology Platforms Wang SJ, Graybill 06. 12. 2008 6
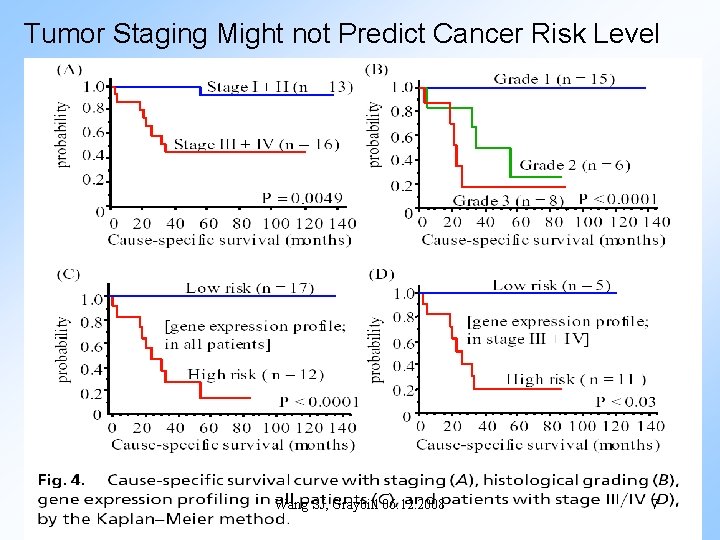
Tumor Staging Might not Predict Cancer Risk Level Wang SJ, Graybill 06. 12. 2008 7
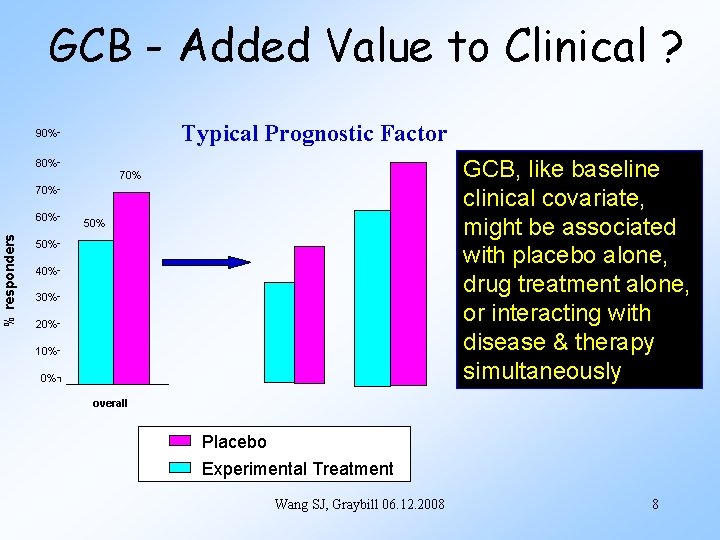
GCB - Added Value to Clinical ? Typical Prognostic Factor 90% 80% GCB, like baseline clinical covariate, might be associated with placebo alone, drug treatment alone, or interacting with disease & therapy simultaneously 70% % responders 60% 50% 40% 30% 20% 10% 0% overall Placebo Experimental Treatment Wang SJ, Graybill 06. 12. 2008 8
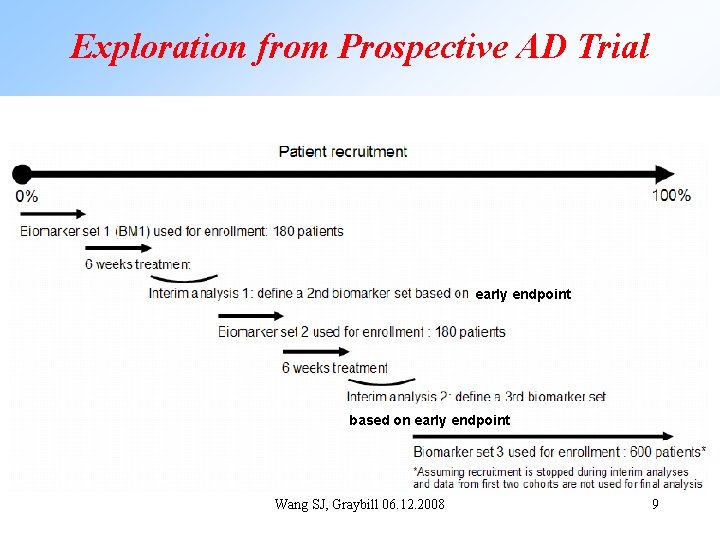
Exploration from Prospective AD Trial early endpoint based on early endpoint Wang SJ, Graybill 06. 12. 2008 9
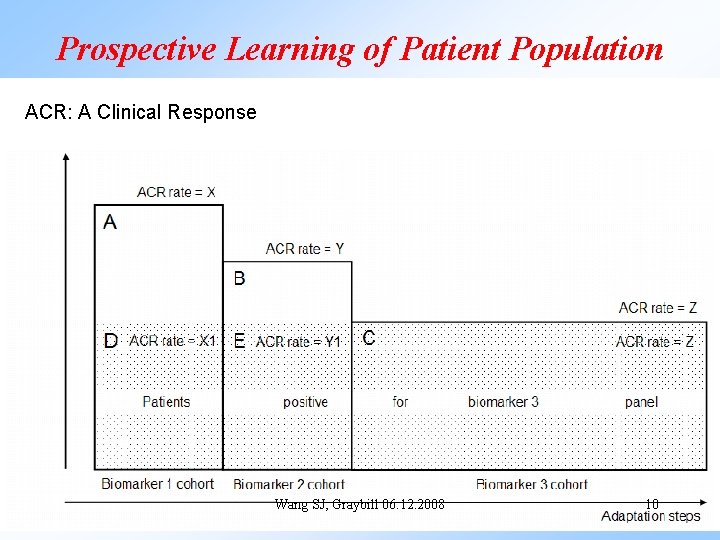
Prospective Learning of Patient Population ACR: A Clinical Response Wang SJ, Graybill 06. 12. 2008 10
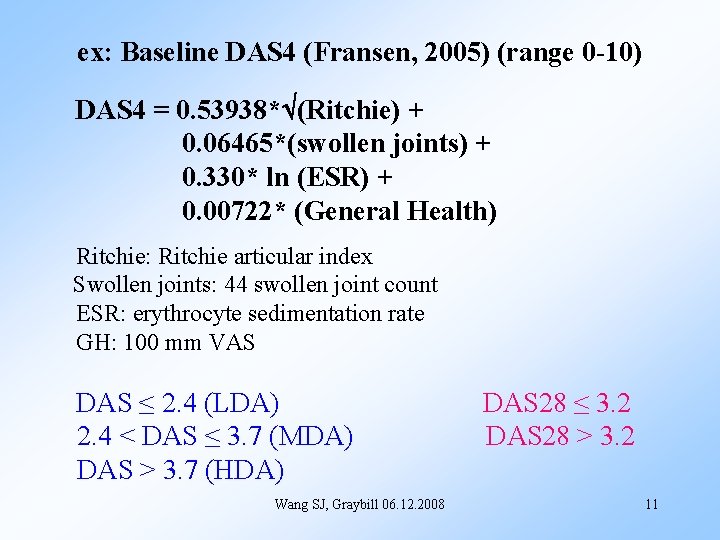
ex: Baseline DAS 4 (Fransen, 2005) (range 0 -10) DAS 4 = 0. 53938* (Ritchie) + 0. 06465*(swollen joints) + 0. 330* ln (ESR) + 0. 