Acute Promyelocytic Leukemia APL By Maged Abd El

Acute Promyelocytic Leukemia (APL) By Maged Abd El Fattah Amine Assistant Lecturer Of Medical Oncology South Egypt Cancer Institute 3. 2014

*Outline: - Hematopoiesis. - Introduction and Epidemiology of APL. - Pathogenesis of APL. - Diagnosis of APL. - Treatment of APL. - Conclusions. 18/3/2014 APL 2

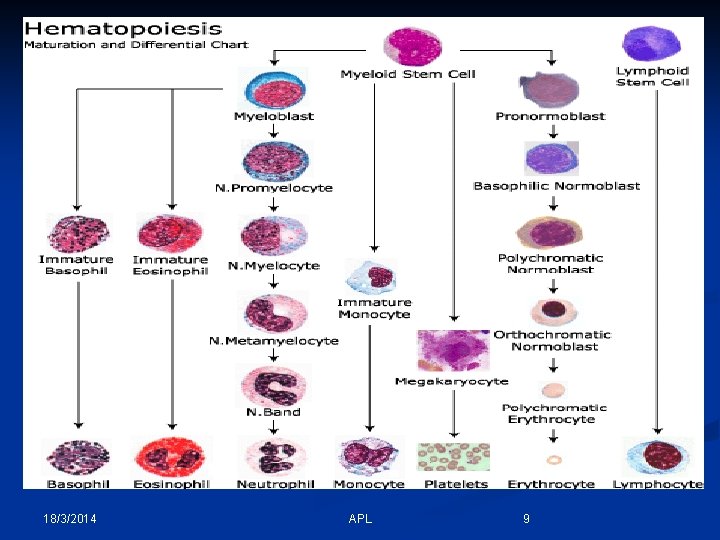
Hematopoiesis 18/3/2014 APL 3
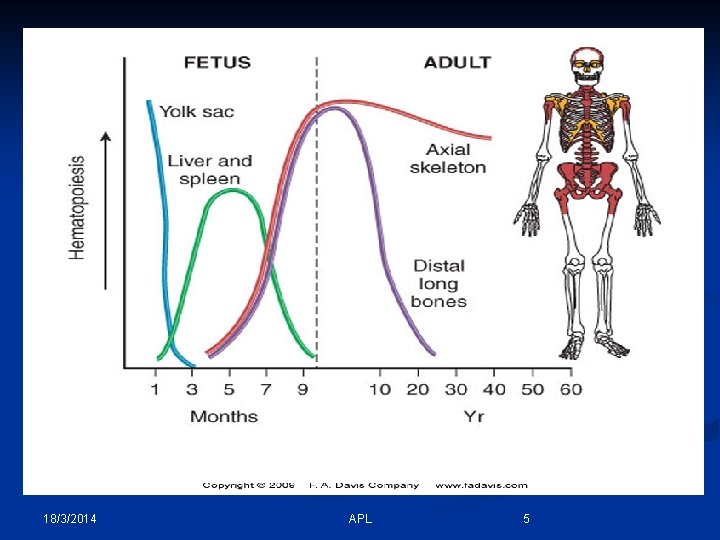
- Hematopoiesis is a term describing the formation and development of blood cells. - The hematopoietic system must have the capacity for self renewal, which involves: 1 - Proliferation of progeny stem cells. 2 - Differentiation and maturation of the stem cells into the functional cellular elements. - Normally hematopoiesis is limited to the bone marrow and the widespread lymphatic system and only mature cells are released into the peripheral blood. 18/3/2014 APL 4
18/3/2014 APL 5

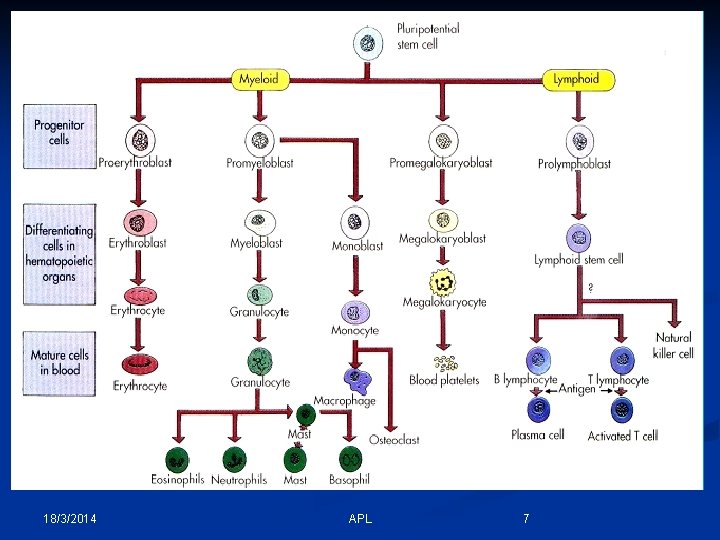
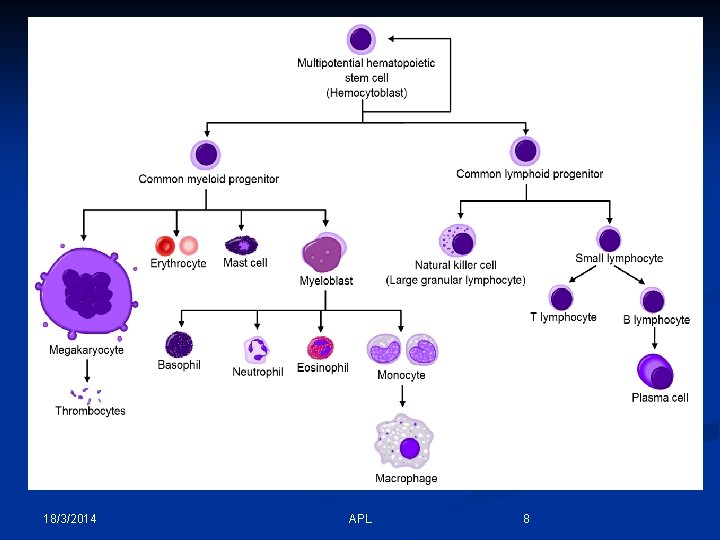
- All cells are derived from a pool of stem cells that are self-renewing. - Pluripotential & multipotential stem cells give rise to committed stem cells for each cell line. - Committed stem cells have receptors for specific growth factors, respond to stimulation by division & maturation (precursor cell stages) into end-stage cells. - Growth factor stimulation increases with need. 18/3/2014 APL 6
18/3/2014 APL 7
18/3/2014 APL 8
18/3/2014 APL 9

Introduction And Epidemiology of APL 18/3/2014 APL 10
- Acute promyelocytic leukemia (APL), is a distinct subtype of acute myeloid leukemia, represents about 10 -12% of adult AML cases, and 8% - 15% of pediatric AML. - The median age is approximately 30 -40 years, which is considerably younger than the other subtypes of AML (70 yrs). - It was first described in 1957 by “Hillestad (Sweden)”, as a hyperacute fatal illness. - laboratory evidence of DIC is present in 70% to 90% of patients at diagnosis or shortly after. - Hemorrhagic events contribute 10% to 15% excess mortality during induction chemotherapy for APL. 18/3/2014 APL 11
- Morphologically, it is identified as AML-M 3 by the French. American-British (FAB) classification. - Cytogenetically (WHO), APL is characterized by a balanced reciprocal translocation abnormality, t(15; 17)(q 22; q 12); PMLRARA. - Currently it is one of the most treatable forms of leukemia with a 12 -yr PFS rate, is estimated to be approximately 70%. 18/3/2014 APL 12

