Adaptive Clinical Trial Designs Health Canadas Regulatory Perspective











- Slides: 16

Adaptive Clinical Trial Designs: Health Canada’s Regulatory Perspective Celia Lourenco, Ph. D Bureau of Gastroenterology, Infection And Viral Diseases 1

Health Canada - HPFB • Reviews and authorises clinical trial applications for pharmaceuticals, biologics, radiopharmaceuticals, medical devices, and natural health products • Reviews and authorises for sale pharmaceuticals, biologics, radiopharmaceuticals, medical devices, and natural health products • Carries out pharmacovigilance in clinical trials and postmarket 2

Main regulatory concerns • Design should be appropriate to answer the scientific question of interest • Sponsor must demonstrate control of Power and Type I error rate over the entire clinical trial • Operational bias – appropriate pre-planning and measures are implemented to avoid operational bias (e. g. , randomization, blinding, secure electronic systems, independent DSMBs, blinded steering committees, etc. ) 3

Adaptive Designs • On Health Canada’s radar for several years (HC working group formed in 2008) • Many seen in clinical trial applications (CTAs) • Some in new drug submissions (NDSs) 4

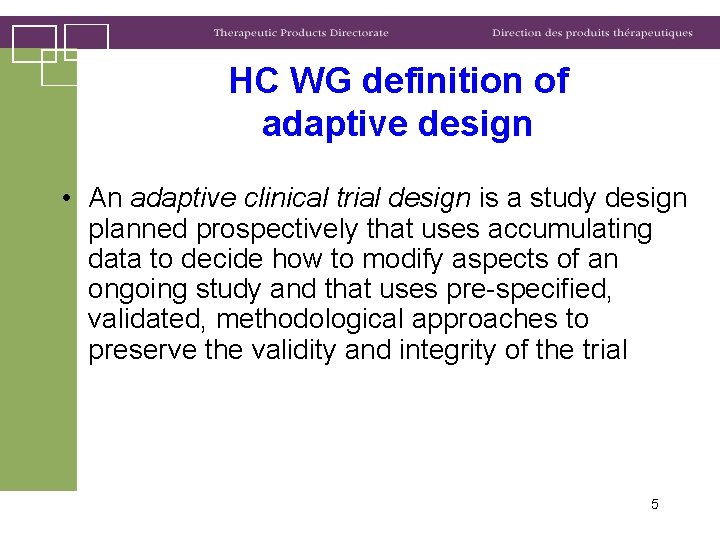
HC WG definition of adaptive design • An adaptive clinical trial design is a study design planned prospectively that uses accumulating data to decide how to modify aspects of an ongoing study and that uses pre-specified, validated, methodological approaches to preserve the validity and integrity of the trial 5

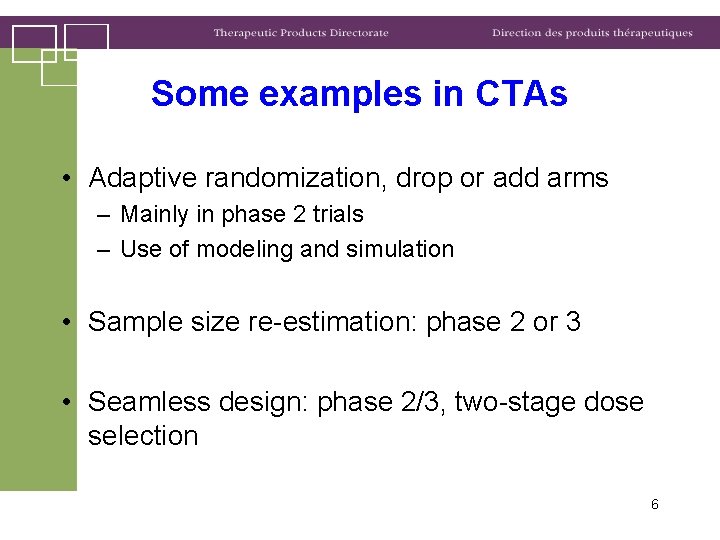
Some examples in CTAs • Adaptive randomization, drop or add arms – Mainly in phase 2 trials – Use of modeling and simulation • Sample size re-estimation: phase 2 or 3 • Seamless design: phase 2/3, two-stage dose selection 6

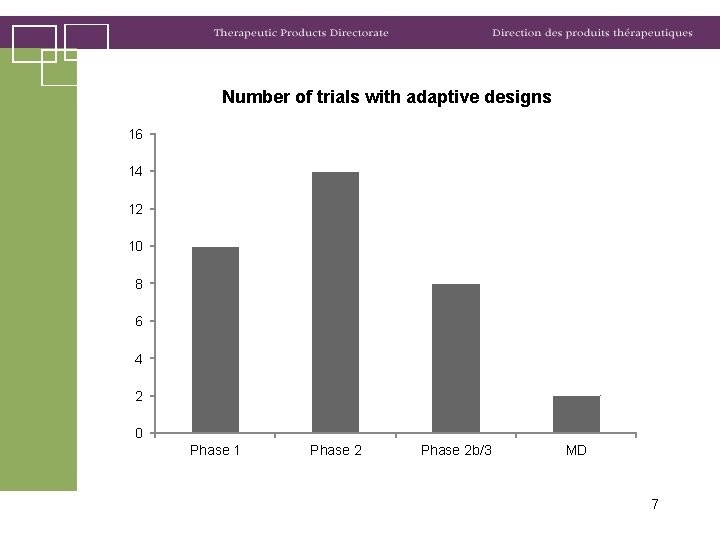
Number of trials with adaptive designs 16 14 12 10 8 6 4 2 0 Phase 1 Phase 2 b/3 MD 7

