ADAPTATION INJURY and DEATH of CELLS Learning Objectives

ADAPTATION, INJURY and DEATH of CELLS

Learning Objectives • 1. List examples of hypertrophy, hyperplasia, atrophy, hypoplasia, metaplasia and dysplasia • 2. List examples of reversible and irreversible cell injury • 3. Diagram abscess, granuloma, renal infarction and fat necrosis • 4. List consequences of ATP loss • 5. List consequences Ca++ increase and release

Pathology: the Study of Disease • Etiology or cause: infection, genetic etc. and often mutifactoral • Pathogenesis: progression of the disease • (Molecular and Morphologic Changes) • Clinical Manifestations: signs and symptoms

Cellular Adaptations • • • Hypertrophy Hyperplasia Atrophy Metaplasia Dysplasia
HYPERTROPHY • Increase in cell size with subsequent increase in organ size

Causes of Hypertrophy 1. Increased functional demand 2. Hormonal stimulation

Hypertrophy of Uterus During Pregnancy
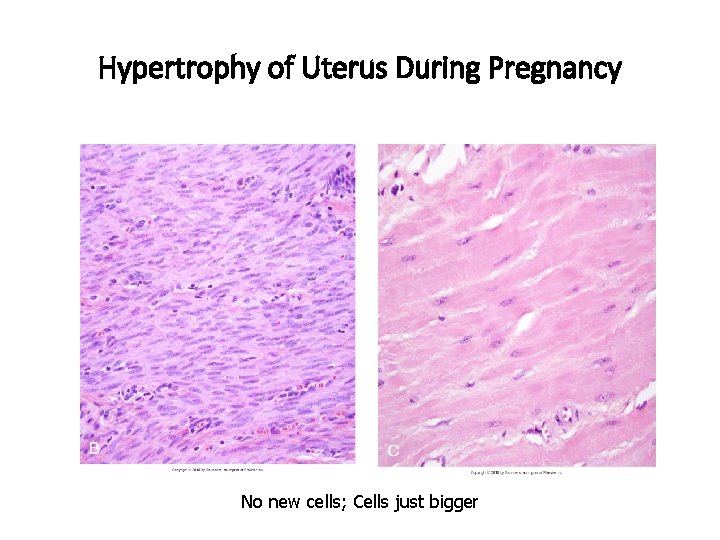
Hypertrophy of Uterus During Pregnancy No new cells; Cells just bigger

• Hypertrophy can be physiologic or pathologic

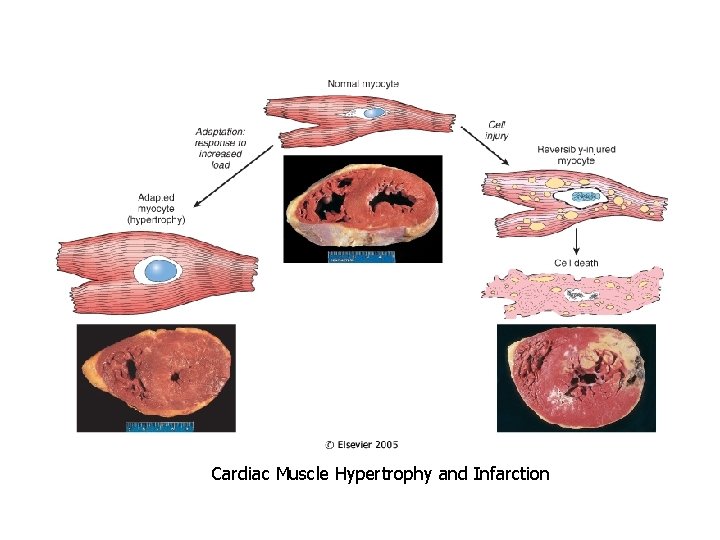
Cardiac Muscle Hypertrophy and Infarction
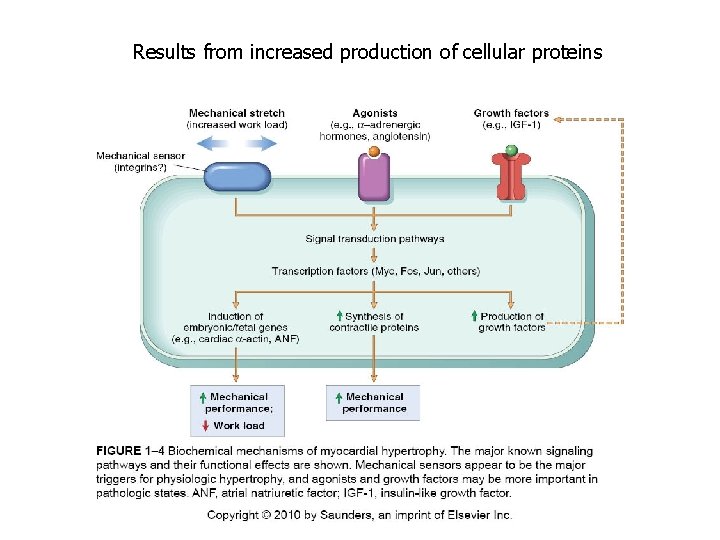
Results from increased production of cellular proteins
HYPERPLASIA • Increase in the number of cells in an organ which may then increase organ size. • Physiologic or Pathologic
PHYSIOLOGIC HYPERPLASIA 1. Hormonal hyperplasiafemale breast at puberty and in pregnancy 2. Compensatory hyperplasialiver regeneration after partial resection
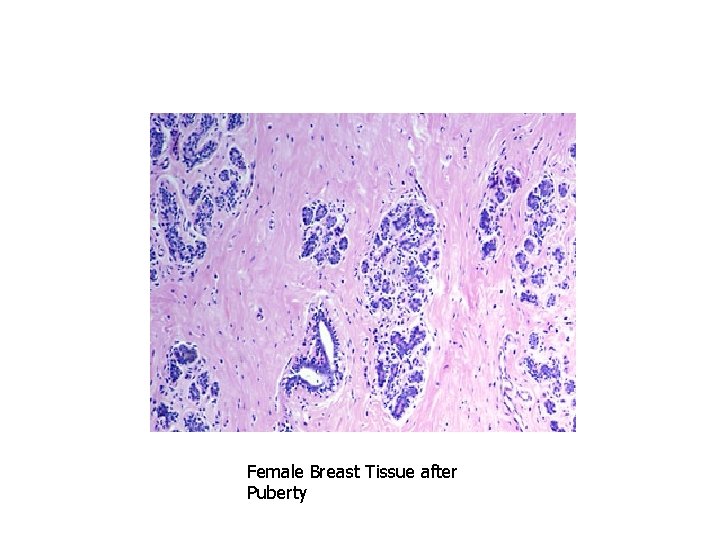
Female Breast Tissue after Puberty
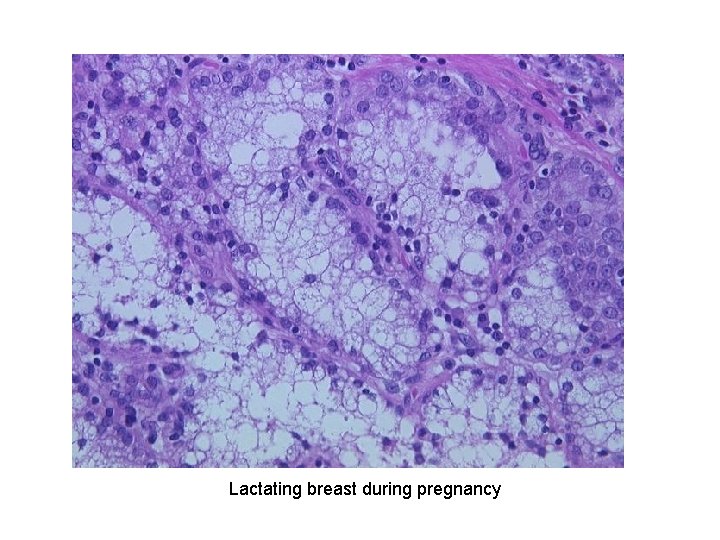
Lactating breast during pregnancy

Causes of Pathologic Hyperplasia 1. Excess hormoneendometrial hyperplasia due to estrogens