Pathogenesis of APL 18/3/2014 APL 13
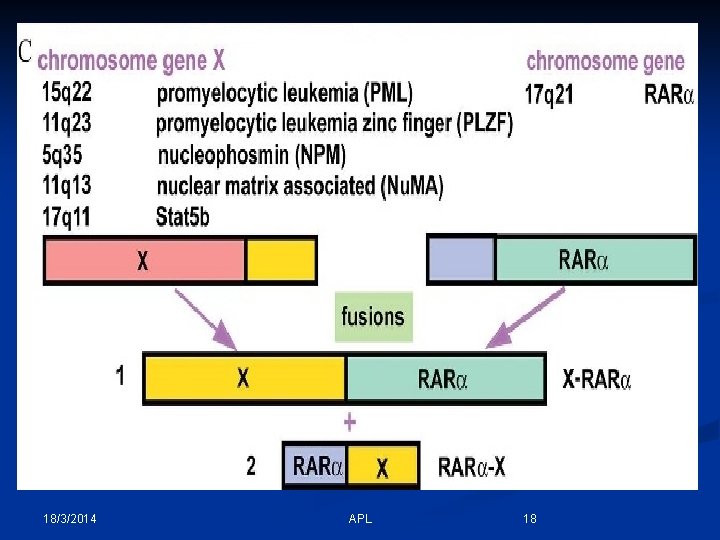
- In Acute promyelocytic leukemia (APL), there is an abnormal accumulation of immature granulocytes called promyelocytes. - APL is characterized a balanced reciprocal translocation abnormality, t(15; 17)(q 22; q 12); PML-RARA, which results in fusion of the retinoic acid receptor (RARA) gene on chromosome 17 with the promyelocytic leukemia (PML) gene on chromosome 15. - The fusion of PML and RARA results in expression of a hybrid protein with altered functions. This fusion protein binds with enhanced affinity to sites on the cell's DNA, blocking transcription and differentiation of granulocytes. 18/3/2014 APL 14
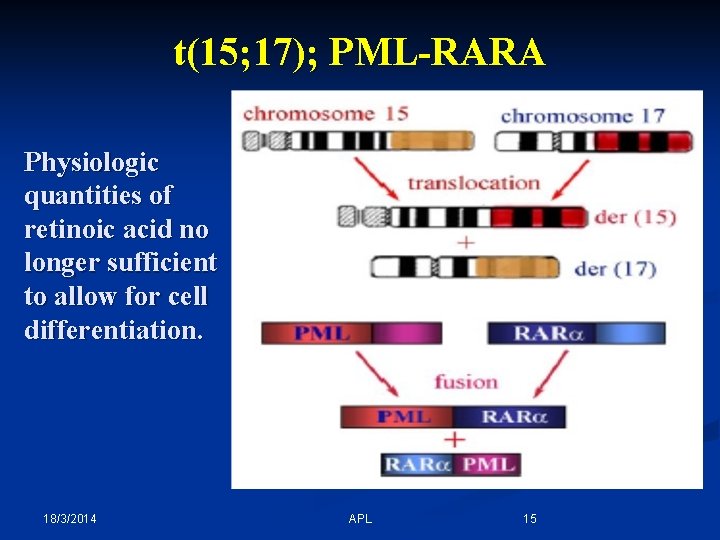
t(15; 17); PML-RARA Physiologic quantities of retinoic acid no longer sufficient to allow for cell differentiation. 18/3/2014 APL 15
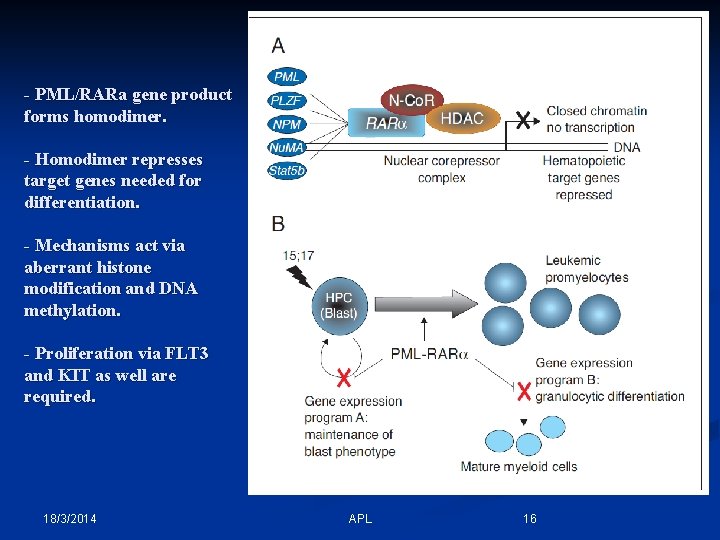
- PML/RARa gene product forms homodimer. - Homodimer represses target genes needed for differentiation. - Mechanisms act via aberrant histone modification and DNA methylation. - Proliferation via FLT 3 and KIT as well are required. 18/3/2014 APL 16
- Although the chromosomal translocation involving RARA is believed to be the initiating event, additional mutations are required for the development of leukemia. - This translocation abnormality could be detected by karyotyping, FISH or PCR techniques, which is useful for both diagnosis and evaluation of minimal residual disease. - Eight other rare gene rearrangements have been described in APL fusing RARA to other genes “Variant chromosomal translocations” [e. g. , t(11; 17), t(5; 17)], can be detected in no less than 5% of APL patients. 18/3/2014 APL 17
18/3/2014 APL 18
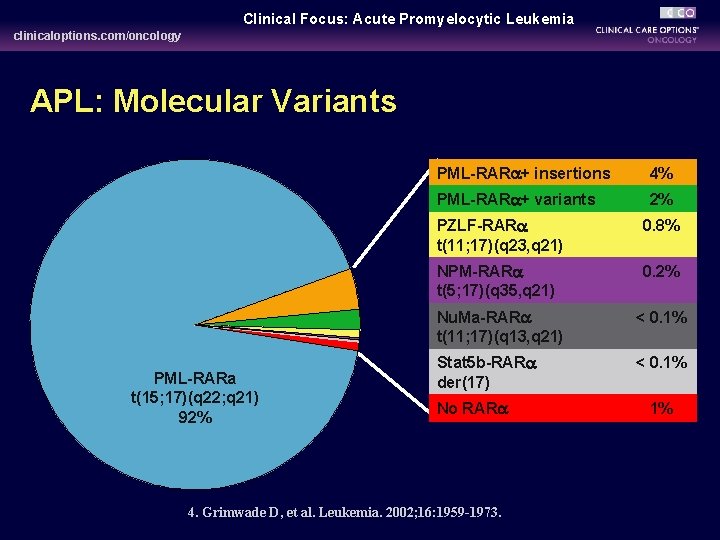
Clinical Focus: Acute Promyelocytic Leukemia clinicaloptions. com/oncology APL: Molecular Variants PML-RARa t(15; 17)(q 22; q 21) 92% PML-RARa+ insertions 4% PML-RARa+ variants 2% PZLF-RARa t(11; 17)(q 23, q 21) 0. 8% NPM-RARa t(5; 17)(q 35, q 21) 0. 2% Nu. Ma-RARa t(11; 17)(q 13, q 21) < 0. 1% Stat 5 b-RARa der(17) < 0. 1% No RARa 4. Grimwade D, et al. Leukemia. 2002; 16: 1959 -1973. 1%

