Acute myeloid leukemia AML Justyna Rybka Department of

Acute myeloid leukemia (AML) Justyna Rybka Department of Haematology, Blood Neoplasms and Bone Marrow Transplantation Wroclaw Medical University Klinika Hematologii, Nowotworów Krwi i Transplantacji Szpiku

AML is a heterogeneous haematological malignancy of the myeloid blood cells 1 Lineage affected in AML causes clonal expansion of blast cells Any myeloid neoplasm with ≥ 20% blasts in the PB or BM may be considered an AML 2 AML = acute myeloid leukaemia BM = bone marrow; PB = peripheral blood 1. NCCN clinical practice guidelines in oncology. Acute myeloid leukaemia. Version 1. 2014. Available at NCCN. org 2. Vardiman JW, et al. Blood 2009; 114: 937– 51
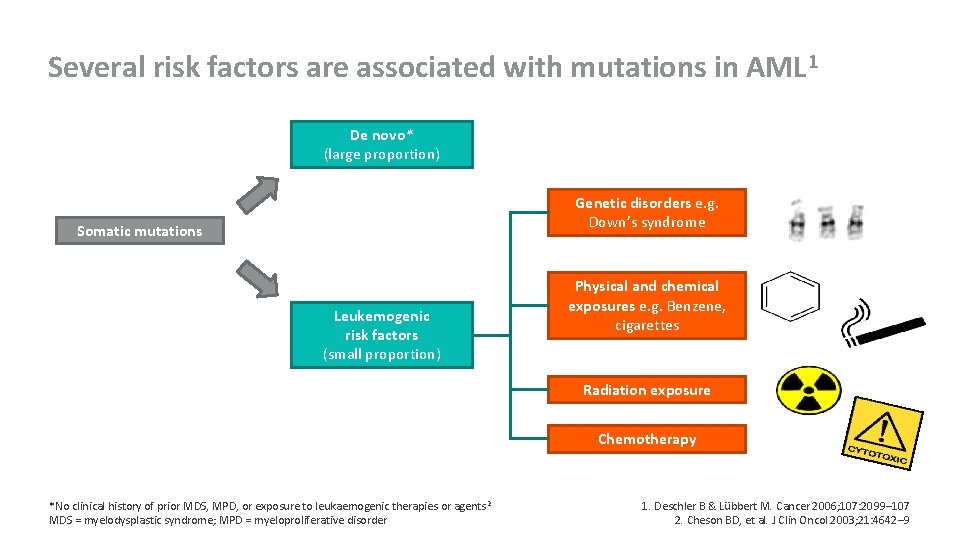
Several risk factors are associated with mutations in AML 1 De novo* (large proportion) Genetic disorders e. g. Down’s syndrome Somatic mutations Leukemogenic risk factors (small proportion) Physical and chemical exposures e. g. Benzene, cigarettes Radiation exposure Chemotherapy *No clinical history of prior MDS, MPD, or exposure to leukaemogenic therapies or agents 2 MDS = myelodysplastic syndrome; MPD = myeloproliferative disorder 1. Deschler B & Lübbert M. Cancer 2006; 107: 2099– 107 2. Cheson BD, et al. J Clin Oncol 2003; 21: 4642– 9
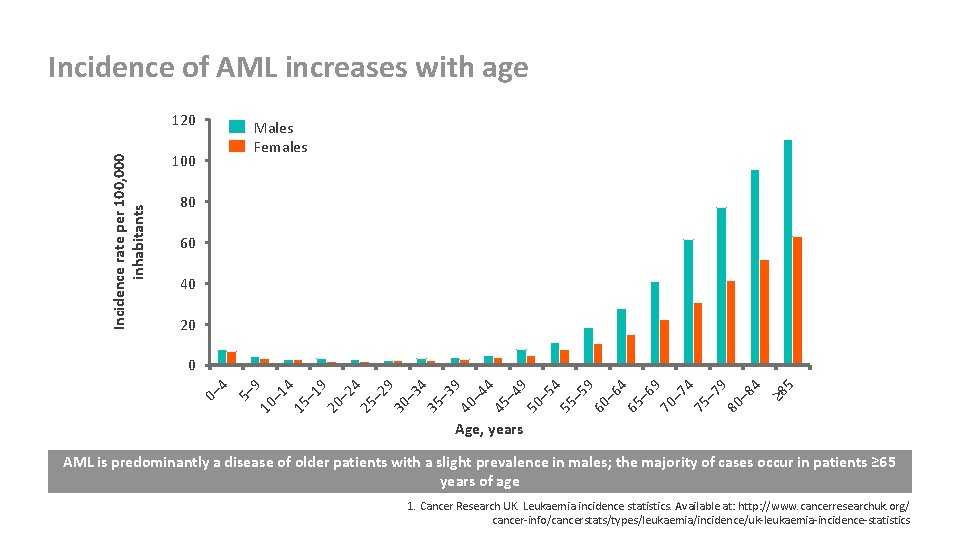
Incidence of AML increases with age Incidence rate per 100, 000 inhabitants 120 Males Females 100 80 60 40 20 10 – 1 4 15 – 1 9 20 – 2 4 25 – 2 9 30 – 3 4 35 – 3 9 40 – 4 4 45 – 4 9 50 – 5 4 55 – 5 9 60 – 6 4 65 – 6 9 70 – 7 4 75 – 7 9 80 – 8 4 ≥ 8 5 5– 9 0– 4 0 Age, years AML is predominantly a disease of older patients with a slight prevalence in males; the majority of cases occur in patients ≥ 65 years of age 1. Cancer Research UK. Leukaemia incidence statistics. Available at: http: //www. cancerresearchuk. org/ cancer-info/cancerstats/types/leukaemia/incidence/uk-leukaemia-incidence-statistics
