Acute myeloid leukemia Malignant clonal disorder of immature













- Slides: 22

Acute myeloid leukemia • Malignant clonal disorder of immature hematopoietic cells characterized by abberant hematopoietic cellular proliferation and maturation. Leukamic blasts may express capabilities for maturation to a variable degree, which lead to morphological heterogeneity

Acute leukemias • Adults: - acute limphoblastic leukemia (ALL) 20% - acute myeloid leukemia (AML) 80%

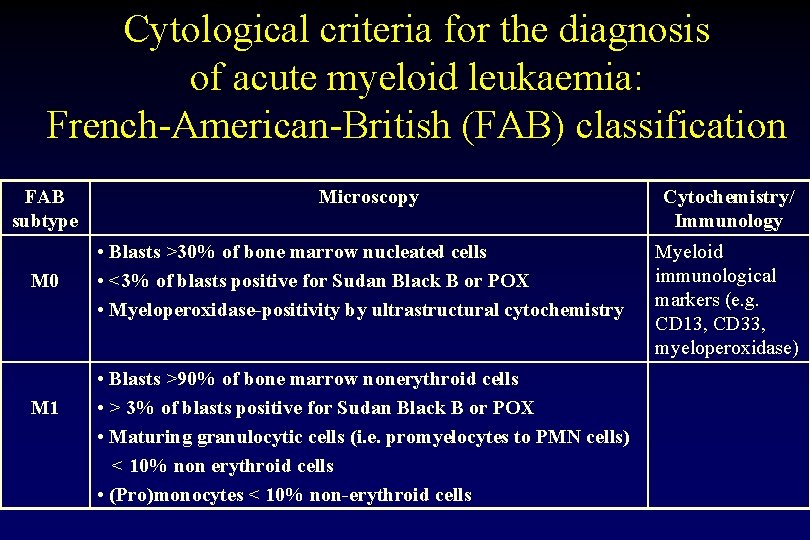
Cytological criteria for the diagnosis of acute myeloid leukaemia: French-American-British (FAB) classification FAB subtype M 0 M 1 Microscopy • Blasts >30% of bone marrow nucleated cells • <3% of blasts positive for Sudan Black B or POX • Myeloperoxidase-positivity by ultrastructural cytochemistry • Blasts >90% of bone marrow nonerythroid cells • > 3% of blasts positive for Sudan Black B or POX • Maturing granulocytic cells (i. e. promyelocytes to PMN cells) < 10% non erythroid cells • (Pro)monocytes < 10% non-erythroid cells Cytochemistry/ Immunology Myeloid immunological markers (e. g. CD 13, CD 33, myeloperoxidase)

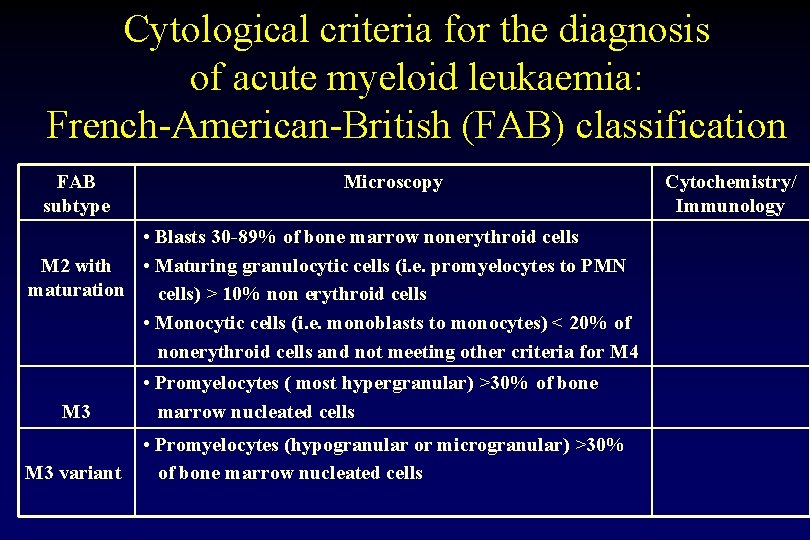
Cytological criteria for the diagnosis of acute myeloid leukaemia: French-American-British (FAB) classification FAB subtype Microscopy • Blasts 30 -89% of bone marrow nonerythroid cells M 2 with • Maturing granulocytic cells (i. e. promyelocytes to PMN maturation cells) > 10% non erythroid cells • Monocytic cells (i. e. monoblasts to monocytes) < 20% of nonerythroid cells and not meeting other criteria for M 4 M 3 variant • Promyelocytes ( most hypergranular) >30% of bone marrow nucleated cells • Promyelocytes (hypogranular or microgranular) >30% of bone marrow nucleated cells Cytochemistry/ Immunology