Diagnosis of APL 18/3/2014 APL 20
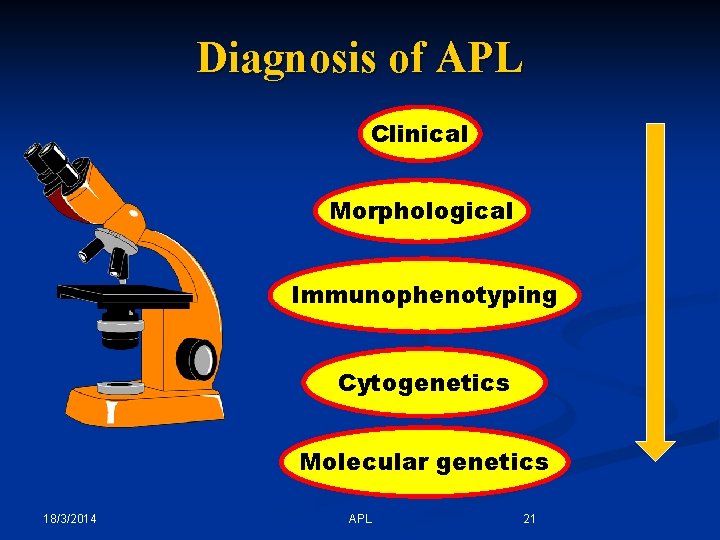
Diagnosis of APL Clinical Morphological Immunophenotyping Cytogenetics Molecular genetics 18/3/2014 APL 21
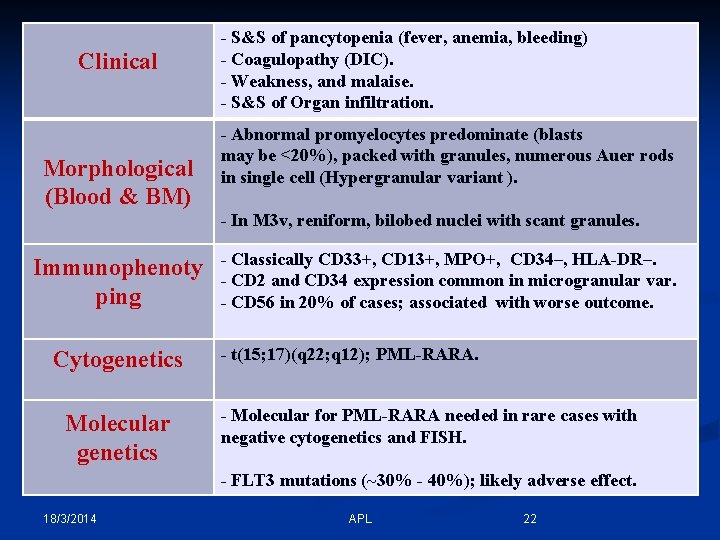
Clinical Morphological (Blood & BM) - S&S of pancytopenia (fever, anemia, bleeding) - Coagulopathy (DIC). - Weakness, and malaise. - S&S of Organ infiltration. - Abnormal promyelocytes predominate (blasts may be <20%), packed with granules, numerous Auer rods in single cell (Hypergranular variant ). - In M 3 v, reniform, bilobed nuclei with scant granules. Immunophenoty ping Cytogenetics Molecular genetics - Classically CD 33+, CD 13+, MPO+, CD 34–, HLA-DR–. - CD 2 and CD 34 expression common in microgranular var. - CD 56 in 20% of cases; associated with worse outcome. - t(15; 17)(q 22; q 12); PML-RARA. - Molecular for PML-RARA needed in rare cases with negative cytogenetics and FISH. - FLT 3 mutations (~30% - 40%); likely adverse effect. 18/3/2014 APL 22

# Karyotype: - Detects translocation variant. # FISH or immunostaining: - Fast – often within 2 -4 hours. - Immunostaining is inexpensive and can be done at smaller centers. # RT-PCR: - Can detect minimal residual disease (MRD). - “Gold Standard”. 18/3/2014 APL 23
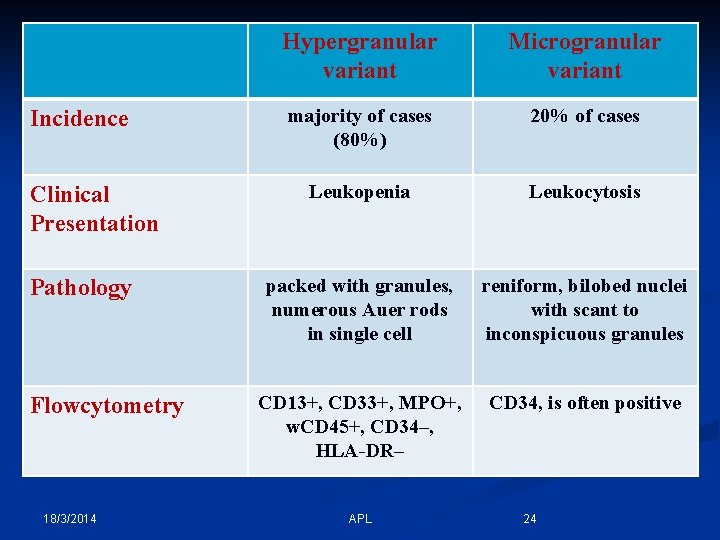
Incidence Clinical Presentation Pathology Flowcytometry 18/3/2014 Hypergranular variant Microgranular variant majority of cases (80%) 20% of cases Leukopenia Leukocytosis packed with granules, numerous Auer rods in single cell reniform, bilobed nuclei with scant to inconspicuous granules CD 13+, CD 33+, MPO+, w. CD 45+, CD 34–, HLA-DR– CD 34, is often positive APL 24
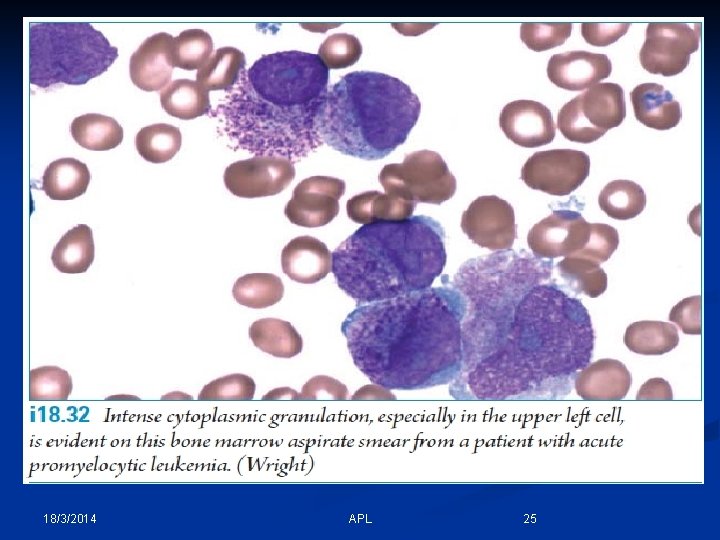
18/3/2014 APL 25
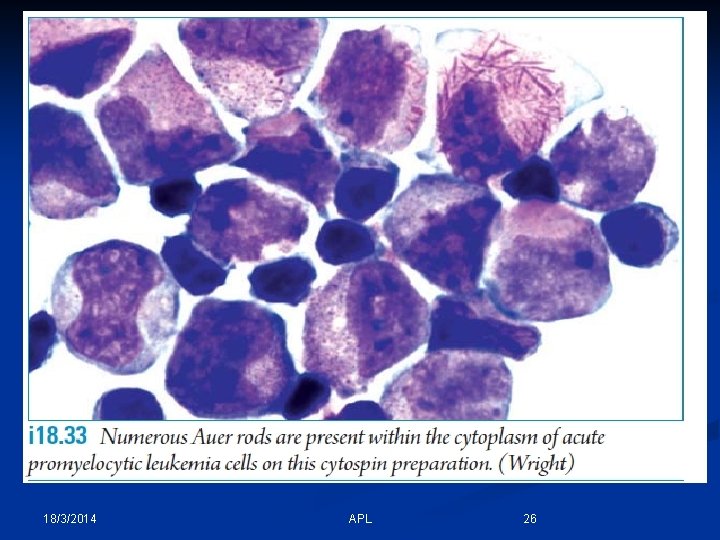
18/3/2014 APL 26