00722* (General Health) Ritchie: Ritchie articular index Swollen joints: 44 swollen joint count ESR: erythrocyte sedimentation rate GH: 100 mm VAS DAS ≤ 2. 4 (LDA) 2. 4 < DAS ≤ 3. 7 (MDA) DAS > 3. 7 (HDA) Wang SJ, Graybill 06. 12. 2008 DAS 28 ≤ 3. 2 DAS 28 > 3. 2 11
Adaptive Designs in Adequate and Well-Controlled Setting · When a (composite) genomic biomarker is developed (not a preliminary biomarker panel that is continually refined), preliminary utility of biomarker as a classifier needs analytic validation and feasibility study · To prospectively assess the biomarker’s clinical utility, adaptive design in adequate and wellcontrolled setting may be considered Wang SJ, Graybill 06. 12. 2008 12
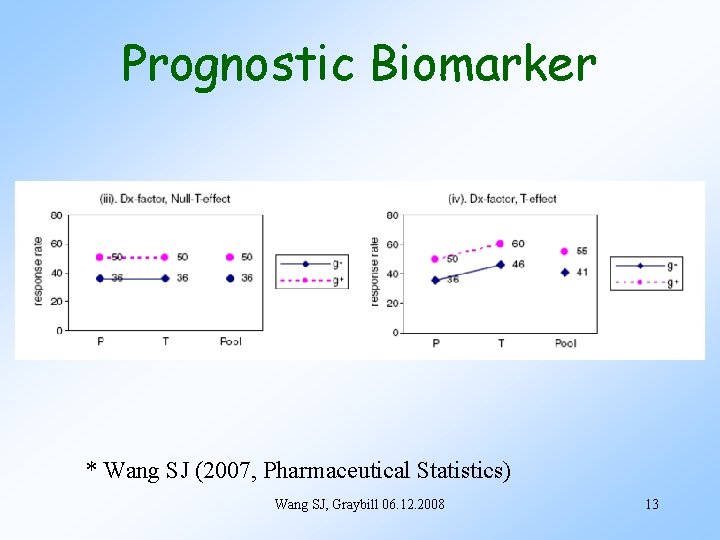
Prognostic Biomarker * Wang SJ (2007, Pharmaceutical Statistics) Wang SJ, Graybill 06. 12. 2008 13
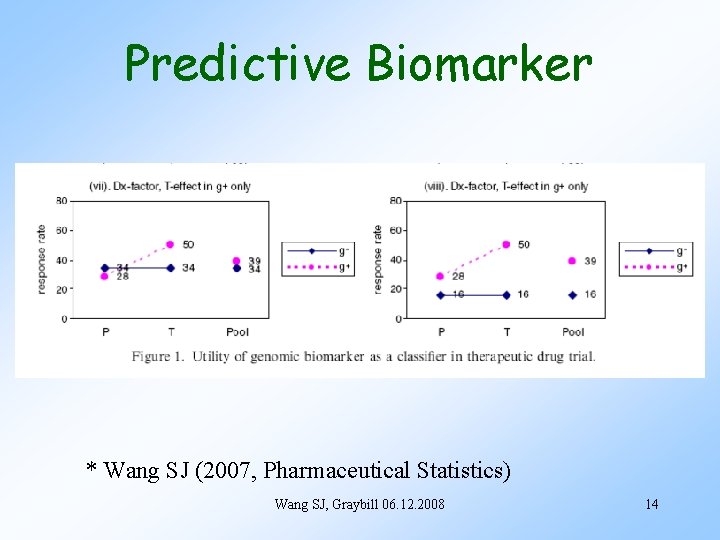
Predictive Biomarker * Wang SJ (2007, Pharmaceutical Statistics) Wang SJ, Graybill 06. 12. 2008 14
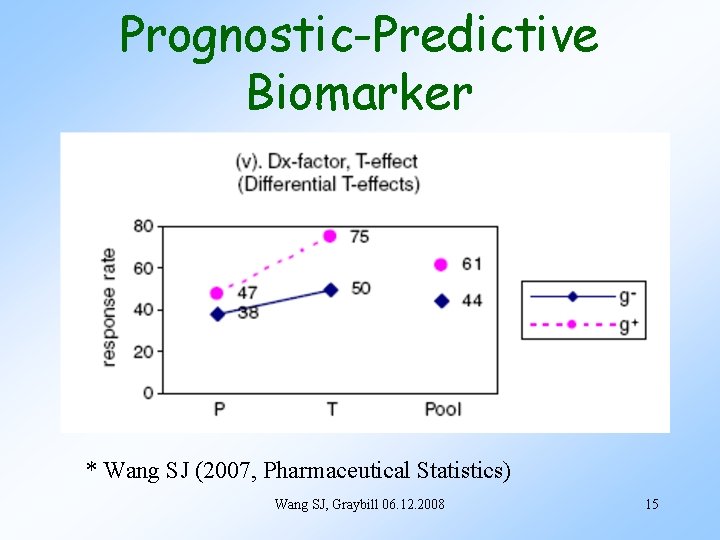
Prognostic-Predictive Biomarker * Wang SJ (2007, Pharmaceutical Statistics) Wang SJ, Graybill 06. 12. 2008 15