• Hyperplasia is NOT a neoplastic process, but it may be fertlie soil for malignancy • “Atypical Hyperplasia” in the endometrium carries an increased risk for development of endometrial adenocarcinoma
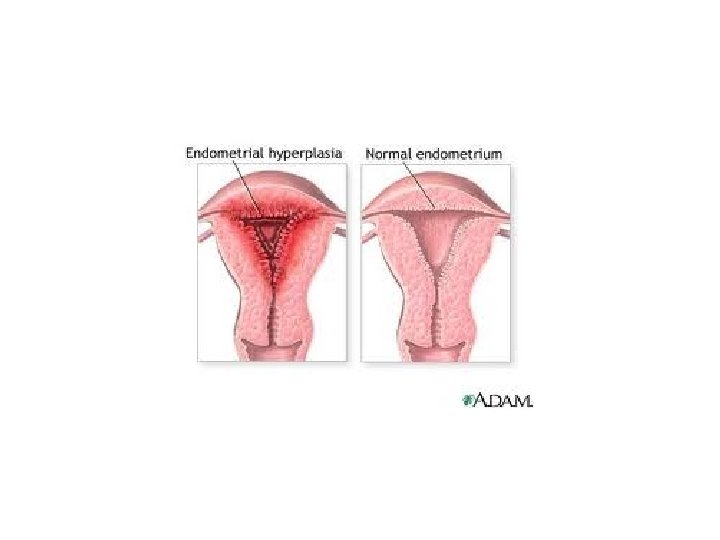
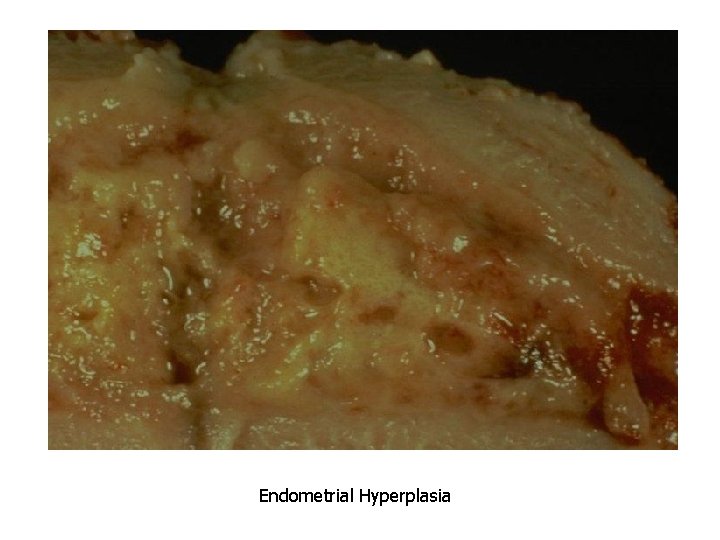
Endometrial Hyperplasia
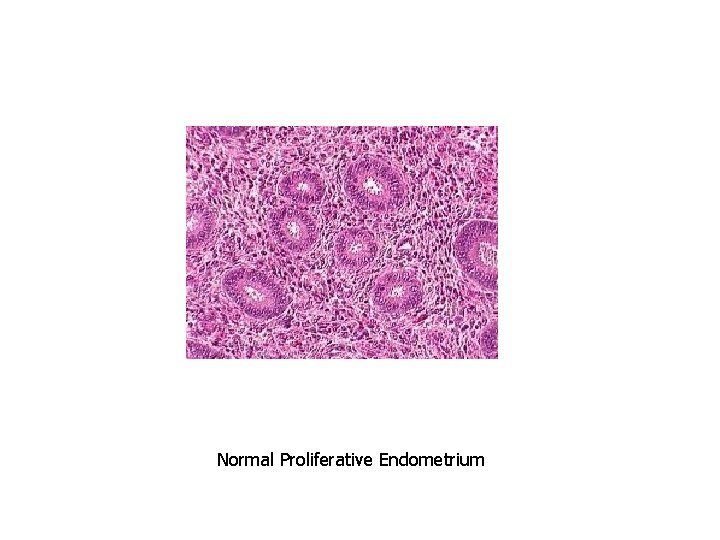
Normal Proliferative Endometrium
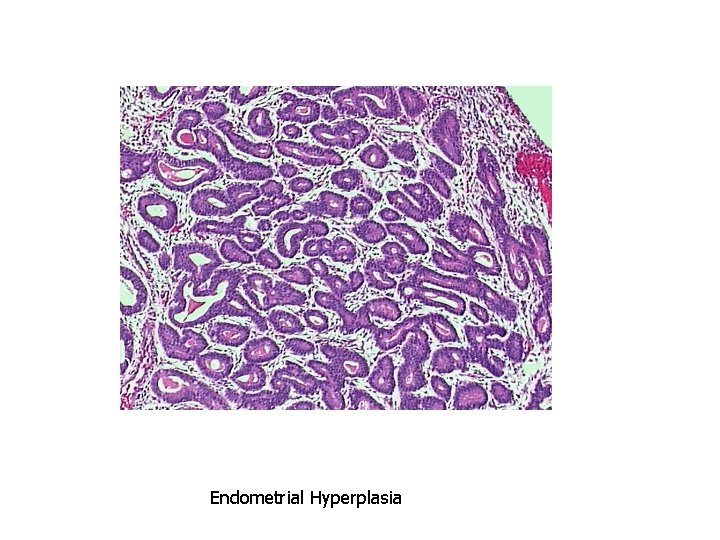
Endometrial Hyperplasia
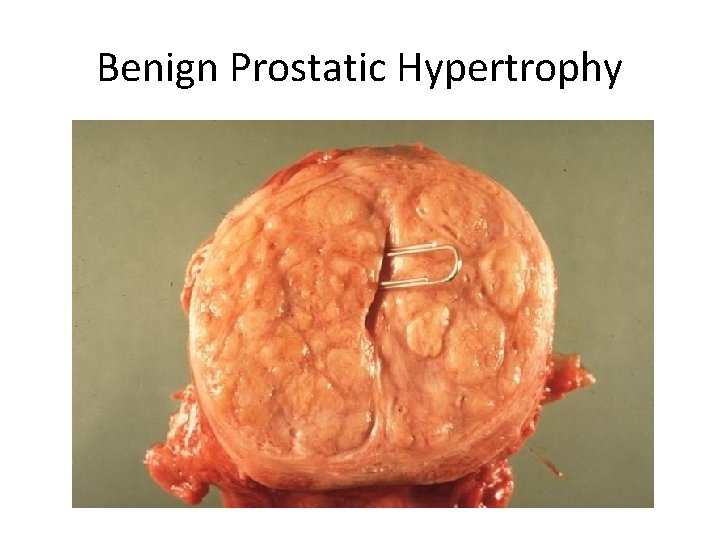
Benign Prostatic Hypertrophy

ATROPHY • Decrease in the size of a cell or organ by loss of cell substance (both size and number)
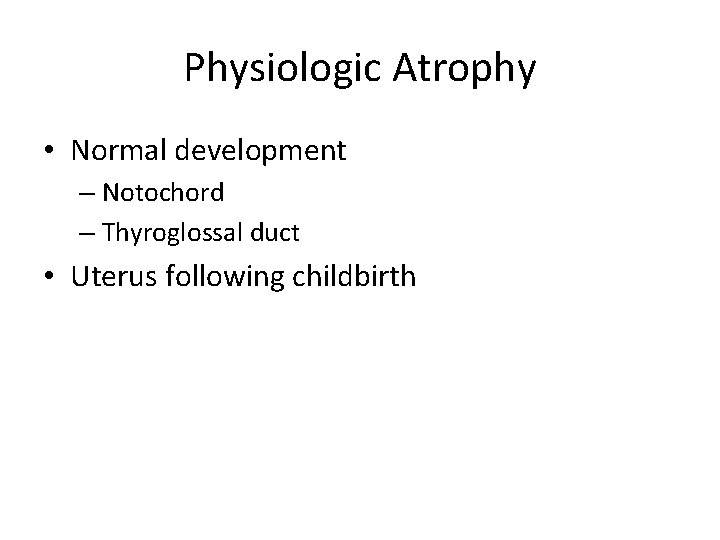
Physiologic Atrophy • Normal development – Notochord – Thyroglossal duct • Uterus following childbirth

Causes of Pathologic Atrophy 1. 2. 3. 4. 5. 6. Decreased workload Loss of innervation Decreased blood supply Inadequate nutrition Loss of endocrine stimulation Pressure

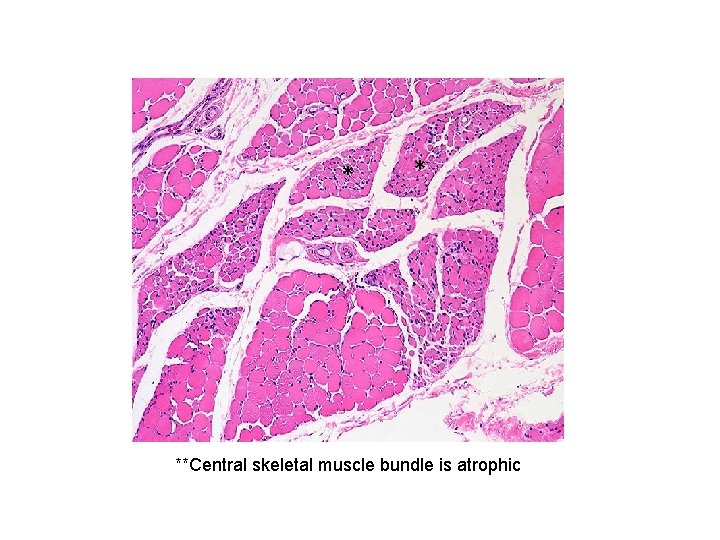
* * **Central skeletal muscle bundle is atrophic
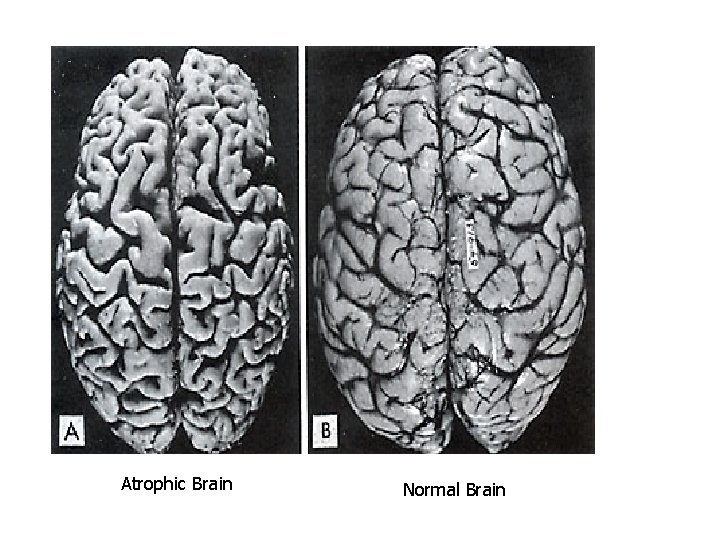
Atrophic Brain Normal Brain

Atrophy results from both… • Decreased protein synthesis • Increased protein degradation
Protein degradation is important in atrophy A. Lysosomes with hydrolytic enzymes B. The ubiquitin-proteasome pathway

HYPOPLASIA • Incomplete development of an organ so that it fails to reach adult size
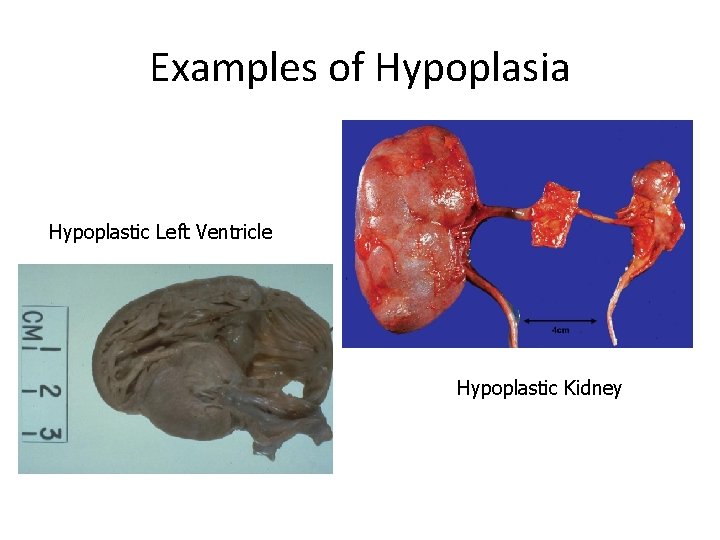
Examples of Hypoplasia Hypoplastic Left Ventricle Hypoplastic Kidney

METAPLASIA • A reversible change in which one ADULT cell type is replaced by another ADULT cell type