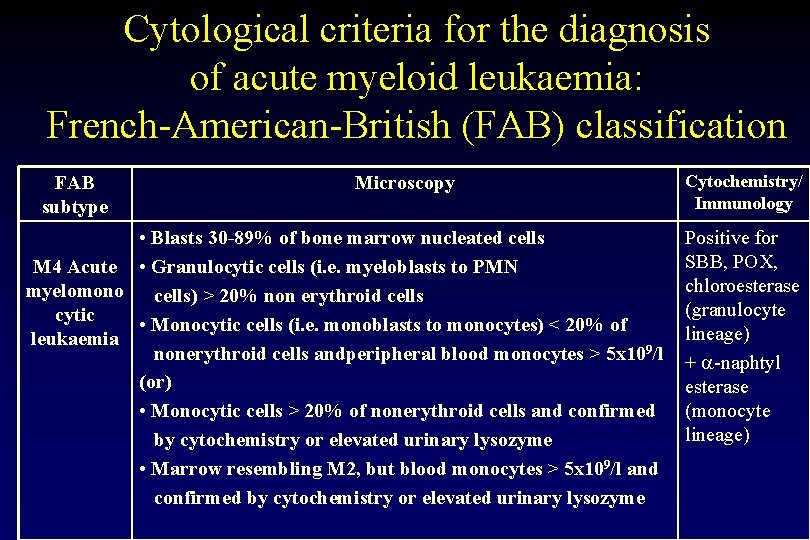
Cytological criteria for the diagnosis of acute myeloid leukaemia: French-American-British (FAB) classification FAB subtype Microscopy • Blasts 30 -89% of bone marrow nucleated cells M 4 Acute • Granulocytic cells (i. e. myeloblasts to PMN myelomono cells) > 20% non erythroid cells cytic • Monocytic cells (i. e. monoblasts to monocytes) < 20% of leukaemia nonerythroid cells andperipheral blood monocytes > 5 x 109/l (or) • Monocytic cells > 20% of nonerythroid cells and confirmed by cytochemistry or elevated urinary lysozyme • Marrow resembling M 2, but blood monocytes > 5 x 109/l and confirmed by cytochemistry or elevated urinary lysozyme Cytochemistry/ Immunology Positive for SBB, POX, chloroesterase (granulocyte lineage) + -naphtyl esterase (monocyte lineage)

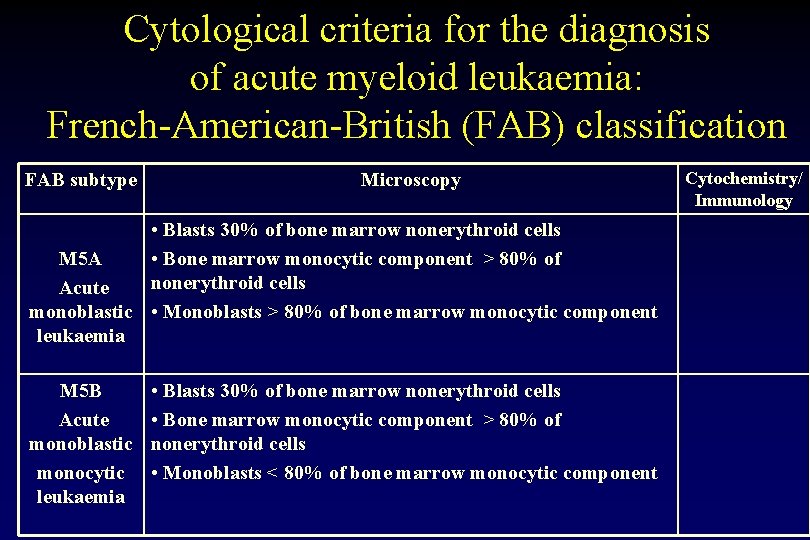
Cytological criteria for the diagnosis of acute myeloid leukaemia: French-American-British (FAB) classification FAB subtype Microscopy • Blasts 30% of bone marrow nonerythroid cells M 5 A • Bone marrow monocytic component > 80% of nonerythroid cells Acute monoblastic • Monoblasts > 80% of bone marrow monocytic component leukaemia M 5 B Acute monoblastic monocytic leukaemia • Blasts 30% of bone marrow nonerythroid cells • Bone marrow monocytic component > 80% of nonerythroid cells • Monoblasts < 80% of bone marrow monocytic component Cytochemistry/ Immunology