Prospective Testing of Biomarker Sensitive Patient Subset A Study Adequate to Support Effectiveness Claims Should Reflect a Clear Prior Hypothesis Documented In The Protocol *FDA Guidance on “providing clinical evidence of effectiveness for human drug and biological products” for Industry, 1998 Wang SJ, Graybill 06. 12. 2008 16
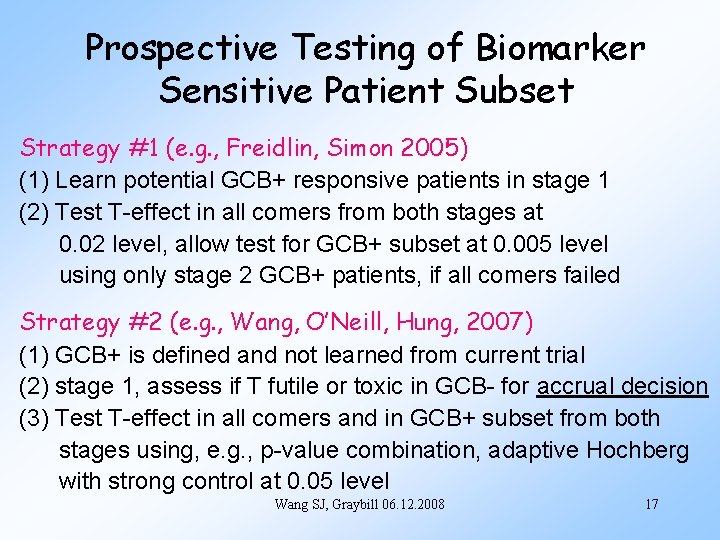
Prospective Testing of Biomarker Sensitive Patient Subset Strategy #1 (e. g. , Freidlin, Simon 2005) (1) Learn potential GCB+ responsive patients in stage 1 (2) Test T-effect in all comers from both stages at 0. 02 level, allow test for GCB+ subset at 0. 005 level using only stage 2 GCB+ patients, if all comers failed Strategy #2 (e. g. , Wang, O’Neill, Hung, 2007) (1) GCB+ is defined and not learned from current trial (2) stage 1, assess if T futile or toxic in GCB- for accrual decision (3) Test T-effect in all comers and in GCB+ subset from both stages using, e. g. , p-value combination, adaptive Hochberg with strong control at 0. 05 level Wang SJ, Graybill 06. 12. 2008 17
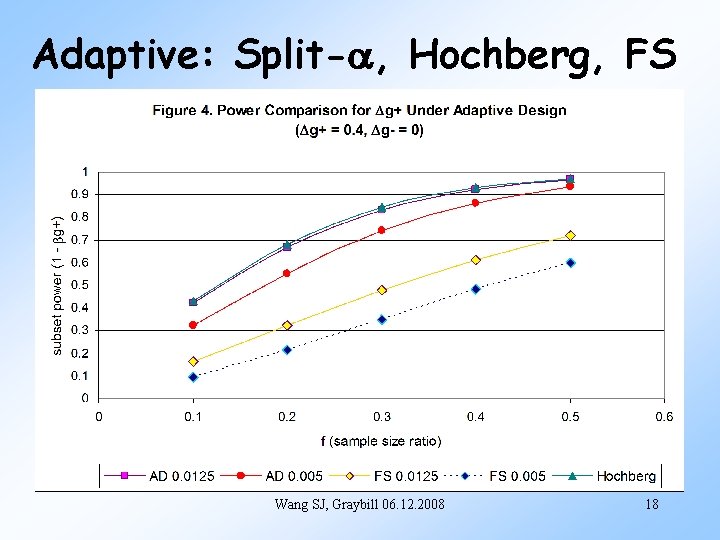