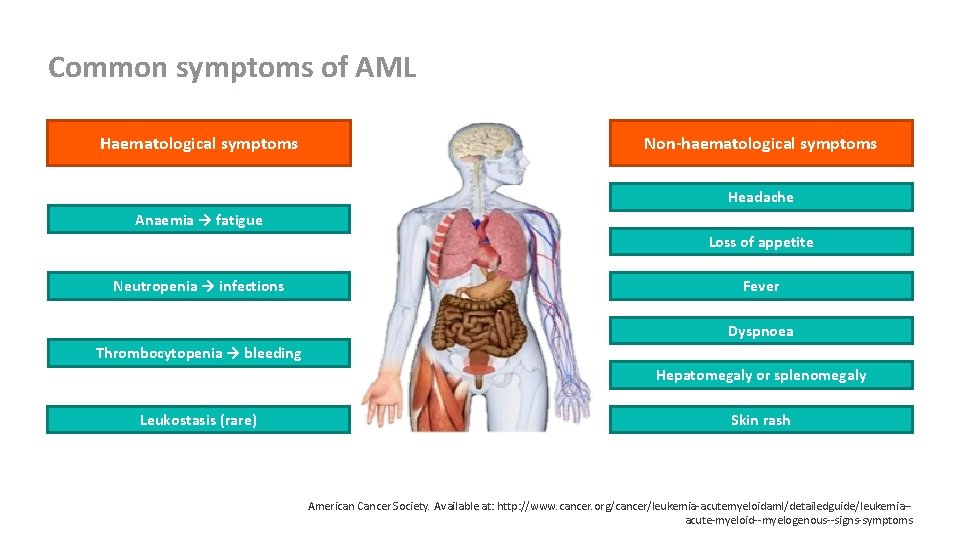
Common symptoms of AML Haematological symptoms Non-haematological symptoms Headache Anaemia → fatigue Loss of appetite Neutropenia → infections Fever Dyspnoea Thrombocytopenia → bleeding Hepatomegaly or splenomegaly Leukostasis (rare) Skin rash American Cancer Society. Available at: http: //www. cancer. org/cancer/leukemia-acutemyeloidaml/detailedguide/leukemia-acute-myeloid--myelogenous--signs-symptoms
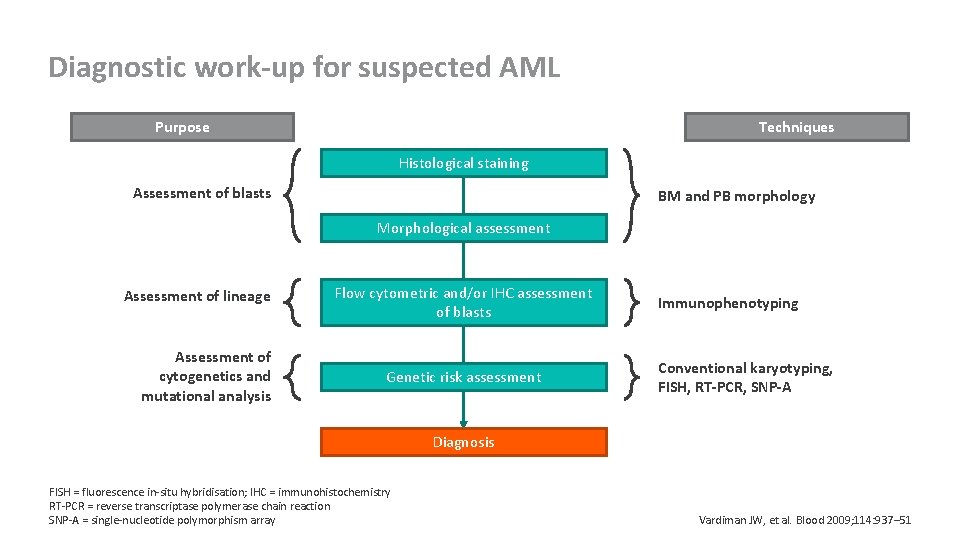
Diagnostic work-up for suspected AML Purpose Techniques Histological staining Assessment of blasts BM and PB morphology Morphological assessment Assessment of lineage Assessment of cytogenetics and mutational analysis Flow cytometric and/or IHC assessment of blasts Genetic risk assessment Immunophenotyping Conventional karyotyping, FISH, RT-PCR, SNP-A Diagnosis FISH = fluorescence in-situ hybridisation; IHC = immunohistochemistry RT-PCR = reverse transcriptase polymerase chain reaction SNP-A = single-nucleotide polymorphism array Vardiman JW, et al. Blood 2009; 114: 937– 51
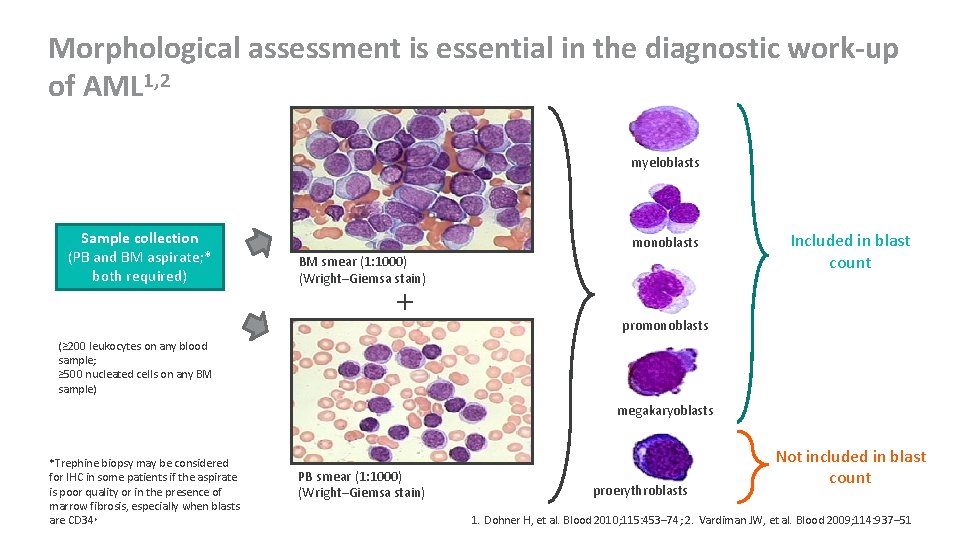
Morphological assessment is essential in the diagnostic work-up of AML 1, 2 myeloblasts Sample collection (PB and BM aspirate; * both required) monoblasts BM smear (1: 1000) (Wright–Giemsa stain) + Included in blast count promonoblasts (≥ 200 leukocytes on any blood sample; ≥ 500 nucleated cells on any BM sample) megakaryoblasts *Trephine biopsy may be considered for IHC in some patients if the aspirate is poor quality or in the presence of marrow fibrosis, especially when blasts are CD 34+ PB smear (1: 1000) (Wright–Giemsa stain) proerythroblasts Not included in blast count 1. Dohner H, et al. Blood 2010; 115: 453– 74; 2. Vardiman JW, et al. Blood 2009; 114: 937– 51
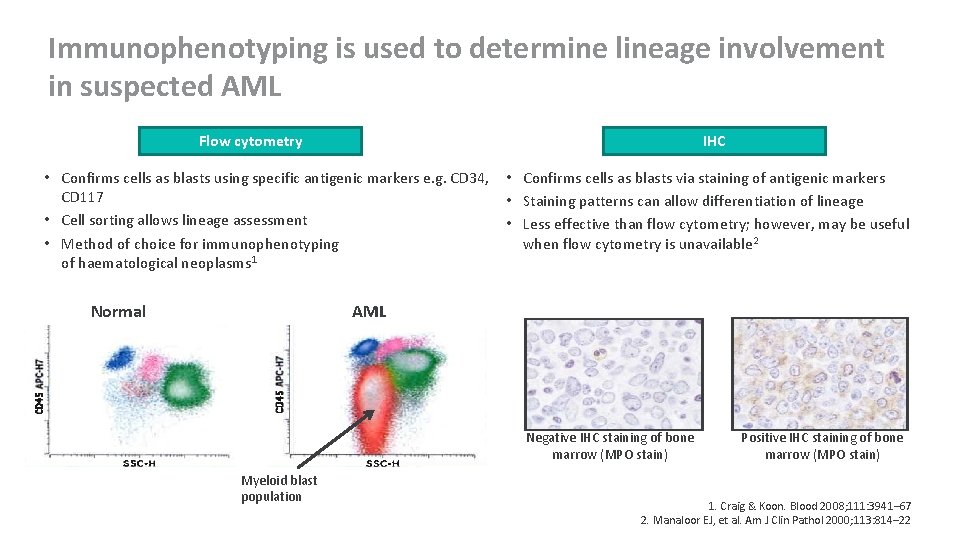