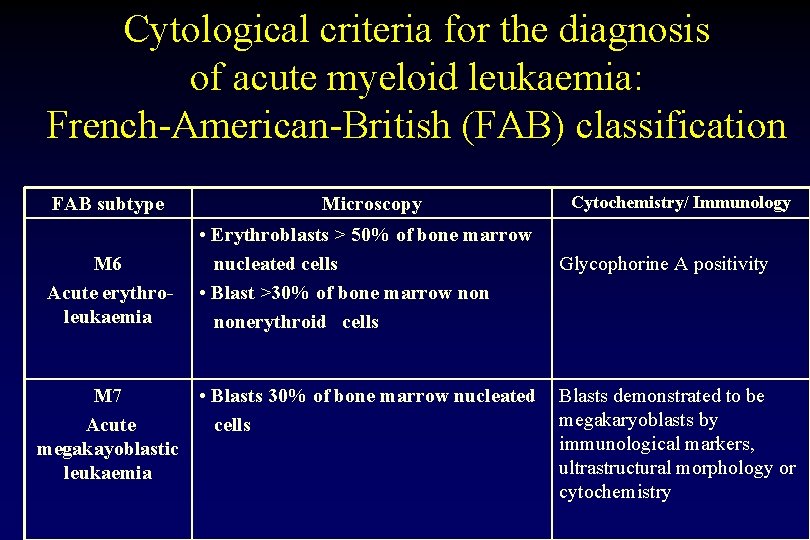
Cytological criteria for the diagnosis of acute myeloid leukaemia: French-American-British (FAB) classification FAB subtype M 6 Acute erythroleukaemia M 7 Acute megakayoblastic leukaemia Microscopy • Erythroblasts > 50% of bone marrow nucleated cells • Blast >30% of bone marrow nonerythroid cells • Blasts 30% of bone marrow nucleated cells Cytochemistry/ Immunology Glycophorine A positivity Blasts demonstrated to be megakaryoblasts by immunological markers, ultrastructural morphology or cytochemistry

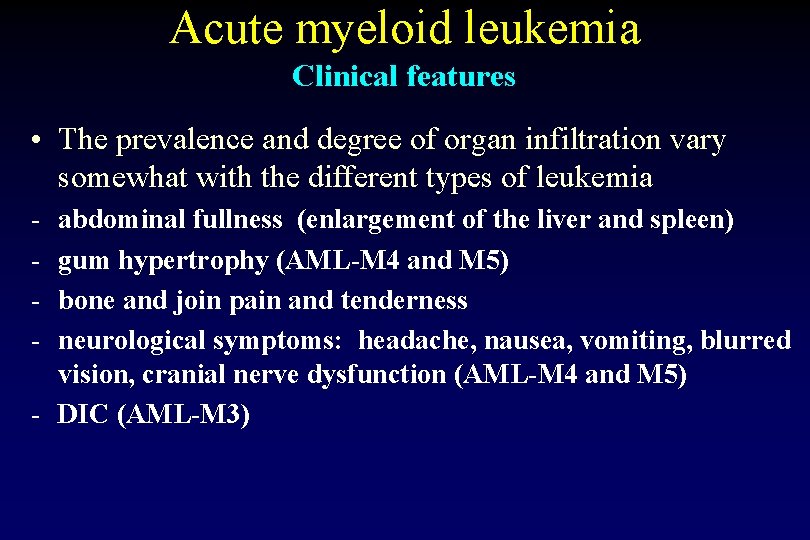
Acute myeloid leukemia Clinical features • Suddent onset of the disease and very fast progression • If not treated death after a few months • Most of the common systemic manifestations, such a fatigue, weakness, fever and weight loss, are non-specific

Acute myeloid leukemia Clinical features • Infiltration of bone marrow by leukemic cells supression of normal hematopoietic progenitor cells growth granulocytopenia, thrombocytopenia and anemia - infection of skin, mucous membranes, gums, respiratory, GI and GU tracts - bleeding in skin, mucous membranes, gums, GI and GU tracts - fatigue, weakness

Acute myeloid leukemia Clinical features • The prevalence and degree of organ infiltration vary somewhat with the different types of leukemia - abdominal fullness (enlargement of the liver and spleen) gum hypertrophy (AML-M 4 and M 5) bone and join pain and tenderness neurological symptoms: headache, nausea, vomiting, blurred vision, cranial nerve dysfunction (AML-M 4 and M 5) - DIC (AML-M 3)