18/3/2014 APL 27
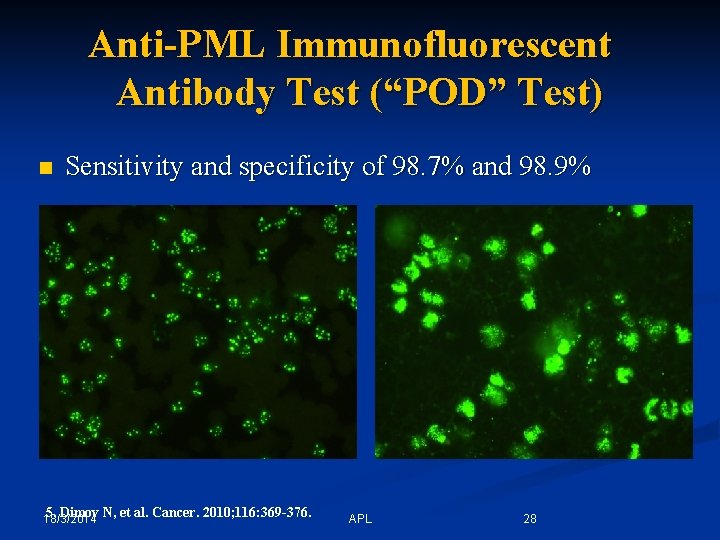
Anti-PML Immunofluorescent Antibody Test (“POD” Test) n Sensitivity and specificity of 98. 7% and 98. 9% 5. Dimov N, et al. Cancer. 2010; 116: 369 -376. 18/3/2014 APL 28