Immunophenotyping is used to determine lineage involvement in suspected AML Flow cytometry IHC • Confirms cells as blasts using specific antigenic markers e. g. CD 34, CD 117 • Cell sorting allows lineage assessment • Method of choice for immunophenotyping of haematological neoplasms 1 Normal • Confirms cells as blasts via staining of antigenic markers • Staining patterns can allow differentiation of lineage • Less effective than flow cytometry; however, may be useful when flow cytometry is unavailable 2 AML Negative IHC staining of bone marrow (MPO stain) Myeloid blast population Positive IHC staining of bone marrow (MPO stain) 1. Craig & Koon. Blood 2008; 111: 3941– 67 2. Manaloor EJ, et al. Am J Clin Pathol 2000; 113: 814– 22
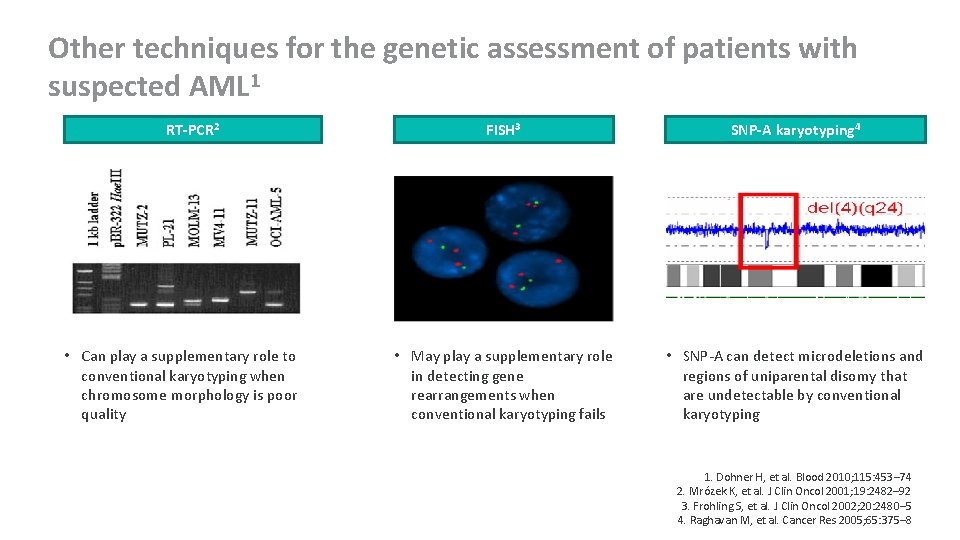
Other techniques for the genetic assessment of patients with suspected AML 1 RT-PCR 2 • Can play a supplementary role to conventional karyotyping when chromosome morphology is poor quality FISH 3 SNP-A karyotyping 4 • May play a supplementary role in detecting gene rearrangements when conventional karyotyping fails • SNP-A can detect microdeletions and regions of uniparental disomy that are undetectable by conventional karyotyping 1. Dohner H, et al. Blood 2010; 115: 453– 74 2. Mrózek K, et al. J Clin Oncol 2001; 19: 2482– 92 3. Frohling S, et al. J Clin Oncol 2002; 20: 2480– 5 4. Raghavan M, et al. Cancer Res 2005; 65: 375– 8
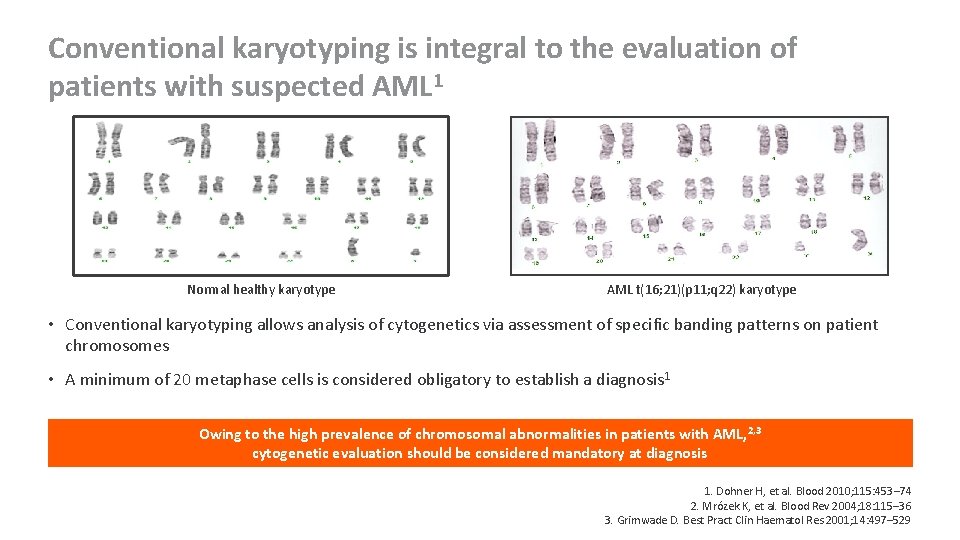
Conventional karyotyping is integral to the evaluation of patients with suspected AML 1 Normal healthy karyotype AML t(16; 21)(p 11; q 22) karyotype • Conventional karyotyping allows analysis of cytogenetics via assessment of specific banding patterns on patient chromosomes • A minimum of 20 metaphase cells is considered obligatory to establish a diagnosis 1 Owing to the high prevalence of chromosomal abnormalities in patients with AML, 2, 3 cytogenetic evaluation should be considered mandatory at diagnosis 1. Dohner H, et al. Blood 2010; 115: 453– 74 2. Mrózek K, et al. Blood Rev 2004; 18: 115– 36 3. Grimwade D. Best Pract Clin Haematol Res 2001; 14: 497– 529