Adaptive: Split-a, Hochberg, FS Wang SJ, Graybill 06. 12. 2008 18
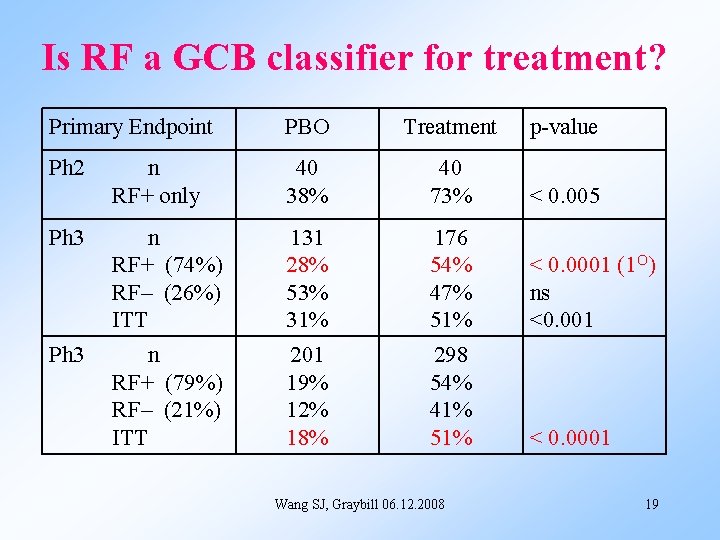
Is RF a GCB classifier for treatment? Primary Endpoint PBO Treatment p-value Ph 2 n RF+ only 40 38% 40 73% < 0. 005 n RF+ (74%) RF– (26%) ITT 131 28% 53% 31% 176 54% 47% 51% < 0. 0001 (1 O) ns <0. 001 n RF+ (79%) RF– (21%) ITT 201 19% 12% 18% 298 54% 41% 51% < 0. 0001 Ph 3 Wang SJ, Graybill 06. 12. 2008 19
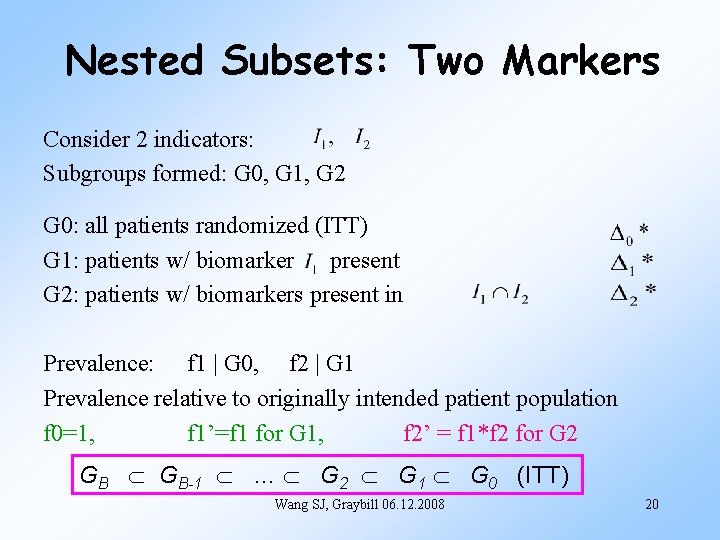
Nested Subsets: Two Markers Consider 2 indicators: Subgroups formed: G 0, G 1, G 2 G 0: all patients randomized (ITT) G 1: patients w/ biomarker present G 2: patients w/ biomarkers present in Prevalence: f 1 | G 0, f 2 | G 1 Prevalence relative to originally intended patient population f 0=1, f 1’=f 1 for G 1, f 2’ = f 1*f 2 for G 2 GB GB-1 … G 2 G 1 G 0 (ITT) Wang SJ, Graybill 06. 12. 2008 20
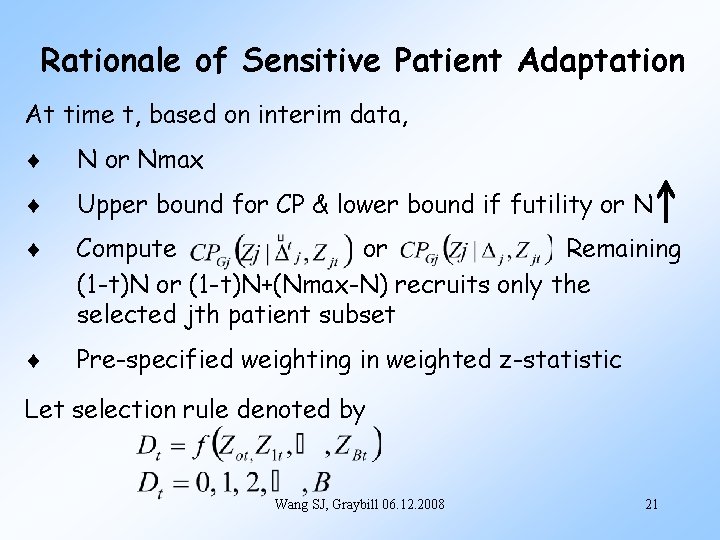
Rationale of Sensitive Patient Adaptation At time t, based on interim data, N or Nmax Upper bound for CP & lower bound if futility or N Compute or Remaining (1 -t)N or (1 -t)N+(Nmax-N) recruits only the selected jth patient subset Pre-specified weighting in weighted z-statistic Let selection rule denoted by Wang SJ, Graybill 06. 12. 2008 21