• C a u s e d Metaplasia b y : –C h r o n i c i r r i
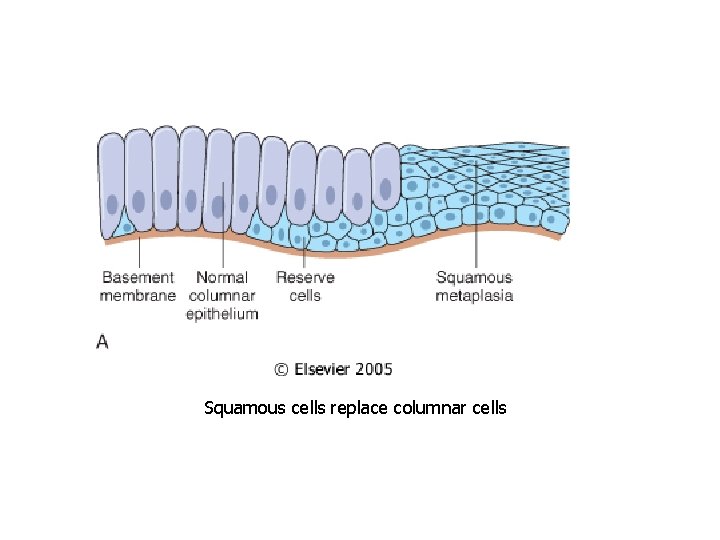
Squamous cells replace columnar cells
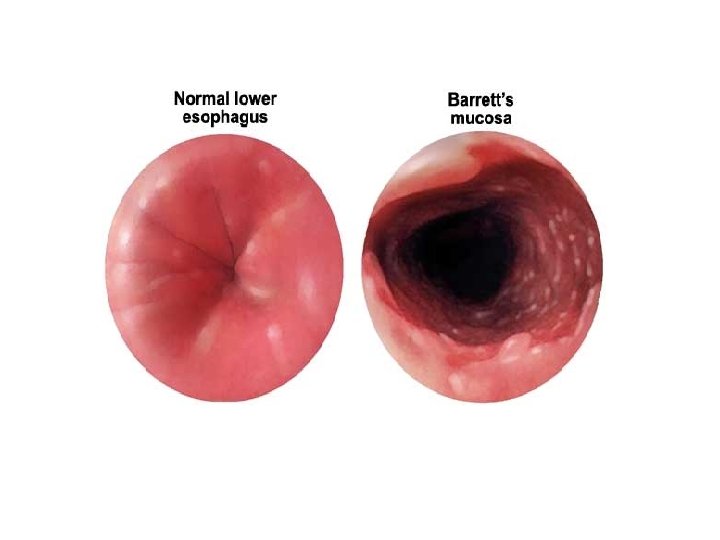
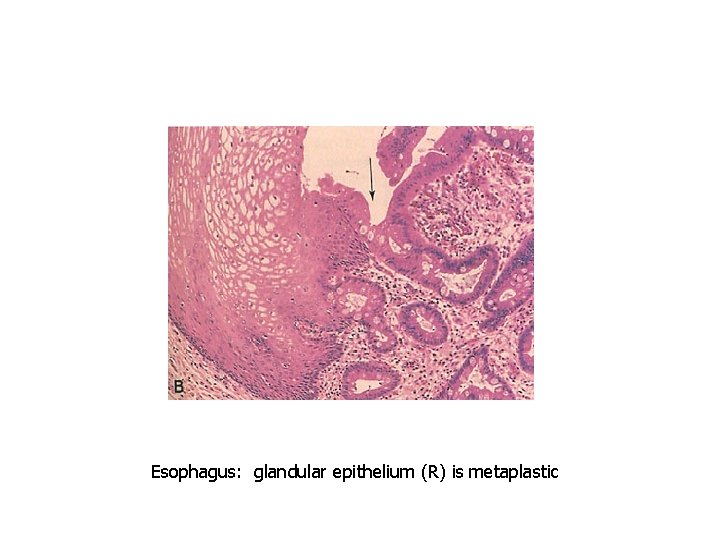
Esophagus: glandular epithelium (R) is metaplastic