The French–American–British classification was originally used to classify AML Description Blast count M 0 – minimally differentiated ≥ 30% blasts <3% blasts MPO+, SBB+ M 1 – without maturation ≥ 30% blasts <3% blasts MPO+, SBB+ <10% maturing myeloids (promyelocyte and beyond) M 2 – with maturation ≥ 30% blasts ≥ 10% maturing myeloids M 3 – promyelocytic ≥ 30% neoplastic promyelocytes and blasts M 4 – myelomonocytic ≥ 30% blast equivalents (myeloblasts, monoblasts, promonocytes) M 5 a – monoblastic (>80% monoblasts) M 5 b – monocytic (<80% monoblasts) ≥ 30% blast equivalents (myeloblasts, monoblasts, promonocytes) ≥ 80% NSE+ monocytic elements <20% MPO+, SBB+ myeloid elements M 6 – erythroid M 6 a – erythroleukaemia M 6 b – pure erythroid leukaemia ≥ 50% erythroid elements ≥ 30% of nonerythroid elements are myeloid blasts ≥ 80% immature erythroid elements M 7 – megakaryocytic ≥ 30% blasts ≥ 50% blasts should be of megakaryocytic lineage FAB = French–American–British; MPO = myeloperoxidase; SBB = Sudan black B; NSE = nonspecific esterase Bennett JM, et al. Br J Haematol 1982; 51: 189– 99
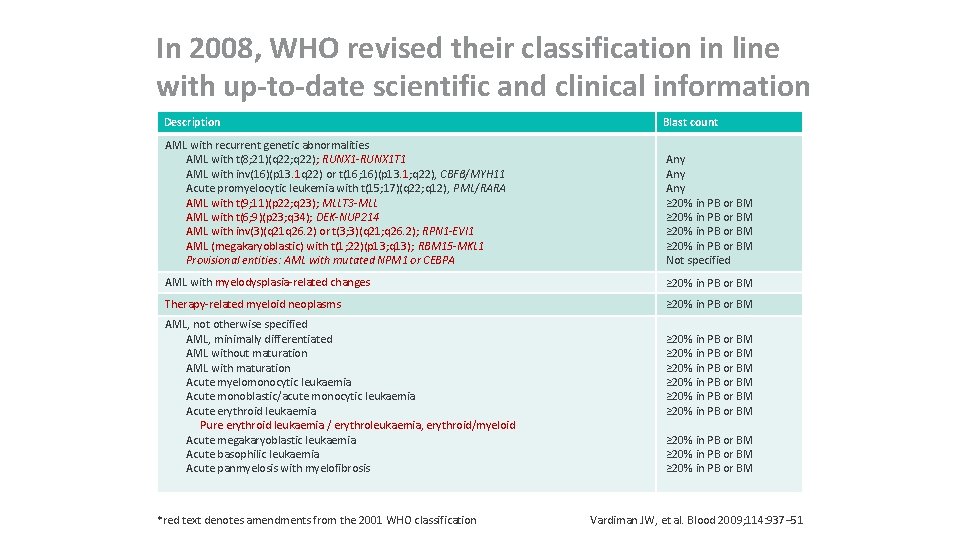
In 2008, WHO revised their classification in line with up-to-date scientific and clinical information Description Blast count AML with recurrent genetic abnormalities AML with t(8; 21)(q 22; q 22); RUNX 1 -RUNX 1 T 1 AML with inv(16)(p 13. 1 q 22) or t(16; 16)(p 13. 1; q 22), CBFβ/MYH 11 Acute promyelocytic leukemia with t(15; 17)(q 22; q 12), PML/RARA AML with t(9; 11)(p 22; q 23); MLLT 3 -MLL AML with t(6; 9)(p 23; q 34); DEK-NUP 214 AML with inv(3)(q 21 q 26. 2) or t(3; 3)(q 21; q 26. 