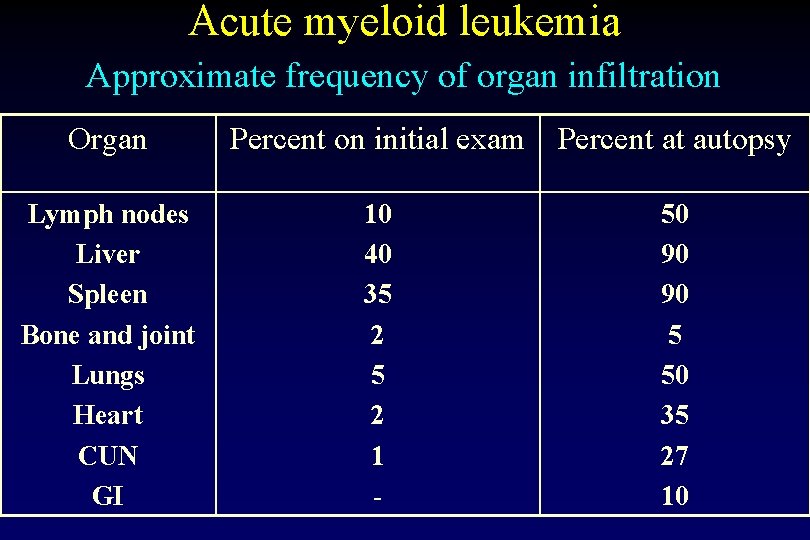
Acute myeloid leukemia Approximate frequency of organ infiltration Organ Percent on initial exam Percent at autopsy Lymph nodes Liver Spleen Bone and joint Lungs Heart CUN GI 10 40 35 2 1 - 50 90 90 5 50 35 27 10

Acute myeloid leukemia • The diagnosis of AML is primarily based on morphological (< 30% of basts and suppression of other lineages) and cytochemical criteria • Immunophentyping, cytogenetic analysis and molecular examination are employed to add specific information for a more precise diagnosis (e. g. to identify undifferentiated leukemias as being myeloid)





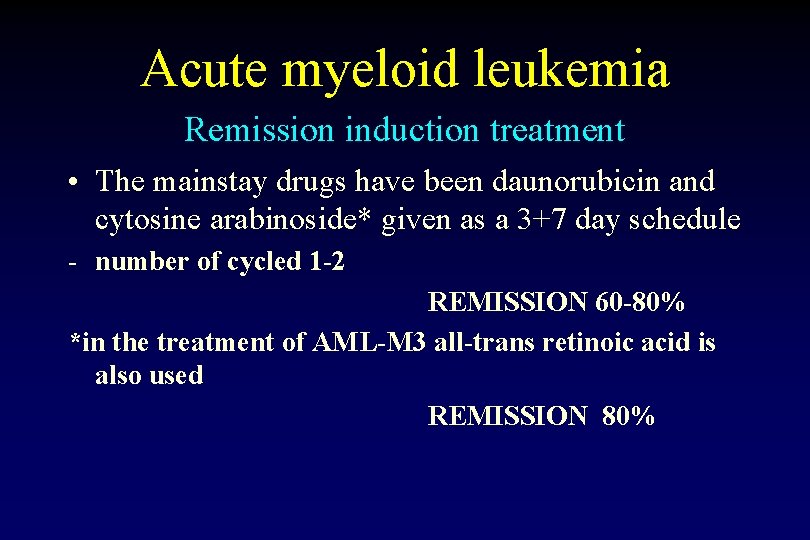
Acute myeloid leukemia Remission induction treatment • The mainstay drugs have been daunorubicin and cytosine arabinoside* given as a 3+7 day schedule - number of cycled 1 -2 REMISSION 60 -80% *in the treatment of AML-M 3 all-trans retinoic acid is also used REMISSION 80%

Acute myeloid leukemia The aims of the induction treatment • obtain the complete remission (RC)* and restoration of polyclonal hemopoiesis * defined as reduction of the blast cells in the marrow < 5% (inapparent) and normalzation of the picture of the peripheral blood However, monoclonal hemopoiesis is still present!

Acute myeloid leukemia Principle of the treatment • CNS prophylaxis/treatment - if clinical symptoms suggest meningeal leukemia AML-M 4 or 5 patients < 18 years old combination of drugs administered intrathecally (Ara-C plus Fenicort, MTX plus Fenicort) or CNS radiotherapy

Acute myeloid leukemia Post-remission chemotherapy The aims of the intensification treatment: - elimination of residual disease - prolongation of the time of remission

Acute myeloid leukemia risk groups • Good risk disease - t(8; 12), t(15; 17) inv 16 • Standard risk disease • Poor risk disease - abnormalities of chromosome 5, complex changes, monosomy 7 and 3 q-