Hyperplasia and Metaplasia are not premalignant changes, however they are “fertile fields” for Dysplasia which is a premalignant change
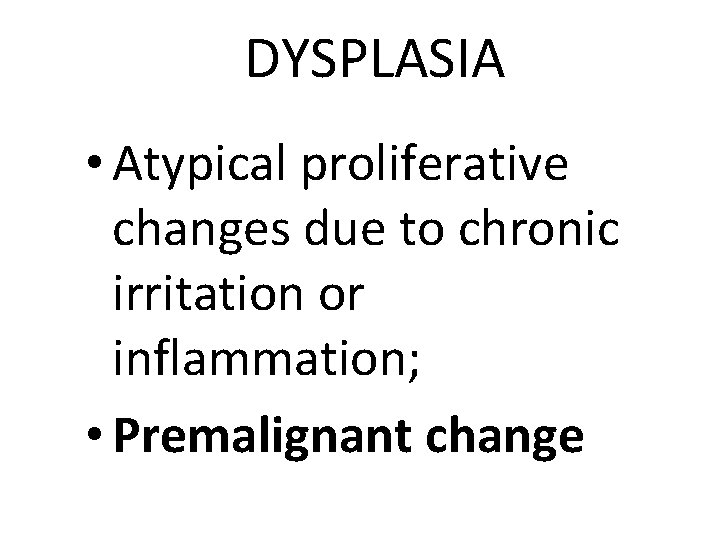
DYSPLASIA • Atypical proliferative changes due to chronic irritation or inflammation; • Premalignant change
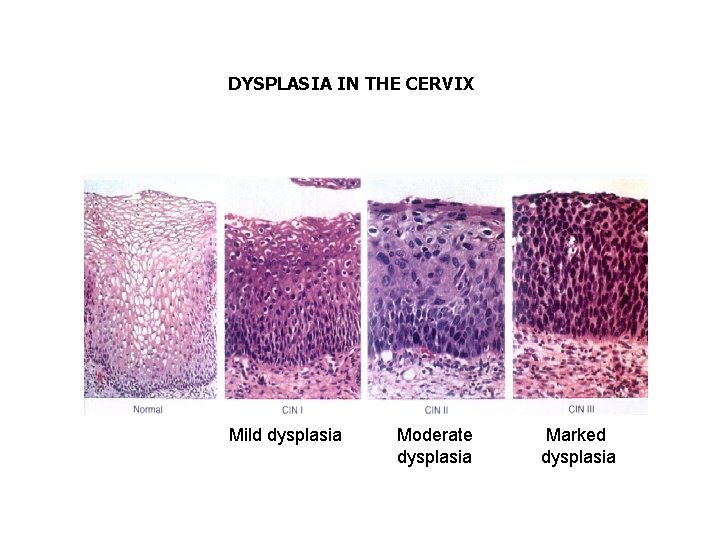
DYSPLASIA IN THE CERVIX Mild dysplasia Moderate dysplasia Marked dysplasia
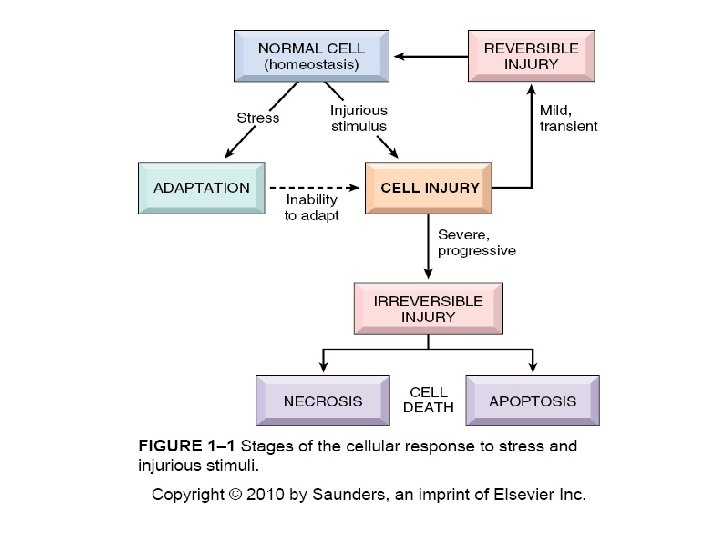
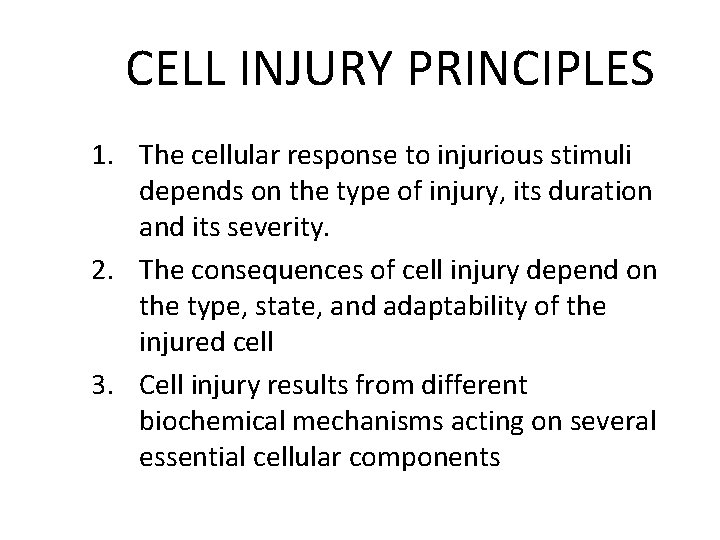
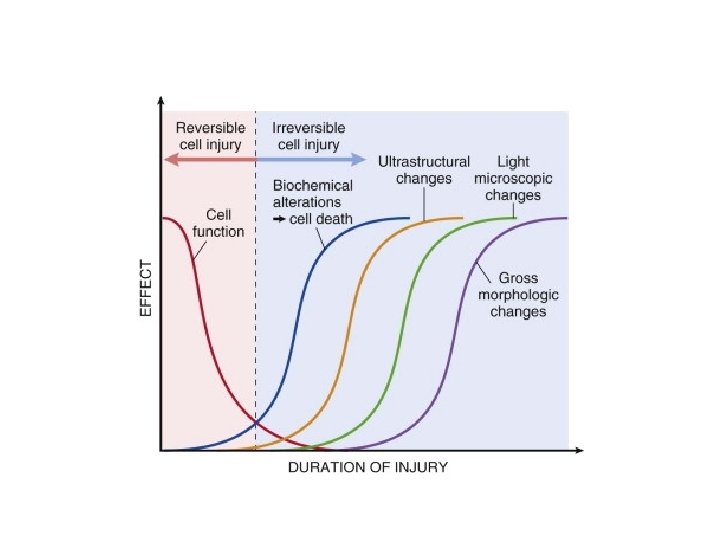
CELL INJURY PRINCIPLES 1. The cellular response to injurious stimuli depends on the type of injury, its duration and its severity. 2. The consequences of cell injury depend on the type, state, and adaptability of the injured cell 3. Cell injury results from different biochemical mechanisms acting on several essential cellular components
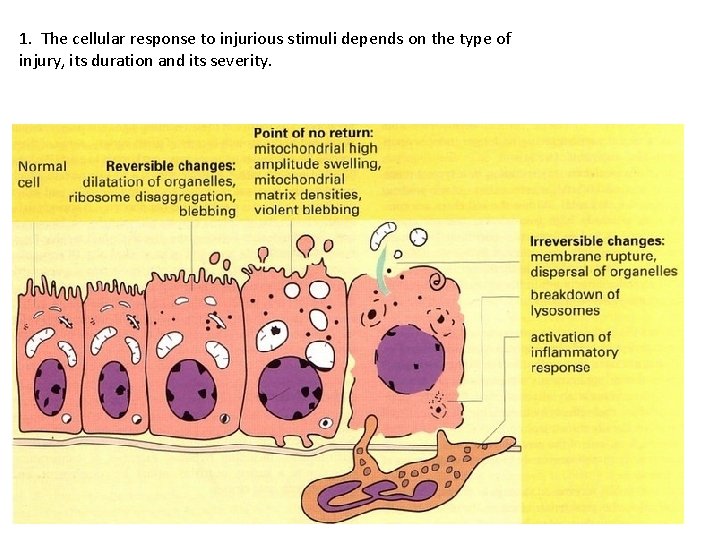
1. The cellular response to injurious stimuli depends on the type of injury, its duration and its severity.
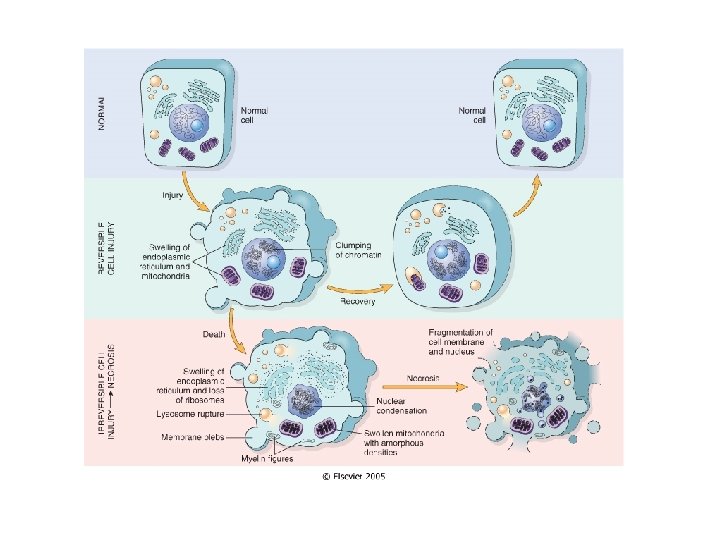
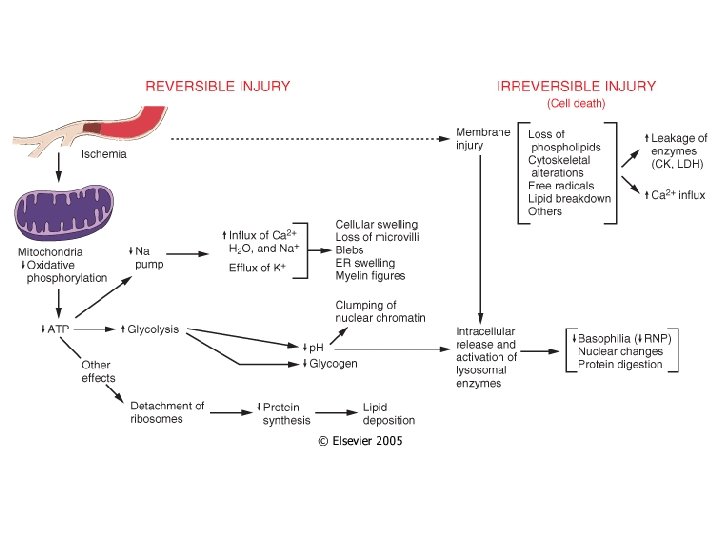
Cellular Changes Secondary to Injury REVERSIBLE • Cellular swelling • Cell membrane blebs • Detached ribosomes • Chromatin clumping IRREVERSIBLE • Lysosomes rupture • Dense bodies in mitochondria • Cell membrane rupture • Karyolysis, karyorrhexis, pyknosis
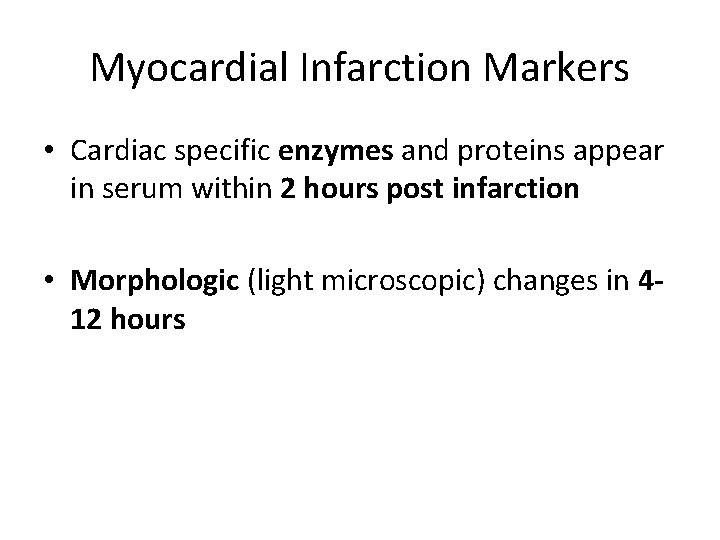
Myocardial Infarction Markers • Cardiac specific enzymes and proteins appear in serum within 2 hours post infarction • Morphologic (light microscopic) changes in 412 hours
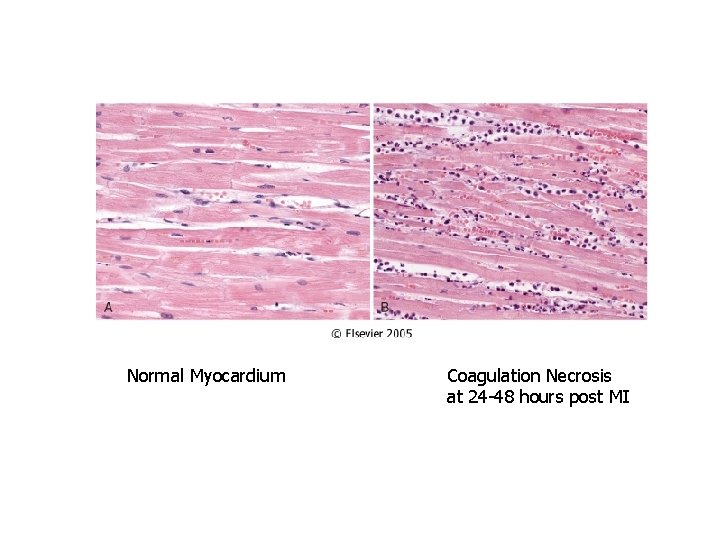
Normal Myocardium Coagulation Necrosis at 24 -48 hours post MI
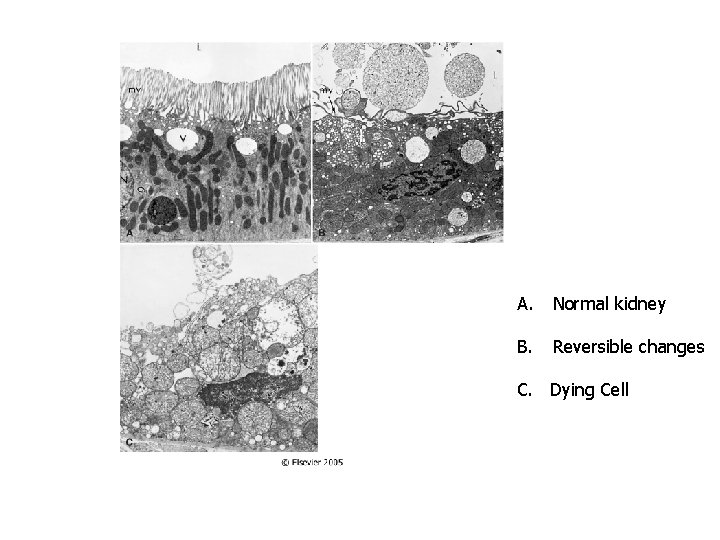
A. Normal kidney B. Reversible changes C. Dying Cell
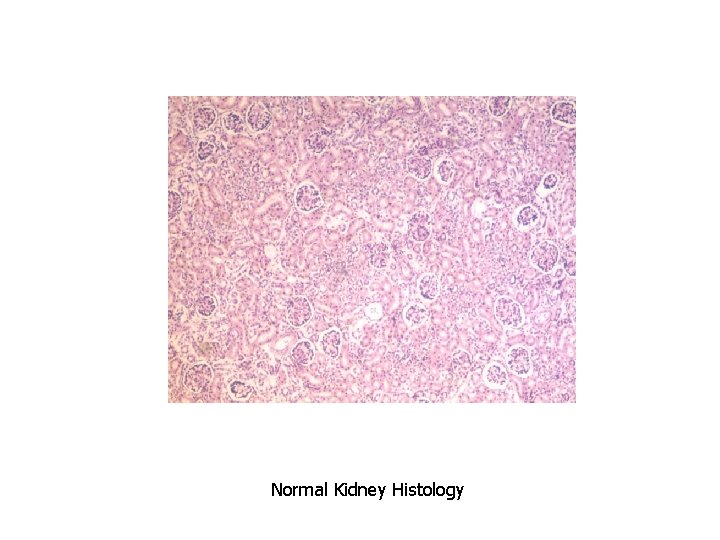
Normal Kidney Histology
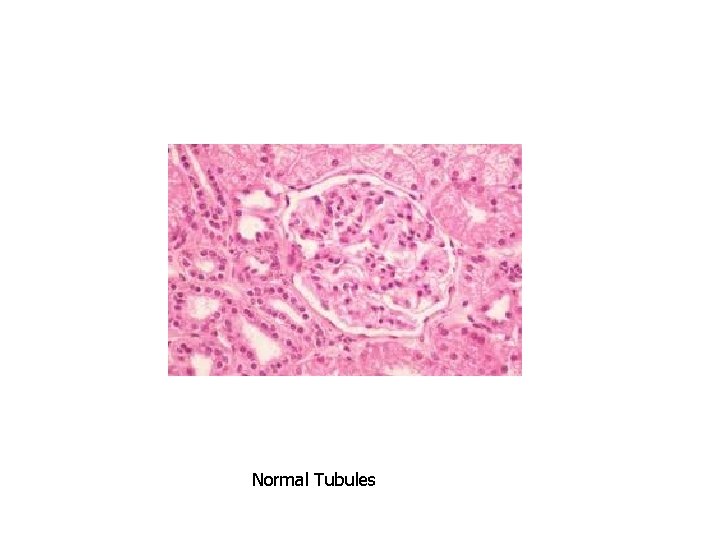
Normal Tubules
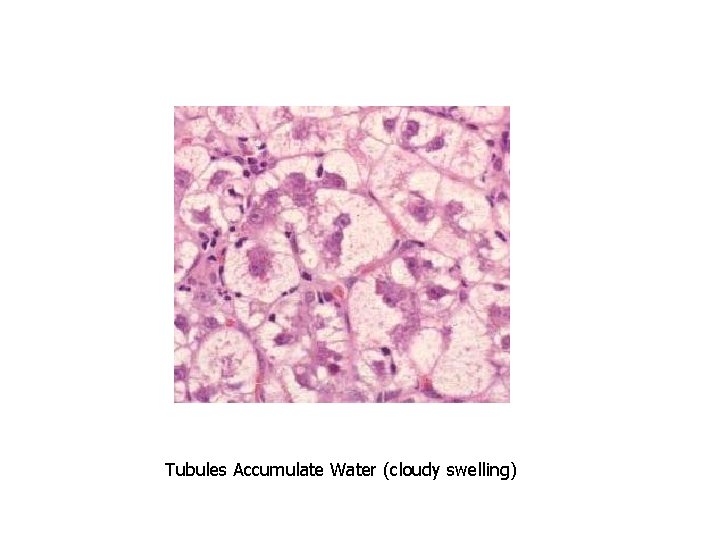
Tubules Accumulate Water (cloudy swelling)