2); RPN 1 -EVI 1 AML (megakaryoblastic) with t(1; 22)(p 13; q 13); RBM 15 -MKL 1 Provisional entities: AML with mutated NPM 1 or CEBPA Any Any ≥ 20% in PB or BM Not specified AML with myelodysplasia-related changes ≥ 20% in PB or BM Therapy-related myeloid neoplasms ≥ 20% in PB or BM AML, not otherwise specified AML, minimally differentiated AML without maturation AML with maturation Acute myelomonocytic leukaemia Acute monoblastic/acute monocytic leukaemia Acute erythroid leukaemia Pure erythroid leukaemia / erythroleukaemia, erythroid/myeloid Acute megakaryoblastic leukaemia Acute basophilic leukaemia Acute panmyelosis with myelofibrosis *red text denotes amendments from the 2001 WHO classification ≥ 20% in PB or BM ≥ 20% in PB or BM ≥ 20% in PB or BM Vardiman JW, et al. Blood 2009; 114: 937– 51
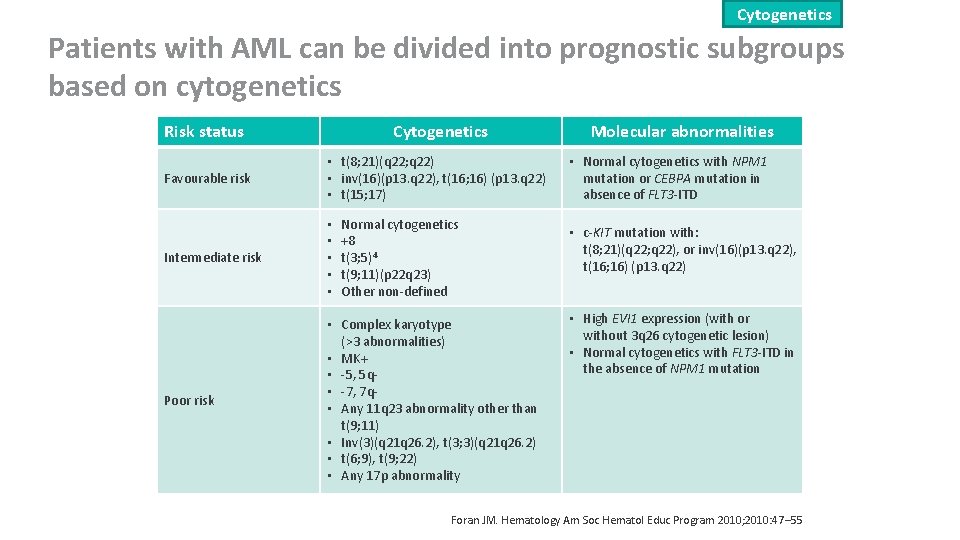
Cytogenetics Patients with AML can be divided into prognostic subgroups based on cytogenetics Risk status Cytogenetics Favourable risk • t(8; 21)(q 22; q 22) • inv(16)(p 13. q 22), t(16; 16) (p 13. q 22) • t(15; 17) Intermediate risk • • • Poor risk • Complex karyotype (>3 abnormalities) • MK+ • -5, 5 q • -7, 7 q • Any 11 q 23 abnormality other than t(9; 11) • Inv(3)(q 21 q 26. 2), t(3; 3)(q 21 q 26. 2) • t(6; 9), t(9; 22) • Any 17 p abnormality Normal cytogenetics +8 t(3; 5)4 t(9; 11)(p 22 q 23) Other non-defined Molecular abnormalities • Normal cytogenetics with NPM 1 mutation or CEBPA mutation in absence of FLT 3 -ITD • c-KIT mutation with: t(8; 21)(q 22; q 22), or inv(16)(p 13. q 22), t(16; 16) (p 13. q 22) • High EVI 1 expression (with or without 3 q 26 cytogenetic lesion) • Normal cytogenetics with FLT 3 -ITD in the absence of NPM 1 mutation Foran JM. Hematology Am Soc Hematol Educ Program 2010; 2010: 47‒ 55

Genetic mutations The most commonly mutated genes in patients with AML Genetic mutation Incidence Clinical impact NPM 1 25– 30% of AML; 45– 64% of CN-AML; approx. 40% with FLT 3 -ITD; 10– 15% with FLT 3 -TKD; 35– 40% with del(9 q) outside of a CK; approx. 15% with trisomy 8; higher prevalence in females • Predicts for achievement of CR and favourable RFS and OS, when present without FLT 3 -ITD CEBPA 10– 18% in CN-AML; approx. 