Number of adaptive trials using Bayesian statistics 12 10 8 6 4 2 0 Phase 1 Phase 2 b/3 MD 8

Some examples in NDSs • Indacaterol: for treatment of COPD - Trial B 2335 S, a 26 -week seamless adaptive design trial that included an initial 2 week dose-ranging phase • Gardasil 9: a vaccine indicated for the prevention of infection caused by the Human Papillomavirus (HPV) types 6, 11, 16, 18, 31, 33, 45, 52 and 58 – A Randomized, International, Double-Blinded (With In-House Blinding), Controlled With GARDASIL™, Dose-Ranging, Tolerability, Immunogenicity, and Efficacy Study of a Multivalent Human Papillomavirus (HPV) L 1 Virus-Like Particle (VLP) Vaccine Administered to 16 - to 26 -Year-Old Women 9

B 2335 s Trial Design 10

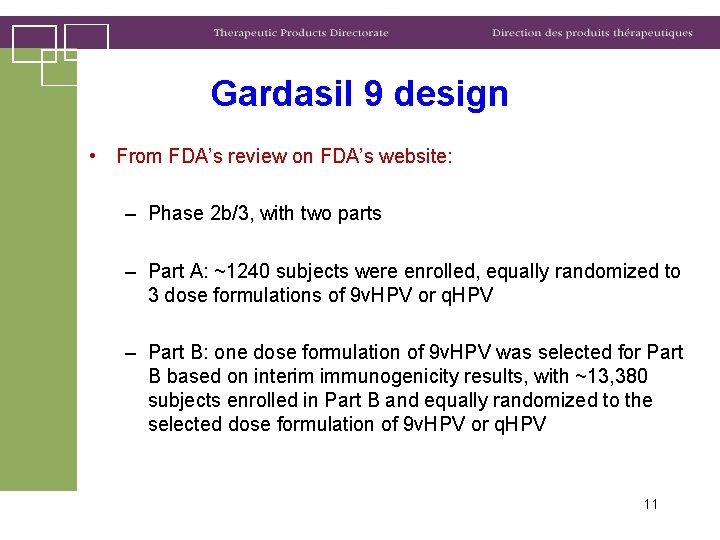
Gardasil 9 design • From FDA’s review on FDA’s website: – Phase 2 b/3, with two parts – Part A: ~1240 subjects were enrolled, equally randomized to 3 dose formulations of 9 v. HPV or q. HPV – Part B: one dose formulation of 9 v. HPV was selected for Part B based on interim immunogenicity results, with ~13, 380 subjects enrolled in Part B and equally randomized to the selected dose formulation of 9 v. HPV or q. HPV 11

Conclusion from case-studies • Adaptive designs present increased complexity • May be difficult to interpret, and make use of new statistical tests/procedures • Increase in use of Bayesian statistics, which involves a different approach in statistical inference • Increase use of simulation – time consuming to validate and can be difficult to interpret what the results mean 12

Why and What of Simulation • Simulation provides the operating characteristics of the trial design • That is the description (a picture) of the likelihood (probabilities) of how the trial will progress down the different paths (planned possible adaptations) to eventual completion or termination of the trial 13

Seeing that Picture • The sponsors should provide that picture • Usually in well designed and planned trials, yes • The question is should regulators look behind the picture and actually check that it was done right? – That means try to duplicate the simulation results – Ask to submit codes, and check/replicate 14

Conclusions & recommendations • Continue to monitor adaptive designs, expect that the topic will continue to evolve • Pre-submission meetings are encouraged • Optimize biostatistics and IT resources to deal with emerging trends such as use of Bayesian statistics • Provide support and encourage attendance of courses and seminars on adaptive design • Biostatistics consult sought (early) for pivotal Adaptive Design trials in submissions 15

THANK YOU!
 Temperatura en las cañadas del teide
Temperatura en las cañadas del teide Canadas landforms
Canadas landforms 90 of the canadian population lives within
90 of the canadian population lives within Whats canadas longest river
Whats canadas longest river Unit 5 food national geographic
Unit 5 food national geographic Who is canadas head of state
Who is canadas head of state What is canada's economic system
What is canada's economic system Iespm granada
Iespm granada Physical regions in canada
Physical regions in canada Canadas climate
Canadas climate Cynchia
Cynchia Rsna clinical trial processor
Rsna clinical trial processor Morpheus clinical trial
Morpheus clinical trial Clinical trial budget example
Clinical trial budget example Novel clinical drug trial design
Novel clinical drug trial design Clinical trials.gov api
Clinical trials.gov api Clinical trial financial management
Clinical trial financial management