2. The consequences of cell injury depend on the type, state, and adaptability of the injured cell
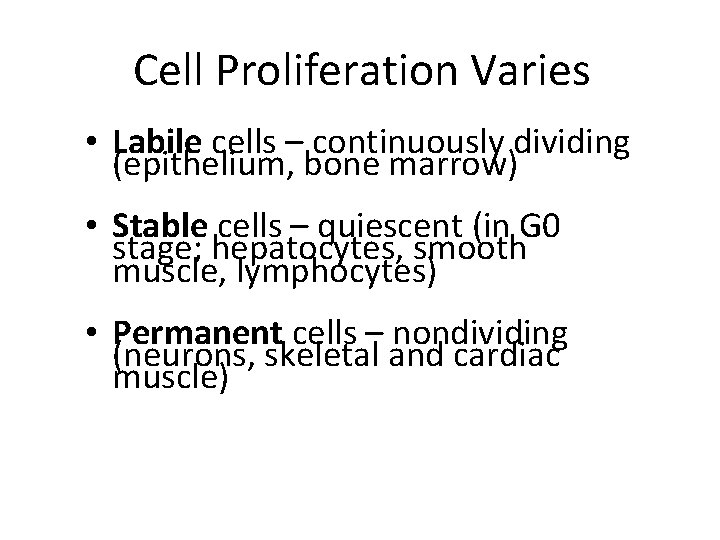
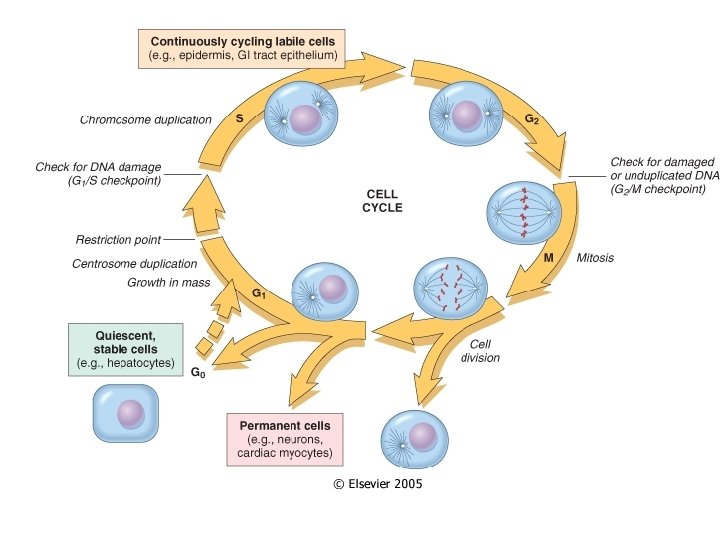
Cell Proliferation Varies • Labile cells – continuously dividing (epithelium, bone marrow) • Stable cells – quiescent (in G 0 stage; hepatocytes, smooth muscle, lymphocytes) • Permanent cells – nondividing (neurons, skeletal and cardiac muscle)
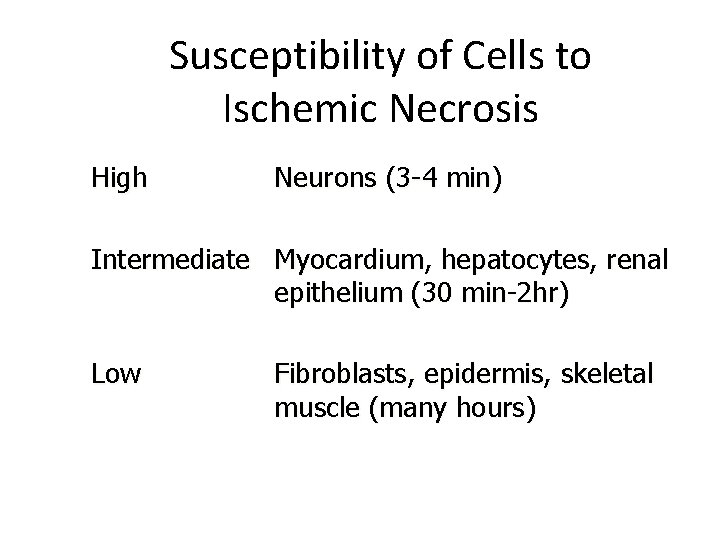
Susceptibility of Cells to Ischemic Necrosis High Neurons (3 -4 min) Intermediate Myocardium, hepatocytes, renal epithelium (30 min-2 hr) Low Fibroblasts, epidermis, skeletal muscle (many hours)
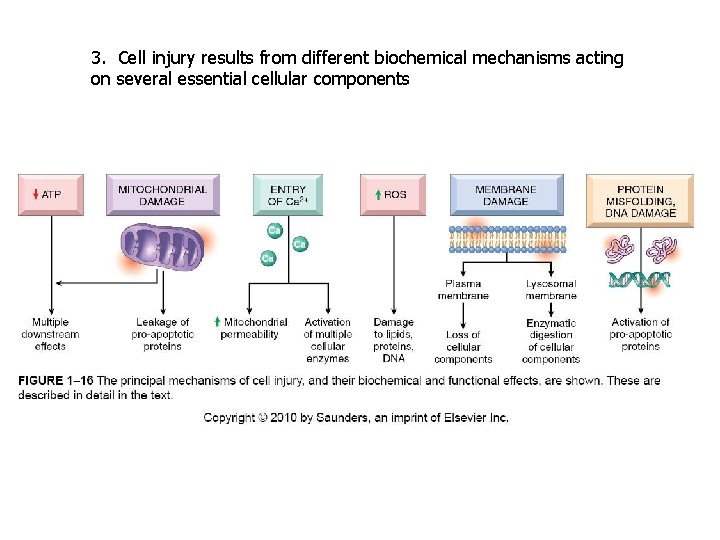
3. Cell injury results from different biochemical mechanisms acting on several essential cellular components
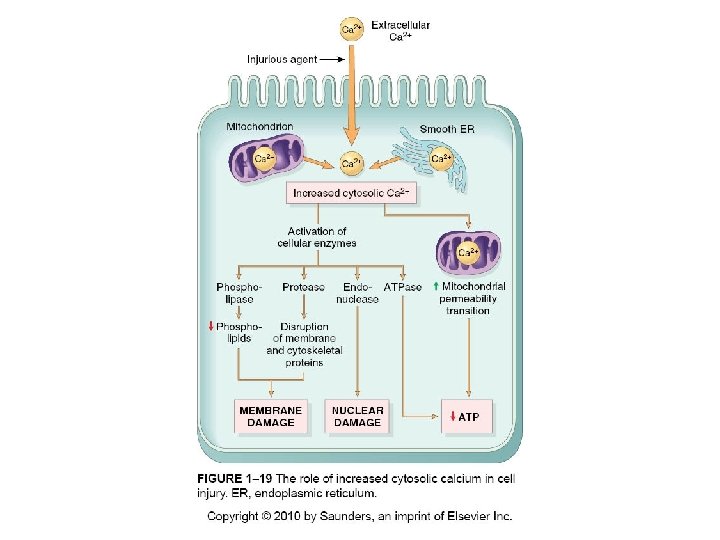
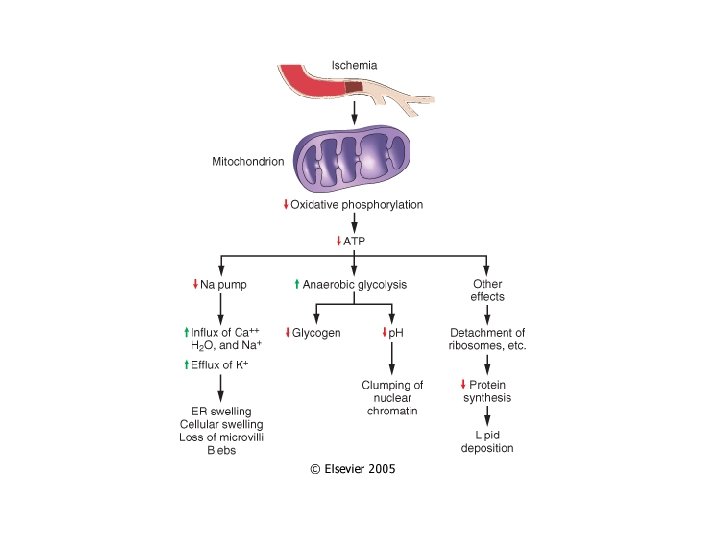
Depletion of ATP • Na+ pump fails Na+ and water enter and K+ is lost • Glycolysis depletes glycogen and lowers p. H (loss of enzyme activity) • Ca++ pump fails- Ca++ into cells (toxic) • Decreased protein synthesis (ribosomes detach) • � Unfolded protein response
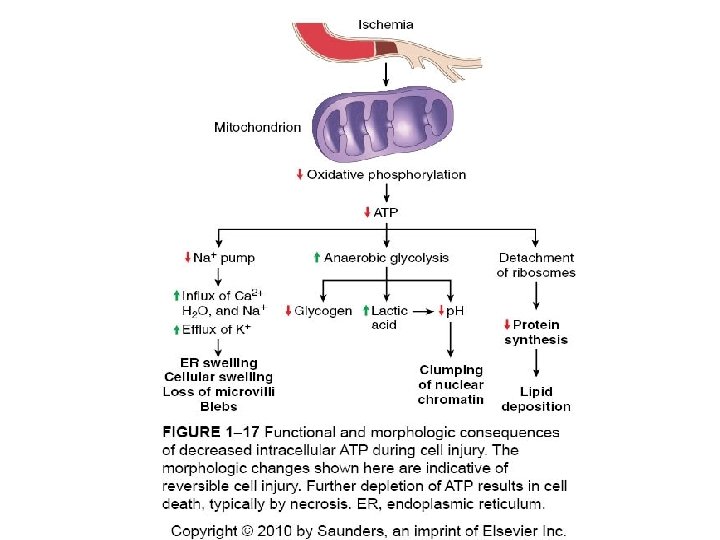
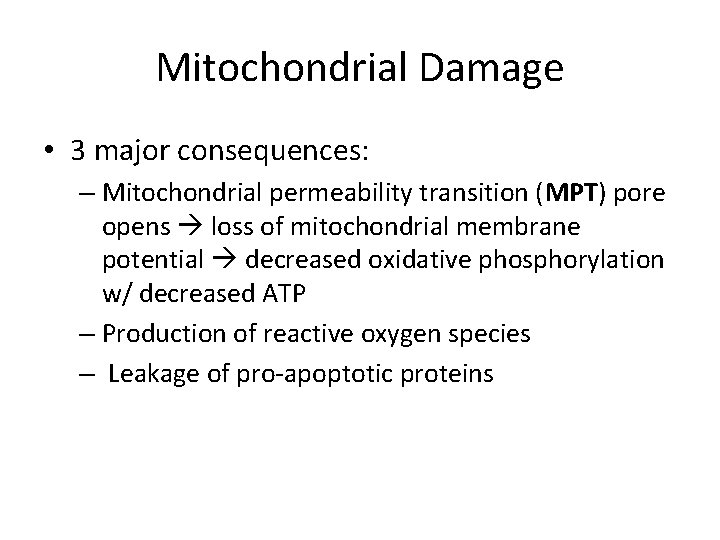
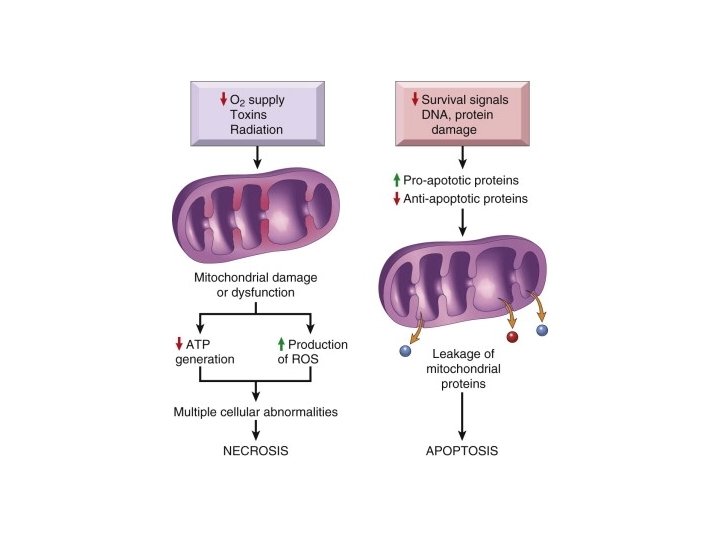
Mitochondrial Damage • 3 major consequences: – Mitochondrial permeability transition (MPT) pore opens loss of mitochondrial membrane potential decreased oxidative phosphorylation w/ decreased ATP – Production of reactive oxygen species – Leakage of pro-apoptotic proteins
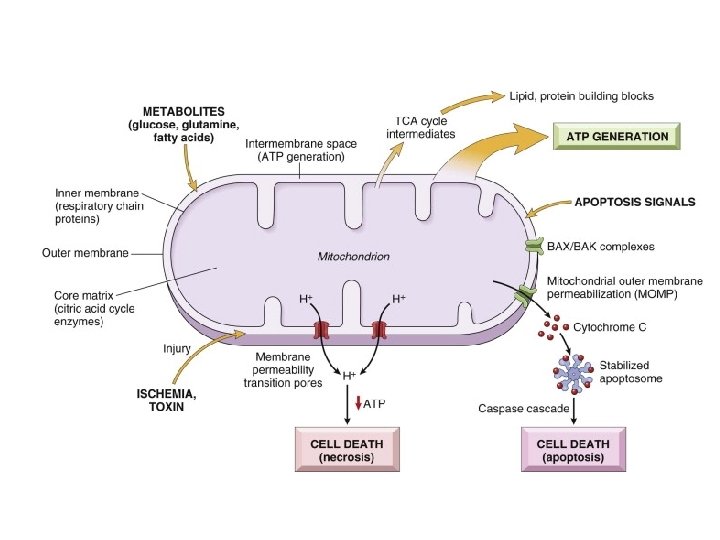
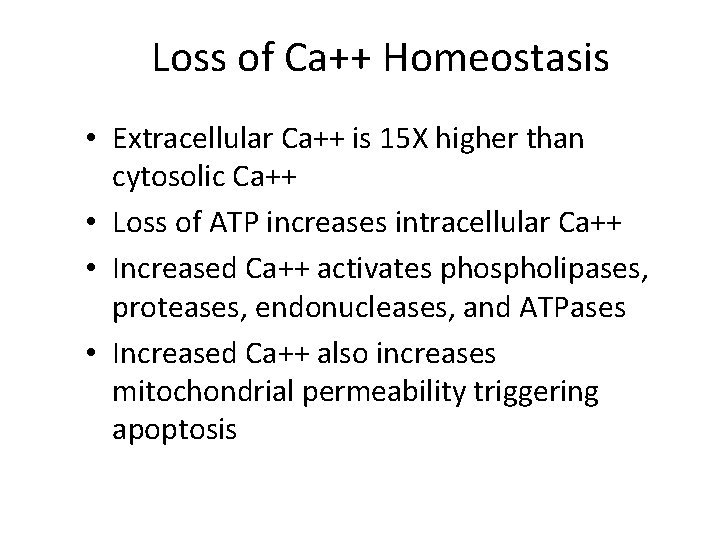
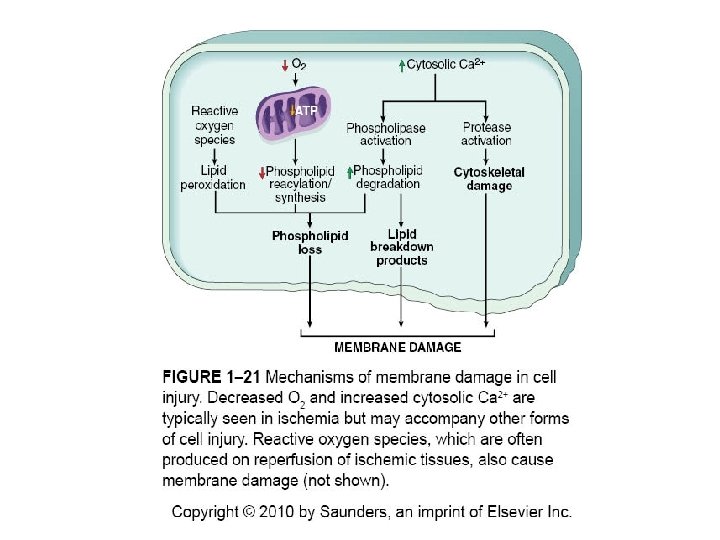
Loss of Ca++ Homeostasis • Extracellular Ca++ is 15 X higher than cytosolic Ca++ • Loss of ATP increases intracellular Ca++ • Increased Ca++ activates phospholipases, proteases, endonucleases, and ATPases • Increased Ca++ also increases mitochondrial permeability triggering apoptosis