40% in del(9 q) outside of a CK • Higher CR rate and favourable RFS and OS FLT 3 -ITD Approx. 20% of AML; 28– 34% of CN-AML • Inferior outcome FLT 3 -TKD 5– 10% of AML; 11– 14% of CN-AML • Trials underway to determine clinical significance MLL-PTD 5– 11% of CN-AML, and ≤ 90% of AML with trisomy 11 • In initial studies: shorter CR duration, inferior RFS and EFS, but not OS NRAS 9– 14% of CN-AML, ≤ 40% in CBF-AML • May predict sensitivity to cytarabine 10– 13% of CN-AML • Most studies: negative prognostic impact • Poor prognosis with post-remission high-dose cytarabine • WT 1 SNP rs 16754 associated with inferior outcome in CN-AML WT 1 CBF-AML = core binding factor acute myeloid leukaemia; EFS = event-free survival PTD = partial tandem duplication; RFS = relapse-free survival TD = internal tandem duplication ; TKD = tyrosine kinase domain Marcucci G, et al. J Clin Oncol 2011; 29: 475– 86

Genetic mutations ELN system proposed for grouping genetic abnormalities in AML* Genetic group Subsets Favourable • • t(8; 21)(q 22; q 22); RUNX 1 -RUNX 1 T 1 inv(16)(p 13. 1; q 22) or t(16; 16)(p 13. 1; q 22); CBFB-MYH 11 Mutated NPM 1 without FLT 3 -ITD (CN-AML) Mutated CEBPA (CN-AML) Intermediate-1 • • • Mutated NPM 1 and FLT 3 -ITD (CN-AML) Wild-type NPM 1 without FLT 3 -ITD (CN-AML) Intermediate-2 • • t(9; 11)(q 22; q 23); MLLT 3 -MLL Cytogenetic abnormalities not classified as favourable or adverse Adverse • • • inv(3)(q 21 q 26. 2) or t(3; 3)(q 21; q 26. 2); RPN 1 -EVI 1 t(6; 9)(p 23; q 34); DEK-NUP 214 – 5 or del(5 q); – 7; abn(17 p); CK† Genetic abnormalities can be incorporated into cytogenetic categories to refine risk assessment *Correlating data from cytogenetic and mutational analyses of NPM 1, CEBPA and FLT 3 with clinical outcome; n number not specified; †≥ 3 abnormalities in the absence of t(15; 17), t(8; 21), inv(16) or Dohner H, et al. Blood 2010; 115: 453– 74 t(16; 16), t(9; 11), t(v; 11)(v; q 23), t(6; 9), inv(3) or t(3; 3); ELN = European Leukemia. Net