Treatment of APL 18/3/2014 APL 29
APL Leukemic Infiltration 18/3/2014 Coagulopathy APL 30
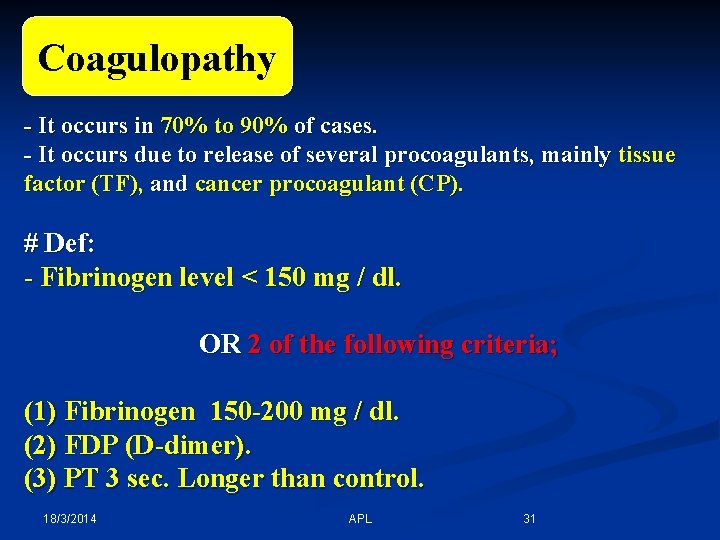
*Coagulopathy: Coagulopathy - It occurs in 70% to 90% of cases. - It occurs due to release of several procoagulants, mainly tissue factor (TF), and cancer procoagulant (CP). # Def: - Fibrinogen level < 150 mg / dl. OR 2 of the following criteria; (1) Fibrinogen 150 -200 mg / dl. (2) FDP (D-dimer). (3) PT 3 sec. Longer than control. 18/3/2014 APL 31
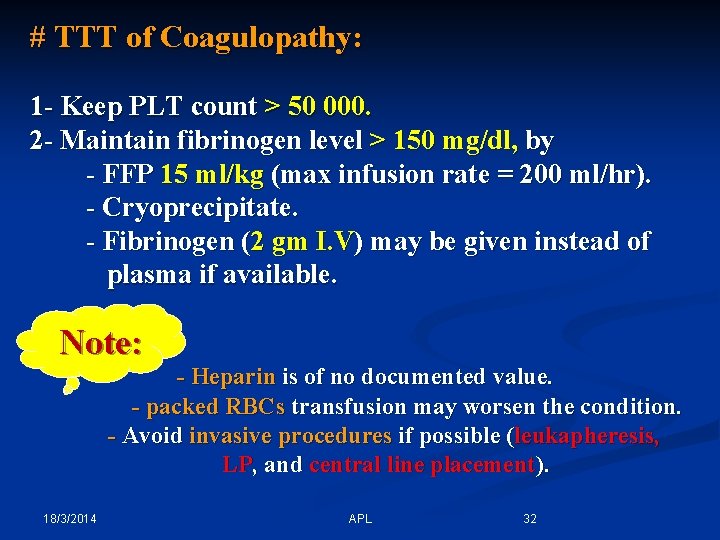
# TTT of Coagulopathy: 1 - Keep PLT count > 50 000. 2 - Maintain fibrinogen level > 150 mg/dl, by - FFP 15 ml/kg (max infusion rate = 200 ml/hr). - Cryoprecipitate. - Fibrinogen (2 gm I. V) may be given instead of plasma if available. Note: - Heparin is of no documented value. - packed RBCs transfusion may worsen the condition. - Avoid invasive procedures if possible (leukapheresis, LP, and central line placement). 18/3/2014 APL 32
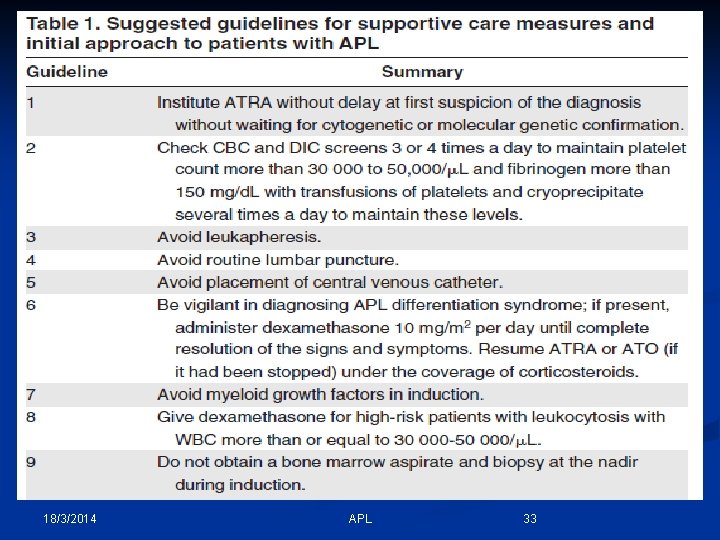
18/3/2014 APL 33
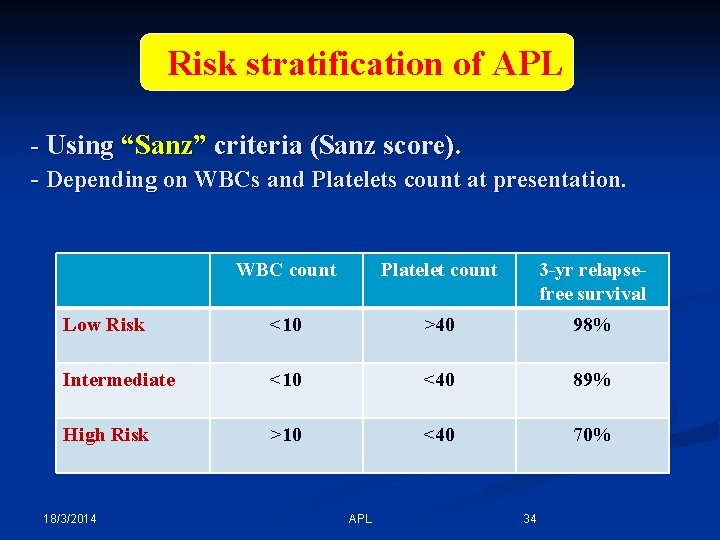
Risk stratification of APL - Using “Sanz” criteria (Sanz score). - Depending on WBCs and Platelets count at presentation. WBC count Platelet count 3 -yr relapsefree survival Low Risk <10 >40 98% Intermediate <10 <40 89% High Risk >10 <40 70% 18/3/2014 APL 34
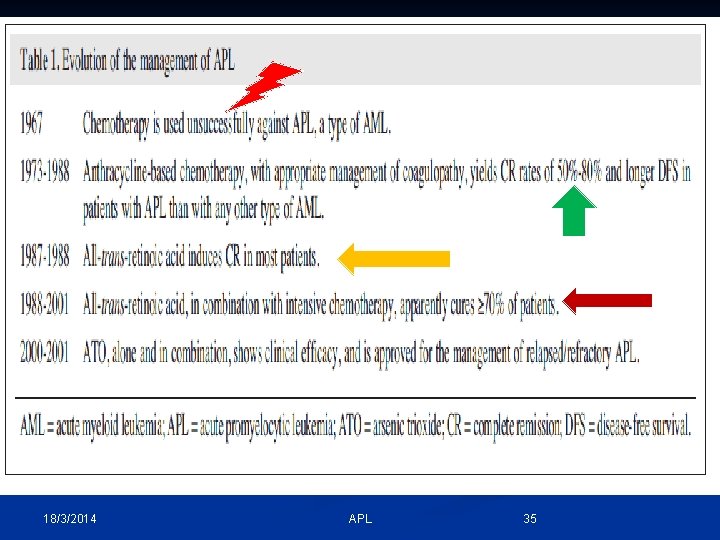
18/3/2014 APL 35
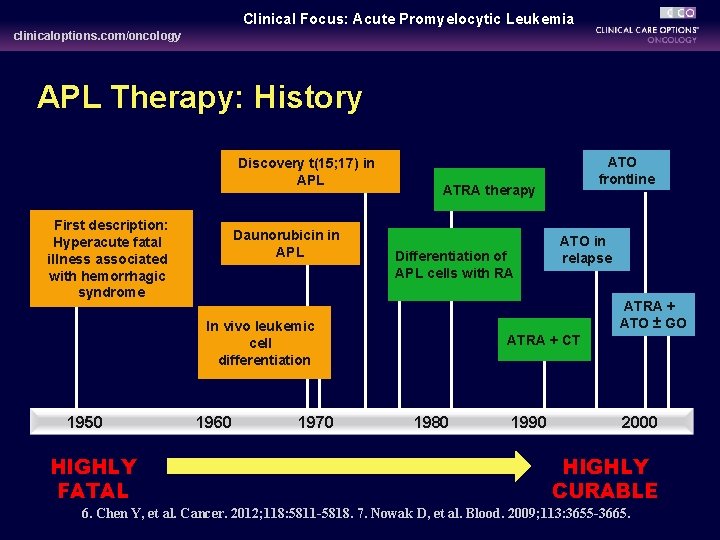
Clinical Focus: Acute Promyelocytic Leukemia clinicaloptions. com/oncology APL Therapy: History Discovery t(15; 17) in APL First description: Hyperacute fatal illness associated with hemorrhagic syndrome Daunorubicin in APL ATRA therapy Differentiation of APL cells with RA HIGHLY FATAL 1960 1970 ATO in relapse ATRA + ATO ± GO In vivo leukemic cell differentiation 1950 ATO frontline ATRA + CT 1980 1990 2000 HIGHLY CURABLE 6. Chen Y, et al. Cancer. 2012; 118: 5811 -5818. 7. Nowak D, et al. Blood. 2009; 113: 3655 -3665.