Free Radical Formation • Single unpaired electron; highly reactive • Normal metabolism produces superoxide anion, hydrogen peroxide and hydroxyl ion; superoxide is produced in neutrophils • Reactive oxygen species (ROS) are a type of free radical • Excess of ROS within cell leads to oxidative stress
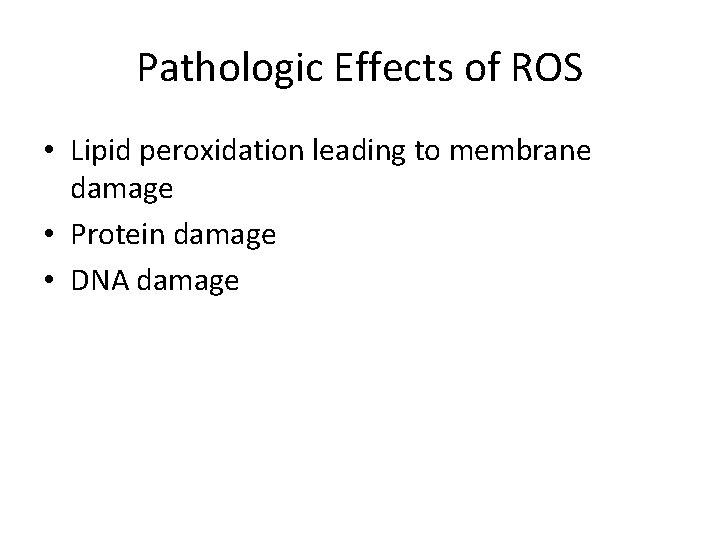
Pathologic Effects of ROS • Lipid peroxidation leading to membrane damage • Protein damage • DNA damage
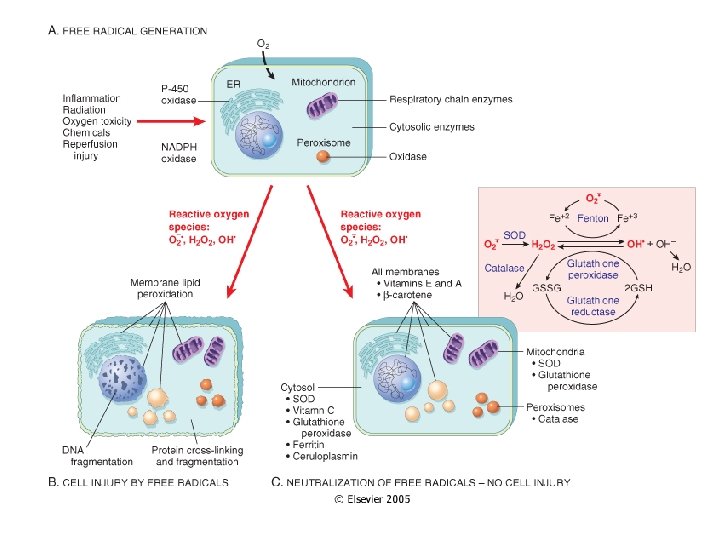
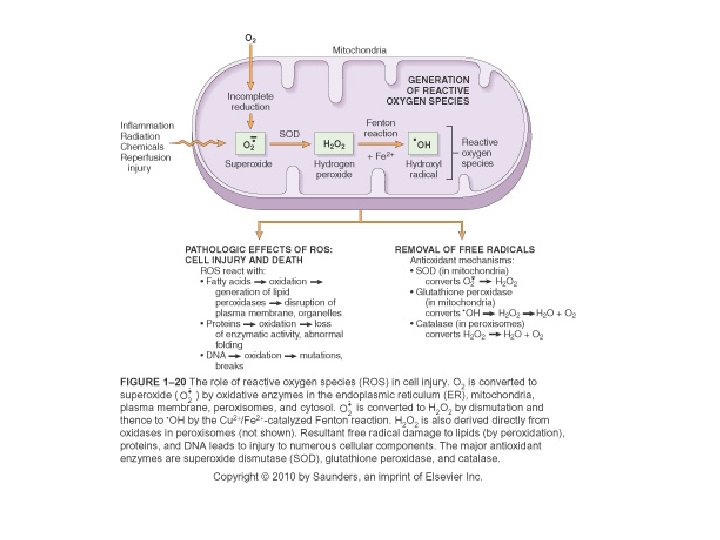
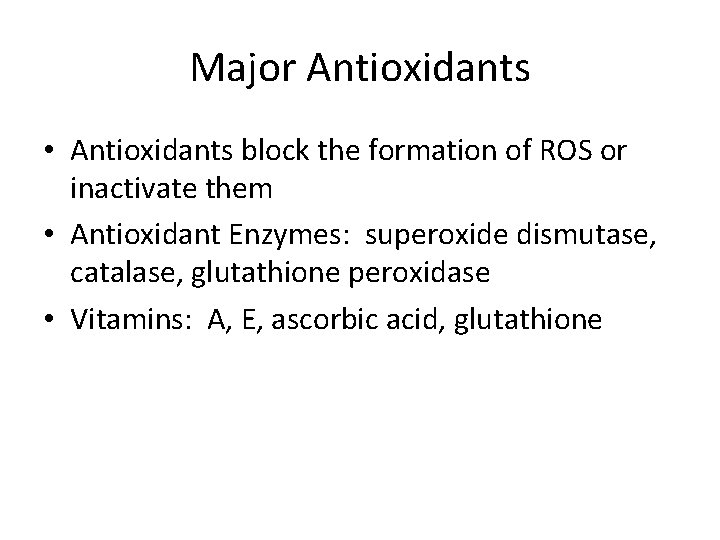
Major Antioxidants • Antioxidants block the formation of ROS or inactivate them • Antioxidant Enzymes: superoxide dismutase, catalase, glutathione peroxidase • Vitamins: A, E, ascorbic acid, glutathione
Membrane Permeability Defects • Plasma membrane • Mitochondrial membrane • Lysosomal membrane- release of RNases, DNases and proteases