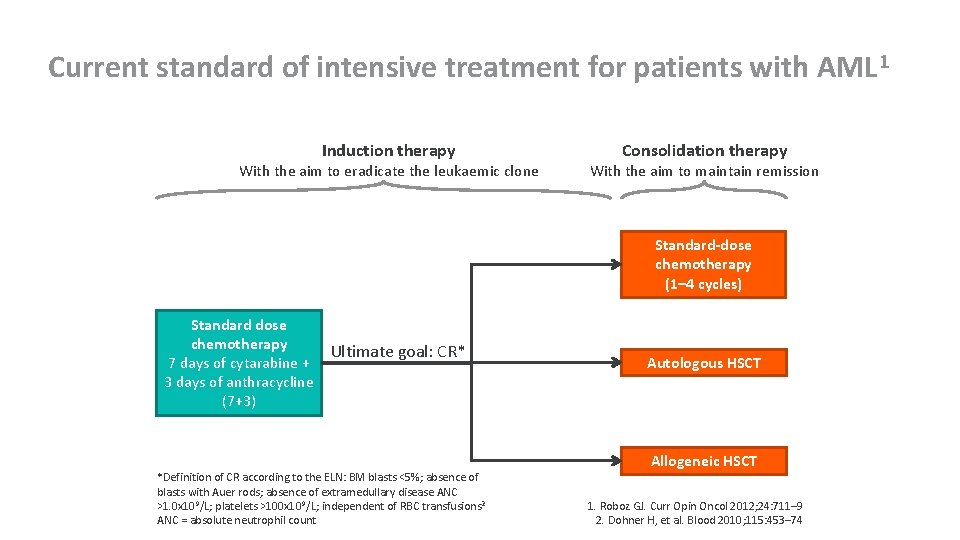
Current standard of intensive treatment for patients with AML 1 Induction therapy With the aim to eradicate the leukaemic clone Consolidation therapy With the aim to maintain remission Standard-dose chemotherapy (1– 4 cycles) Standard dose chemotherapy 7 days of cytarabine + 3 days of anthracycline (7+3) Ultimate goal: CR* *Definition of CR according to the ELN: BM blasts <5%; absence of blasts with Auer rods; absence of extramedullary disease ANC >1. 0 x 109/L; platelets >100 x 10 9/L; independent of RBC transfusions 2 ANC = absolute neutrophil count Autologous HSCT Allogeneic HSCT 1. Roboz GJ. Curr Opin Oncol 2012; 24: 711– 9 2. Dohner H, et al. Blood 2010; 115: 453– 74
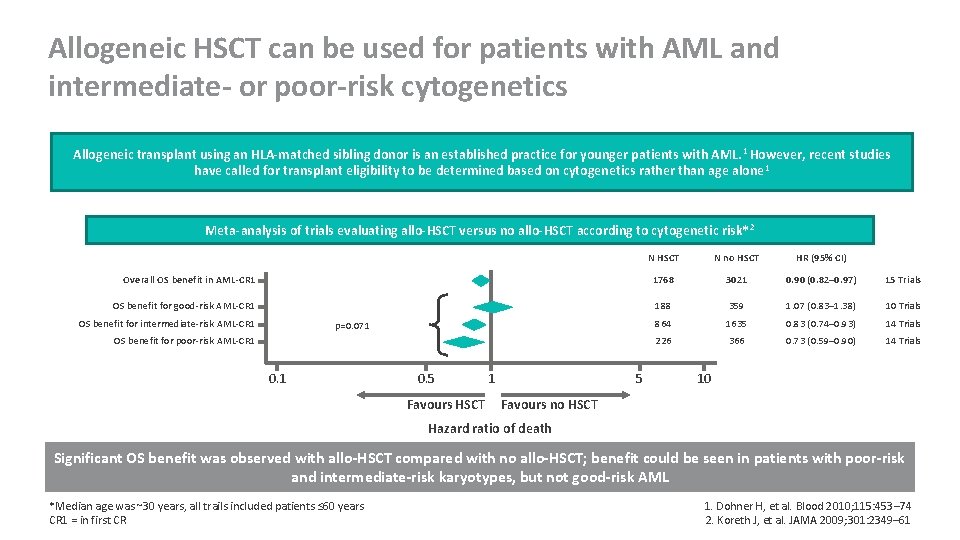
Allogeneic HSCT can be used for patients with AML and intermediate- or poor-risk cytogenetics Allogeneic transplant using an HLA-matched sibling donor is an established practice for younger patients with AML. 1 However, recent studies have called for transplant eligibility to be determined based on cytogenetics rather than age alone 1 Meta-analysis of trials evaluating allo-HSCT versus no allo-HSCT according to cytogenetic risk* 2 N HSCT N no HSCT HR (95% CI) Overall OS benefit in AML-CR 1 1768 3021 0. 90 (0. 82– 0. 97) 15 Trials OS benefit for good-risk AML-CR 1 188 359 1. 07 (0. 83– 1. 38) 10 Trials 864 1635 0. 83 (0. 74– 0. 93) 14 Trials 226 366 0. 73 (0. 59– 0. 90) 14 Trials OS benefit for intermediate-risk AML-CR 1 p=0. 071 OS benefit for poor-risk AML-CR 1 0. 5 Favours HSCT 1 5 10 Favours no HSCT Hazard ratio of death Significant OS benefit was observed with allo-HSCT compared with no allo-HSCT; benefit could be seen in patients with poor-risk and intermediate-risk karyotypes, but not good-risk AML *Median age was ~30 years, all trails included patients ≤ 60 years CR 1 = in first CR 1. Dohner H, et al. Blood 2010; 115: 453– 74 2. Koreth J, et al. JAMA 2009; 301: 2349– 61