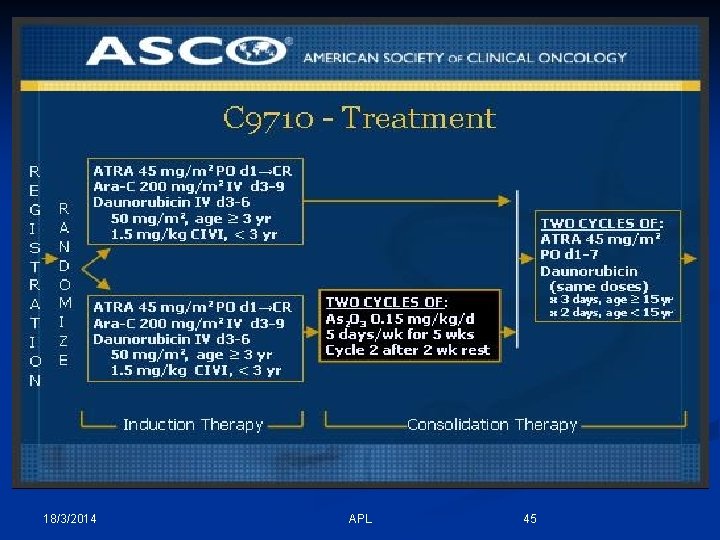
Induction Therapy TTT of APL Consolidation Maintenance 18/3/2014 APL 37
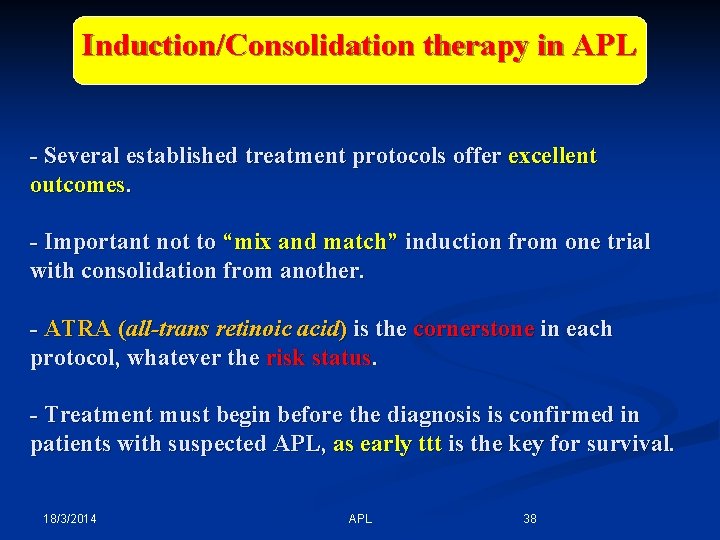
Induction/Consolidation therapy in APL - Several established treatment protocols offer excellent outcomes. - Important not to “mix and match” induction from one trial with consolidation from another. - ATRA (all-trans retinoic acid) is the cornerstone in each protocol, whatever the risk status. - Treatment must begin before the diagnosis is confirmed in patients with suspected APL, as early ttt is the key for survival. 18/3/2014 APL 38
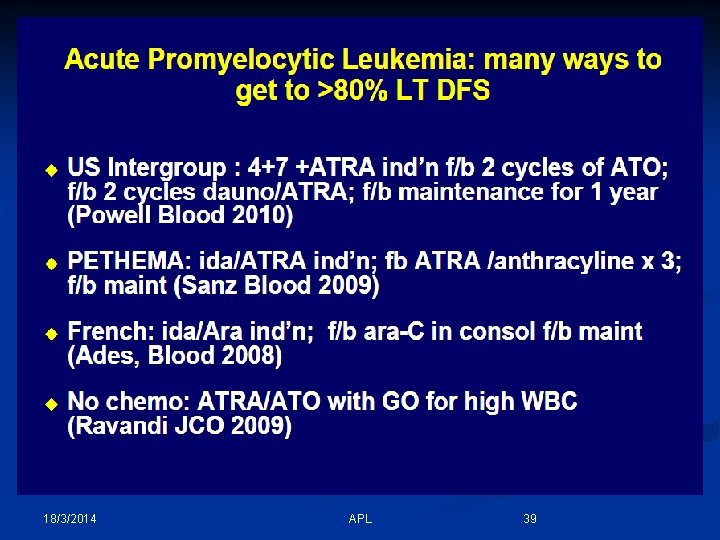
18/3/2014 APL 39
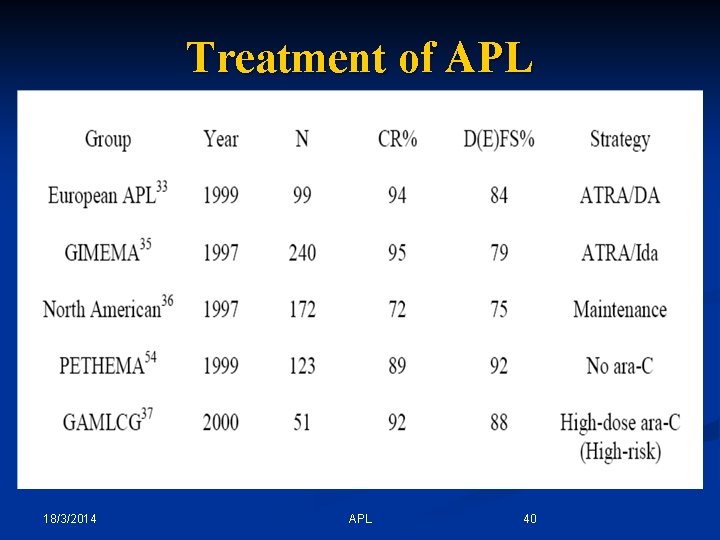
Treatment of APL 18/3/2014 APL 40
![Induction Therapy in APL # ATRA [Vesanoid Caps. (10 mg)]: - 45 mg/m 2 Induction Therapy in APL # ATRA [Vesanoid Caps. (10 mg)]: - 45 mg/m 2](http://slidetodoc.com/presentation_image/427f907ee7bf8327ff204da0597d3ea5/image-41.jpg)
Induction Therapy in APL # ATRA [Vesanoid Caps. (10 mg)]: - 45 mg/m 2 P. O. daily in 2 divided/12 hs, till CR or for a max. of 90 days. #Anthracyclines ( DAN or IDA): - Daunorubicin (50 mg/m 2 I. V. x 4 d), or (60 mg/m 2 I. V. x 3 d). - Idarubicin (12 mg/m 2 I. V. x 2, 4, 6, 8 days). # Cytarabine (Ara-C): 200 mg/m 2 CIVI x 7 days, could be added according to the protocol. 18/3/2014 APL 41
Consolidation Treatment in APL - 2 or 3 cycles of an anthracycline (daunorubicin or idarubicin) plus 1 to 2 weeks of ATRA are administered until molecular CR. - Intermediate- or high-dose Ara-C can be administered depending on age as a first consolidation, as in (GIMEMA, APL 2000 study); or Arsenic Trioxide (ATO) for 2 courses of 25 days each as in (second North American Intergroup C 9710 study). - To consider LP and IT CTR for 5 doses (weekly) in high risk patients. 18/3/2014 APL 42
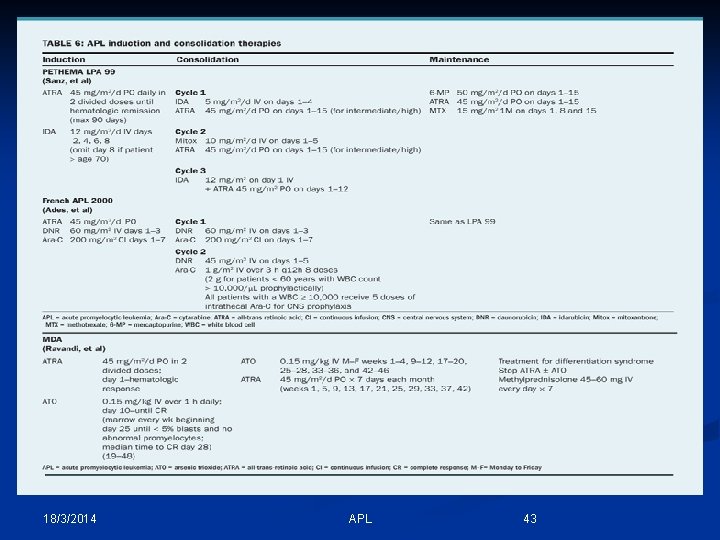
18/3/2014 APL 43
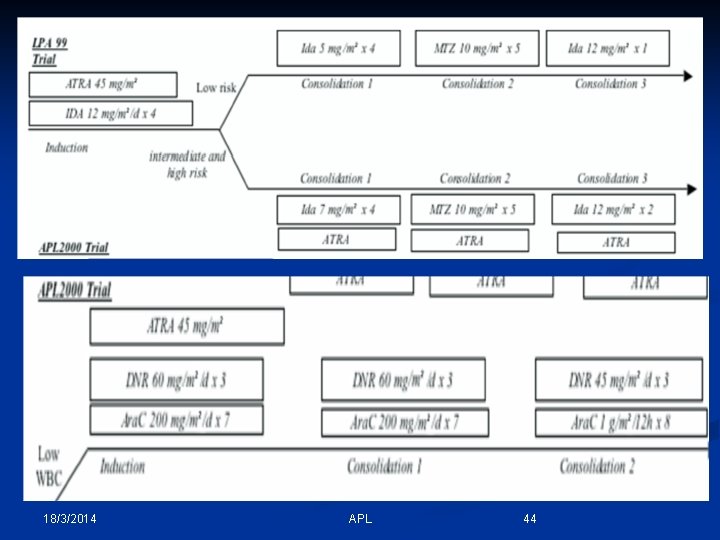
18/3/2014 APL 44
18/3/2014 APL 45
Maintenance Treatment in APL - ATRA and low-dose chemotherapy with 6 mercaptopurine and methotrexate is given for 2 years. -ATRA (45 mg/m 2 P. O/day) for 2 weeks every 3 months. - 6 -MP (60 mg/m 2, P. O/day). - MTX (15 mg/m 2, IM/ week). - There is some debate around maintenance ttt in APL, as some studies showed no difference in DFS and OS, but it is still the standard of care till now. 18/3/2014 APL 46
Treatment of APL in Elderly pts - Elderly patients (> 60 y) have poorer outcomes with standard treatment. - In the PETHEMA trial, the last dose of idarubicin was omitted during induction; consolidation should be altered to liposomal ATRA and ATO. - Another option is ATRA + ATO for induction without anthracyclines. 18/3/2014 APL 47
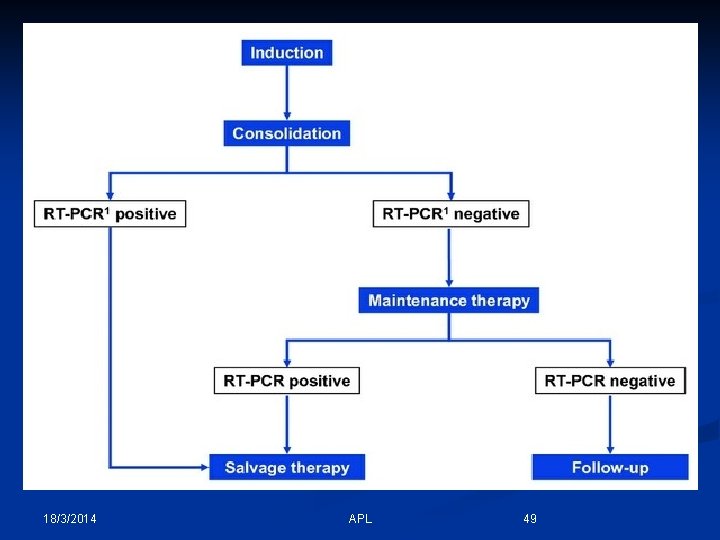
Post- remission Monitoring in APL 1 - Document complete molecular remission by PCR after consolidation. 2 - Monitor PCR every 3 months up to 2 years. 3 - If PCR was –ve, continue maintenance ttt. 4 - If PCR was +ve, repeat within 4 weeks to confirm. 5 - If PCR still +ve, proceed to ttt of relapse. 18/3/2014 APL 48
18/3/2014 APL 49
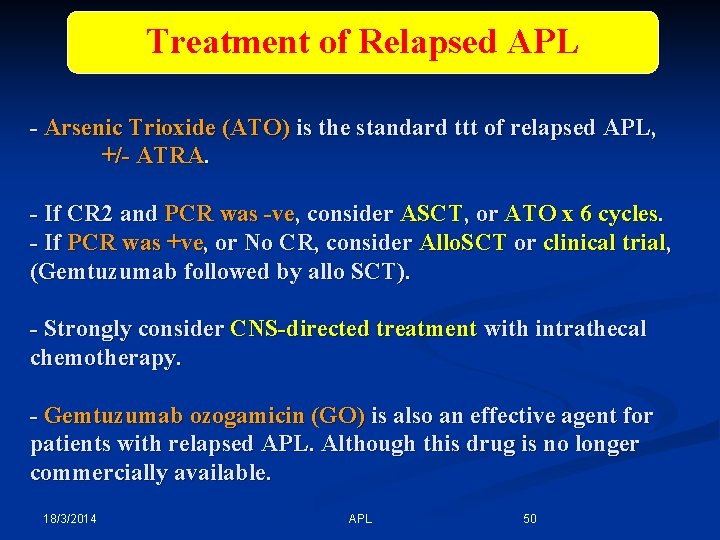
Treatment of Relapsed APL - Arsenic Trioxide (ATO) is the standard ttt of relapsed APL, +/- ATRA. - If CR 2 and PCR was -ve, consider ASCT, or ATO x 6 cycles. - If PCR was +ve, or No CR, consider Allo. SCT or clinical trial, (Gemtuzumab followed by allo SCT). - Strongly consider CNS-directed treatment with intrathecal chemotherapy. - Gemtuzumab ozogamicin (GO) is also an effective agent for patients with relapsed APL. Although this drug is no longer commercially available. 18/3/2014 APL 50
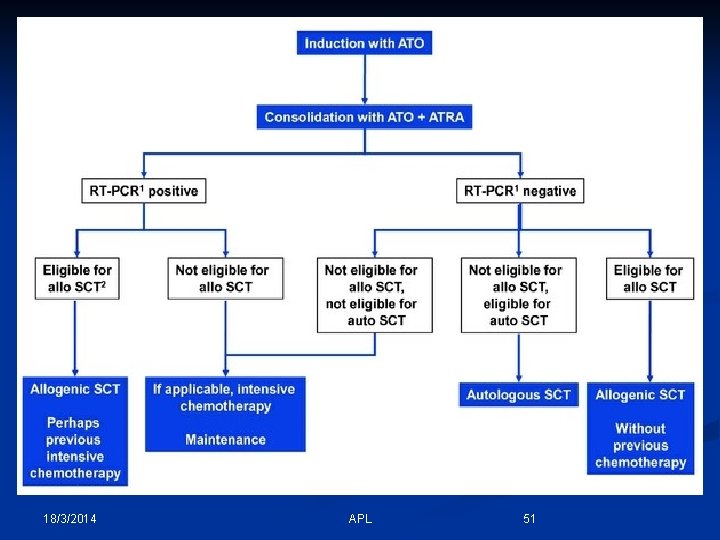
18/3/2014 APL 51
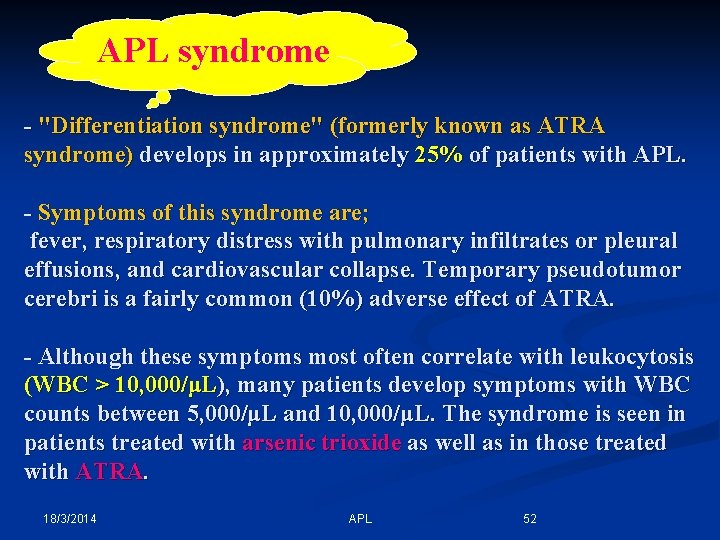
APL syndrome - "Differentiation syndrome" (formerly known as ATRA syndrome) develops in approximately 25% of patients with APL. - Symptoms of this syndrome are; fever, respiratory distress with pulmonary infiltrates or pleural effusions, and cardiovascular collapse. Temporary pseudotumor cerebri is a fairly common (10%) adverse effect of ATRA. - Although these symptoms most often correlate with leukocytosis (WBC > 10, 000/μL), many patients develop symptoms with WBC counts between 5, 000/μL and 10, 000/μL. The syndrome is seen in patients treated with arsenic trioxide as well as in those treated with ATRA. 18/3/2014 APL 52