CAUSES OF CELL INJURY • • Oxygen deprivation Physical agents Chemical agents and drugs Infectious agents Immunologic reactions Genetic derangements Nutritional imbalances
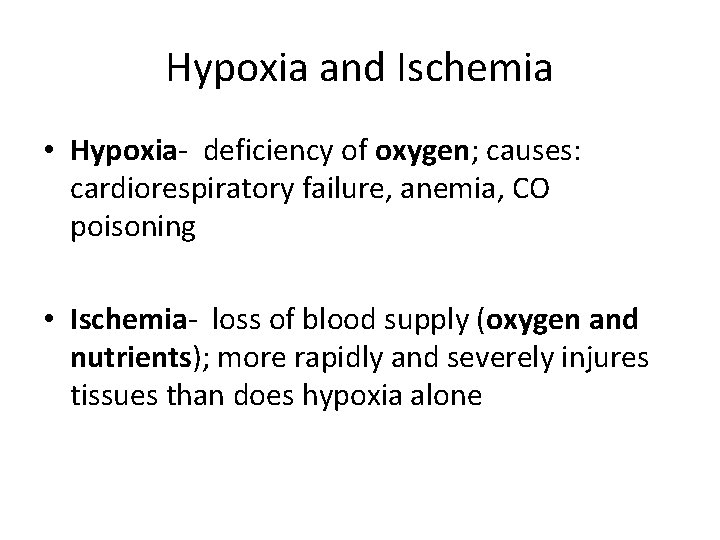
Hypoxia and Ischemia • Hypoxia- deficiency of oxygen; causes: cardiorespiratory failure, anemia, CO poisoning • Ischemia- loss of blood supply (oxygen and nutrients); more rapidly and severely injures tissues than does hypoxia alone

• N e c r o s i s d e a NECROSIS vs APOPTOSIS
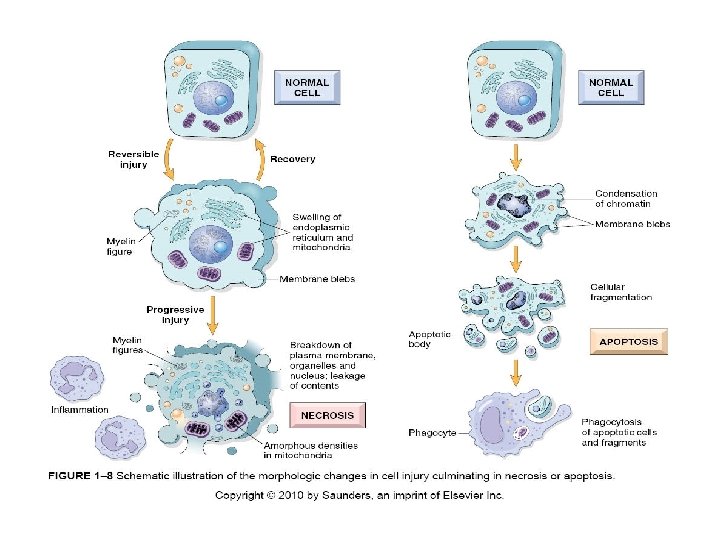
NECROSIS • Morphologic changes in GROUPS of cells that follow the death of living tissue; cells and PMNs leak lytic enzymes • CYTOPLASM: eosinophilia, vacuoles, calcification, myelin figures • NUCLEUS: pyknosis, karyorrhexis, karyolysis