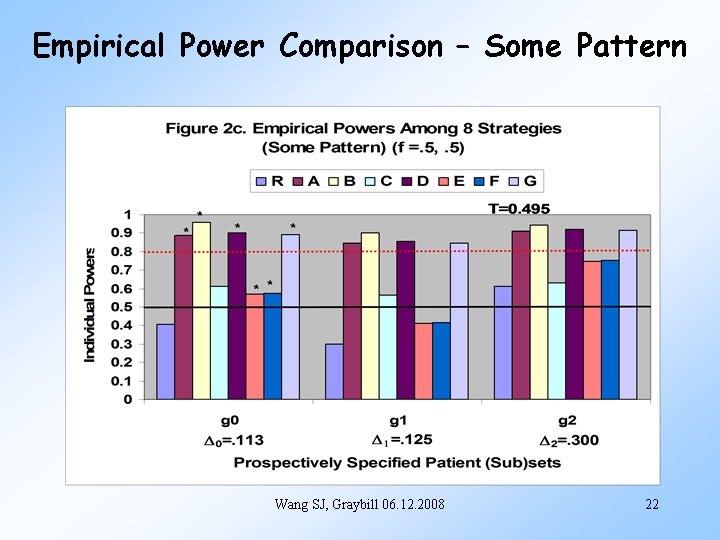
Empirical Power Comparison – Some Pattern Wang SJ, Graybill 06. 12. 2008 22
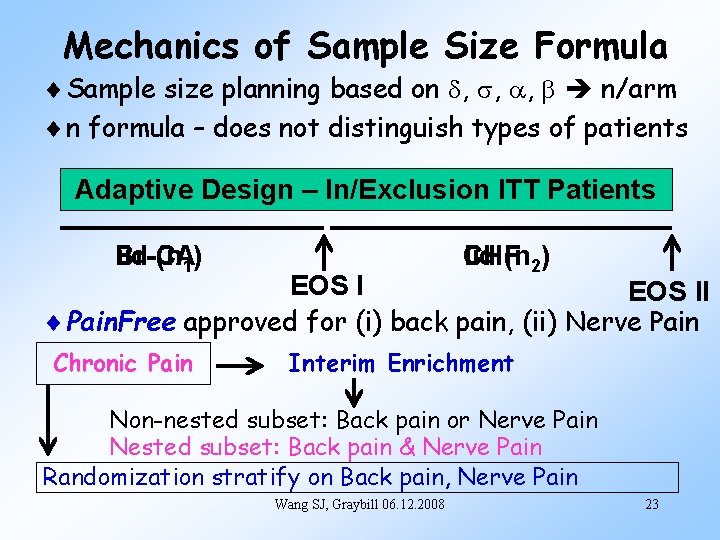
Mechanics of Sample Size Formula Sample size planning based on d, s, a, b n/arm n formula – does not distinguish types of patients Adaptive Design – In/Exclusion ITT Patients Br-CA iid (n 1) CHF iid (n 2) EOS II Pain. Free approved for (i) back pain, (ii) Nerve Pain Chronic Pain Interim Enrichment Non-nested subset: Back pain or Nerve Pain Nested subset: Back pain & Nerve Pain Randomization stratify on Back pain, Nerve Pain Wang SJ, Graybill 06. 12. 2008 23
Concluding Remarks Exploratory biomarker development - flexible AD design For A&WC trials, recommend stratified randomization based on biomarker status to avoid bias Biomarker status for ITT patients should be available Two-stage adaptive design in A&WC setting provides flexibility for assessing sensitive patients prospectively and effectively Improvement from conventional null, sample size caveats Replication of the finding needed Wang SJ, Graybill 06. 12. 2008 24

Some References Cui, Hung, Wang. (1999, Biometrics) Wang, Chen. (2004, Journal of Computational Biology) Wang. (2005, Flexible Design Genomic Drug Trial, NCI-FDA Biomarker Wksp) Wang. (2005, Special report in 1 st Multi-track DIA WKSP, Japan) Tsai, Wang, Chen. (2005, Bioinformatics) Simon, Wang (2006, The Pharmacogenomics Journal (TPJ)) Trepicchio, Essayan, Hall, Schechter, Tezak, Wang, et al. (2006, TPJ) Wang, Cohen, Katz, et al. (2006, TPJ) Chen, Wang, Tsai, Lin (2006, TPJ) Microarray Quality Control Project: (2006, Nature Biotechnology) Wang. (2007, Taiwan Clinical Trials) Wang, O’Neill, Hung. (2007, Pharmaceutical Statistics) Wang. (2007, Pharmaceutical Statistics): Biomarker as a classifier in pharmacugenomics clinical trials: a tribute to 30 th anniversary of PSI (Statistician in the Pharmaceutical Industry) Wang et al. (2008, invited Biometrical J. in progress) Wang SJ, Graybill 06. 12. 2008 25
- Slides: 25