(1) Fever + rigors. (2) Capillary leak (dyspnea, pleural effusion, pericardial effusion, HF). (3) CXR: pulmonary infiltrates. (4) Renal failure. (5) Hypotension. (6) Edema & gain of weight. (7) Lymphadenopathy & tonsillitis. (8) Leucocytosis. 18/3/2014 APL 53
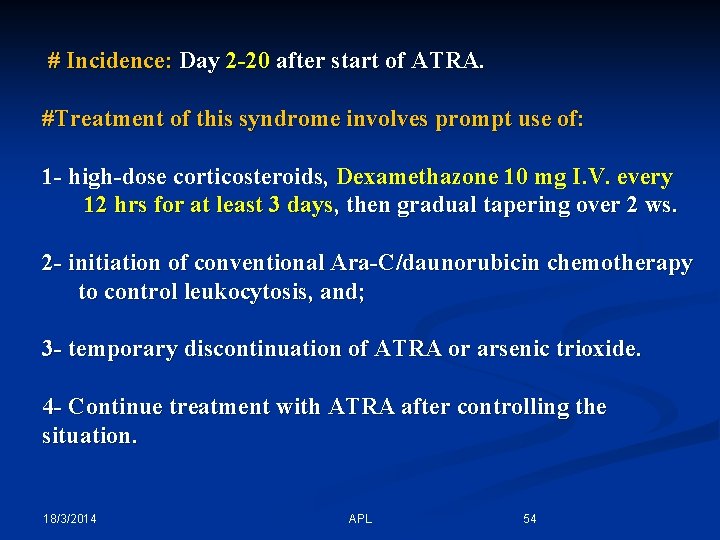
# Incidence: Day 2 -20 after start of ATRA. #Treatment of this syndrome involves prompt use of: 1 - high-dose corticosteroids, Dexamethazone 10 mg I. V. every 12 hrs for at least 3 days, then gradual tapering over 2 ws. 2 - initiation of conventional Ara-C/daunorubicin chemotherapy to control leukocytosis, and; 3 - temporary discontinuation of ATRA or arsenic trioxide. 4 - Continue treatment with ATRA after controlling the situation. 18/3/2014 APL 54