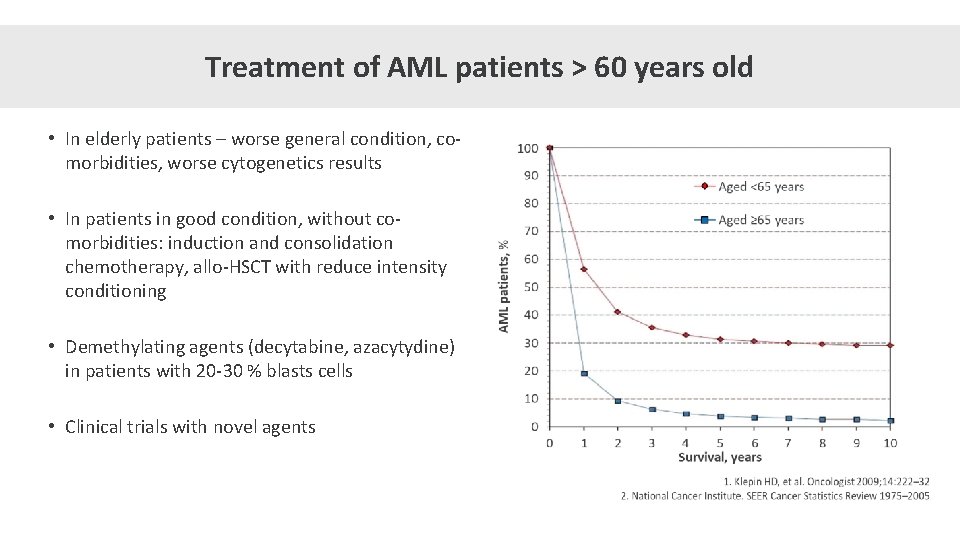
Treatment of AML patients > 60 years old • In elderly patients – worse general condition, comorbidities, worse cytogenetics results • In patients in good condition, without comorbidities: induction and consolidation chemotherapy, allo-HSCT with reduce intensity conditioning • Demethylating agents (decytabine, azacytydine) in patients with 20 -30 % blasts cells • Clinical trials with novel agents

Supportive care options for patients with AML During treatment, most AML patients will receive supportive care to protect against periods of very low blood counts Anti-infectious treatment Antibiotics and anti-fungals are used prophylactically to treat bacterial and fungal infections which can occur due to low WBC counts 1 RBC = red blood cells Myeloid growth factors Transfusions Myeloid growth factors can shorten the duration of neutropenia and reduce the incidence and severity of infections 2 Transfusion of RBC and/or platelets can be used to correct anaemia and prevent bleeding 1 1. http: //www. ucsfhealth. org/conditions/acute_myeloid_leukemia/treatment. html 2. Dohner H, et al. Blood 2010; 115: 453– 74
APL: acute promyelocytic leukemia • 5 -10% of AML • Pathogenesis: translocation of chromosomes 15 and 17, this causes parts of a gene from each of these chromosomes to join and create a fusion gene called PML/RARA • Symptoms of APL: unexplained bleeding or bruising, abnormalities of clotting system (DIC), persistent tiredness, dizziness, paleness, or shortness of breath • The treatment of acute promyelocytic leukemia (APL) is very different than that of other types of acute leukemia. ATRA plus anthracycline-based chemotherapy for induction and consolidation followed by maintenance ATRA with low-dose chemotherapy is currently the standard of care. • Patients must start treatment quickly after being diagnosed.
- Slides: 20