Patterns of Necrosis • Coagulative- hypoxic death (except brain) • Liquefactive- bacterial infections; *also hypoxic death in brain tissue (infarction) • Caseous- tuberculosis • Fat- enzymatic or traumatic damage to fatty tissue; eg. Pancreatitis (enzymatic) • Gangrenous- usually involves lower extremities and often is a type of coagulative necrosis • Fibrinoid- immune complexes in arteries
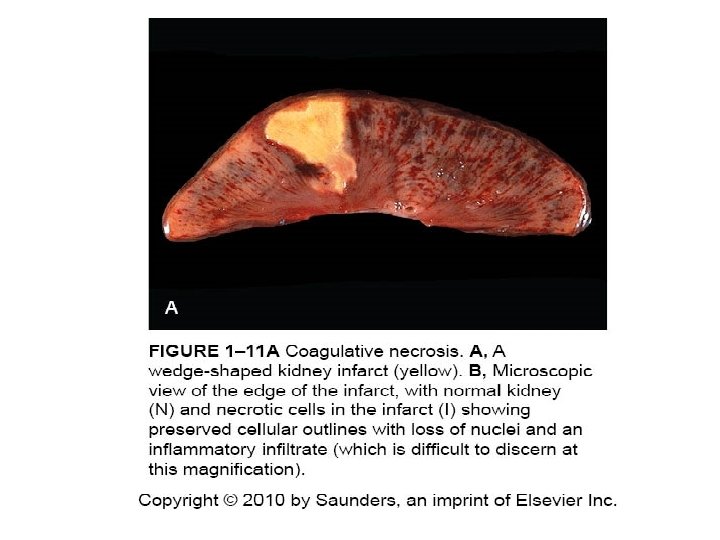
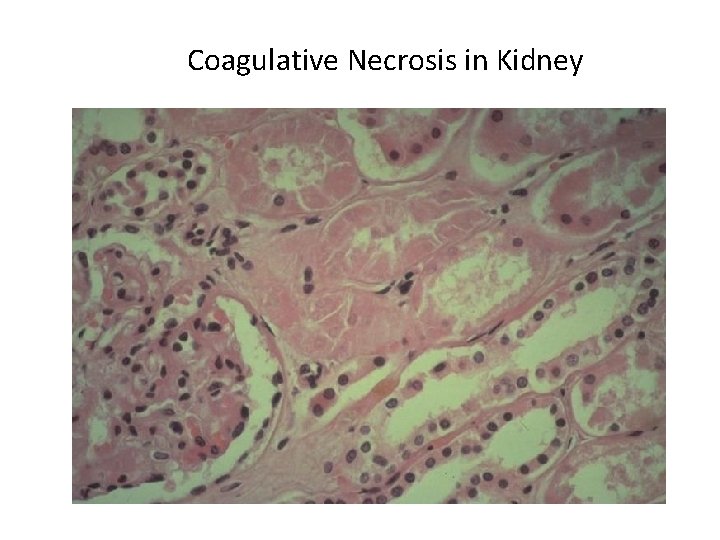
Coagulative Necrosis in Kidney
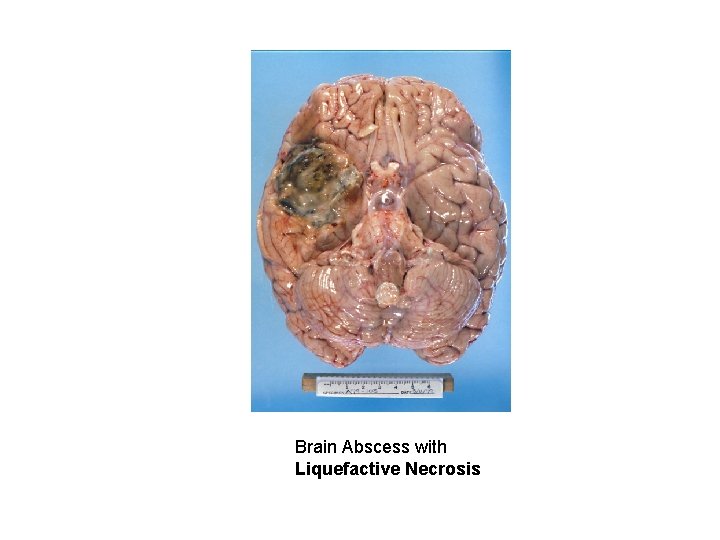
Brain Abscess with Liquefactive Necrosis
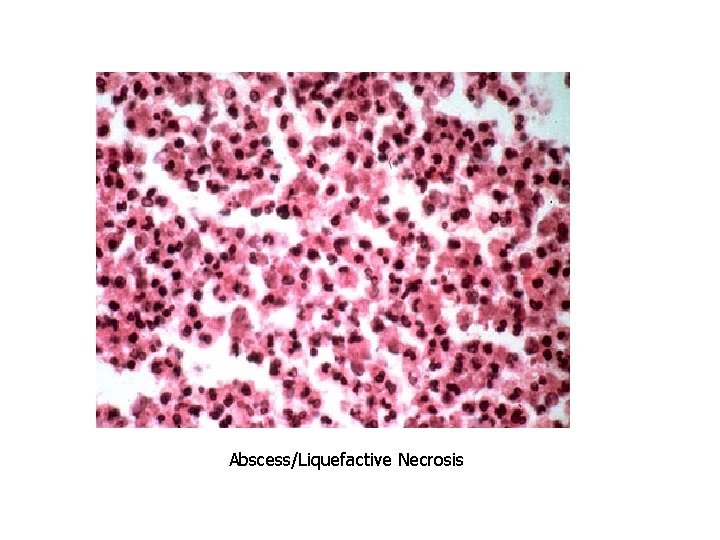
Abscess/Liquefactive Necrosis
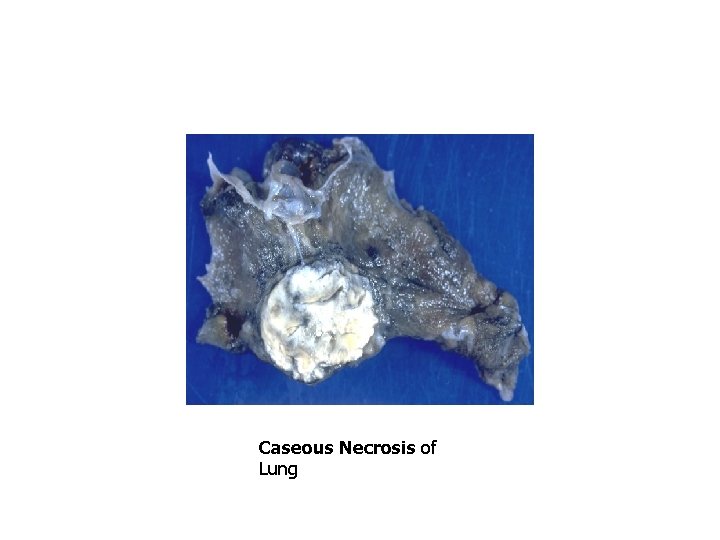
Caseous Necrosis of Lung
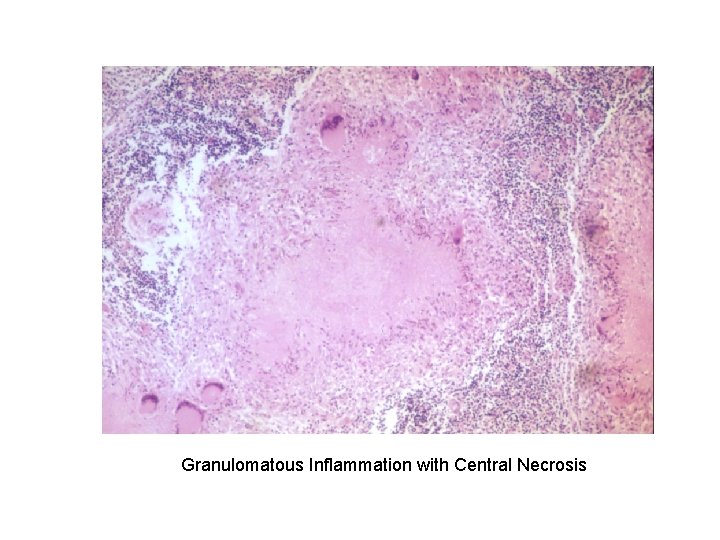
Granulomatous Inflammation with Central Necrosis
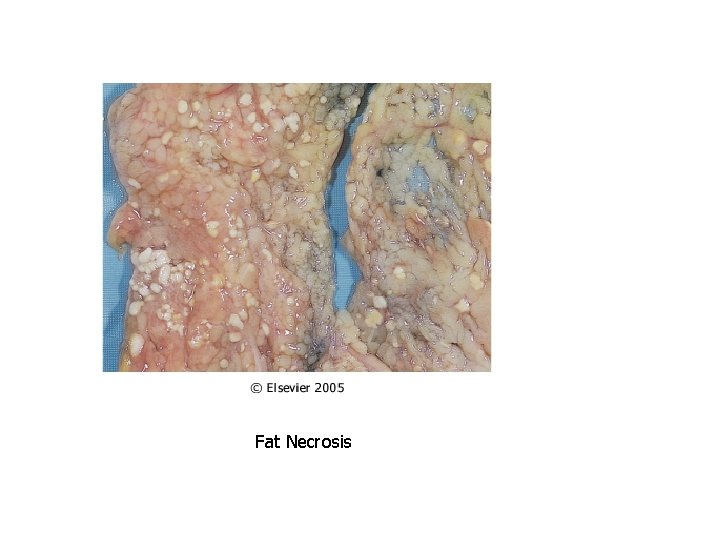
Fat Necrosis
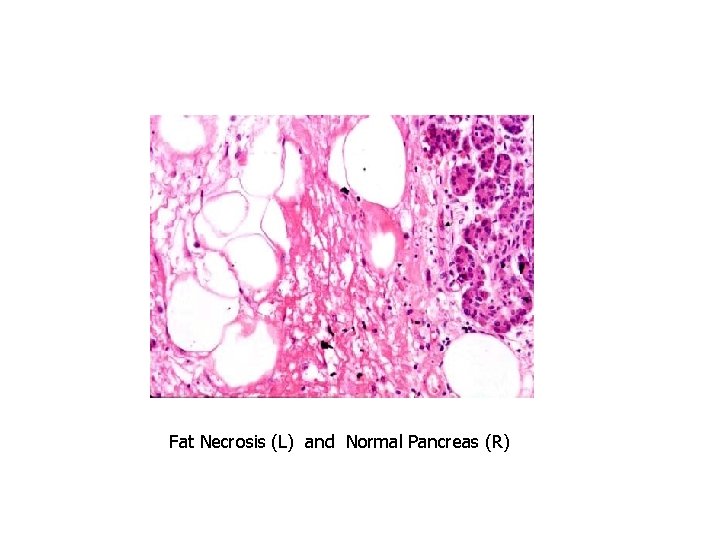
Fat Necrosis (L) and Normal Pancreas (R)
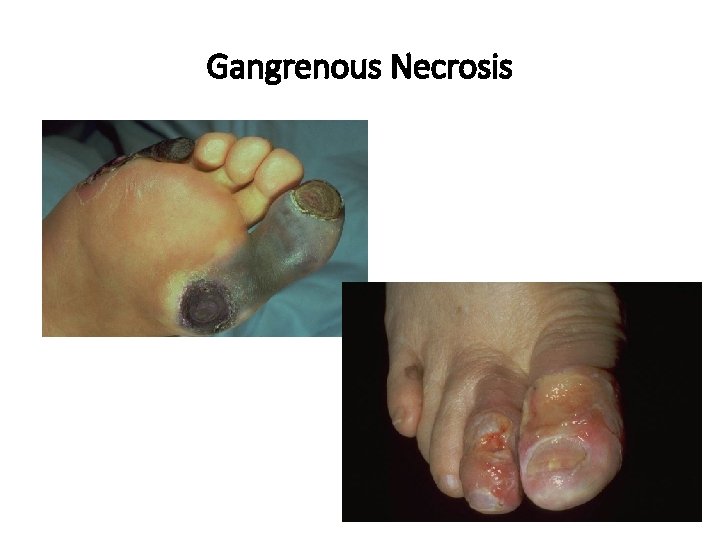
Gangrenous Necrosis
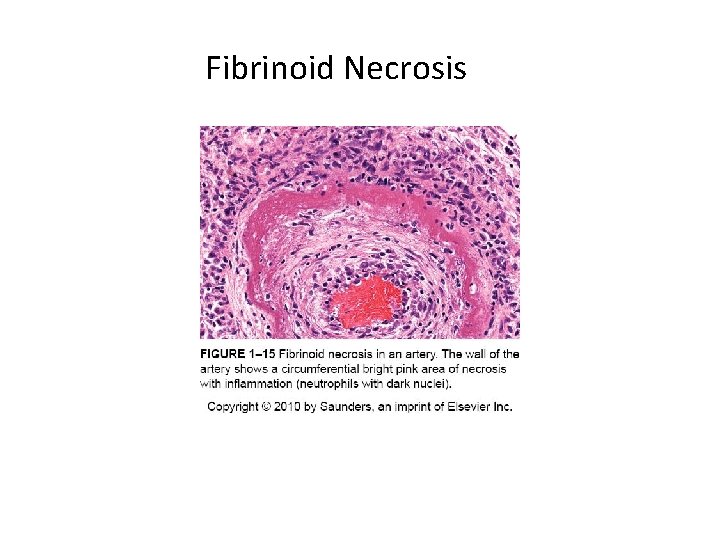
Fibrinoid Necrosis

Questions?
- Slides: 91