Arsenic trioxide adverse effects: - ECG alterations, especially prolongations of the QT interval. - Electrolyte shifts commonly involve potassium and magnesium. potassium should exceed 4 mmol/l and magnesium should be above 1. 8 mg/dl. - Regular ECG checks are indicated. If a QT interval exceeds 500 msec therapy will have to be discontinued due to the increased risk of cardiac arrhythmias (torsade de pointes). Any comedication which might prolong the QT interval in a way similar to ATO should be avoided. - Other frequently occurring, however, not life-threatening adverse effects are nausea, vomiting, exanthema, fatigue, fever, neuropathy, functional liver disorders and increase of transaminase activities, and diarrhea. 18/3/2014 APL 55

Future Directions # Ongoing trials: - US Intergroup trial S 0535, evaluating, Concurrent ATRA, ATO, and GO for induction, then 3 courses of consolidation with daunorubicin plus ATRA, ATO, and GO than maintenance. - GIMEMA[b]/DSIL-APL 0406 protocol, which compares ATRA plus ATO with minimal chemotherapy to standard ATRA plus anthracycline. # Two novel agents: - Oral arsenic appears very effective, and the combination with ATRA will be an attractive strategy. - Tamibarotene (AM-80) that seems less toxic than ATRA. 18/3/2014 APL 56

Conclusions 18/3/2014 APL 57

* Acute promyelocytic leukemia(APL) was first identified as a distinct subtype of acute myeloid leukemia in 1957. * APL is characterized by three features; - accumulation of abnormal promyelocytes. - occurrence of fibrinogenopenia and DIC. - presence of the specific chromosomal translocation t(15; 17)(q 22; q 21). * Currently it is one of the most treatable forms of acute leukemia (shift from highly fatal to highly curable subtype). * Treatment must begin before the diagnosis is confirmed in patients with suspected APL, as early ttt is the key for survival. 18/3/2014 APL 58
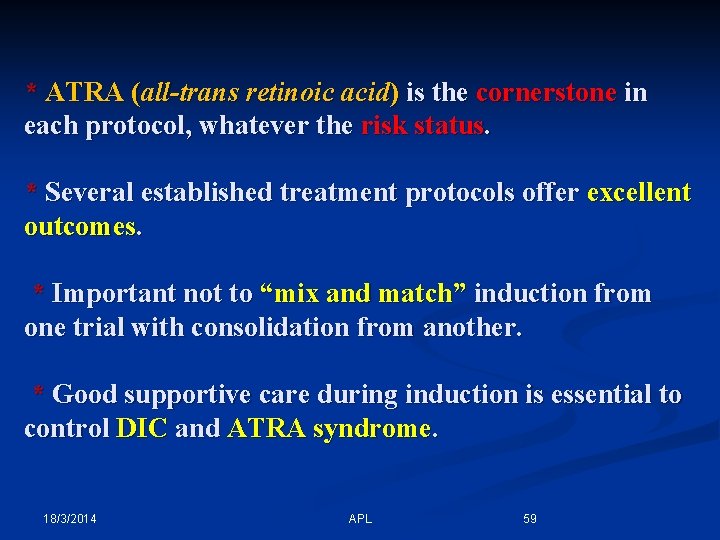
* ATRA (all-trans retinoic acid) is the cornerstone in each protocol, whatever the risk status. * Several established treatment protocols offer excellent outcomes. * Important not to “mix and match” induction from one trial with consolidation from another. * Good supportive care during induction is essential to control DIC and ATRA syndrome. 18/3/2014 APL 59

* To consider evaluation and prophylaxis for CNS involvement after achievement of remission. * Several ongoing trials to evaluate new ttt modalities including low or no CTR. * Novel agents in ttt of APL as Oral arsenic and Tamibarotene (AM-80). 18/3/2014 APL 60

THANK YOU 18/3/2014 APL 61
- Slides: 61