Acute Kidney Injury AKI Pharmacotherapy II Second Semester














- Slides: 70

Acute Kidney Injury (AKI) Pharmacotherapy II Second Semester 2014

References Pharmacotherapy: A Pathophysiologic Approach – Chapter 50 (Assessment) + 51 (ARF), 55 (Drug induced) Pharmacotherapy: Principles and Practice – Chapter 22 Applied Therapeutics: The Clinical Use of Drugs – Chapter 30 Up. To. Date: http: //www. uptodate. com Assessment of kidney function: Serum creatinine; BUN; and GFR Diagnostic approach to the patient with acute or chronic kidney disease Urinalysis in the diagnosis of renal disease Definition of acute kidney injury (acute renal failure) National Kidney Foundation: http: //www. kidney. org The Renal Association. Acute kidney injury. 2011. www. renal. org/Clinical/Guidelines. Section/Acute. Kidney. Injury. aspx Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter. , Suppl. 2012; 2: 1– 138

Introduction Renal function: Maintenance of body composition: Filtration Absorption Secretion Excretion of metabolic end products and foreign substances Production and secretion of enzymes and hormones Activation of vitamin D 3/glucneogenesis/metabolism of insulin, steroids and xenobiotics

Assessment of kidney function 4

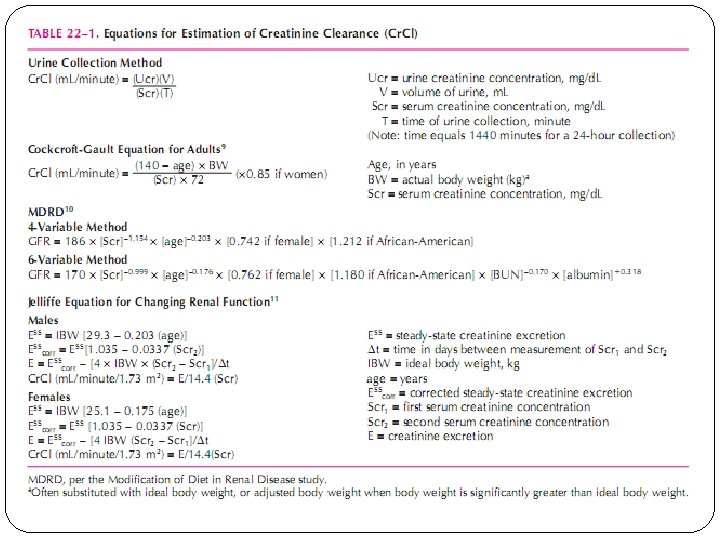
Glomerular filtration rate (GFR) The GFR is equal to the sum of the filtration rates in all of the functioning nephrons the estimation of the GFR gives an approximate measure of the number of functioning nephrons. The GFR is expressed as the volume of plasma filtered across the glomerulus per unit time, based on total renal blood flow (1 L/min/1. 73 m 2)and capillary hemodynamics (plasma volume is 60% of the blood volume). The normal value for GFR depends on age, sex, and body size, and is approximately 130 and 120 m. L/min/1. 73 m 2 for men and women, respectively, with considerable variation even among normal individuals A reduction in GFR implies either progression of the underlying disease or the development of a superimposed and often reversible problem, such as decreased renal perfusion due to volume depletion. An increase in GFR, is indicative of improvement in renal function, or may imply an increase in filtration (hyperfiltration) due to hemodynamic factors A stable GFR in patients with renal disease implies stable disease.



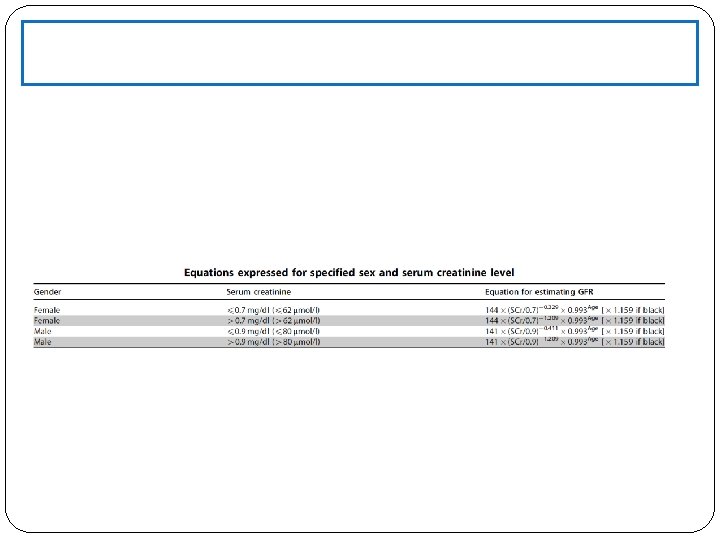
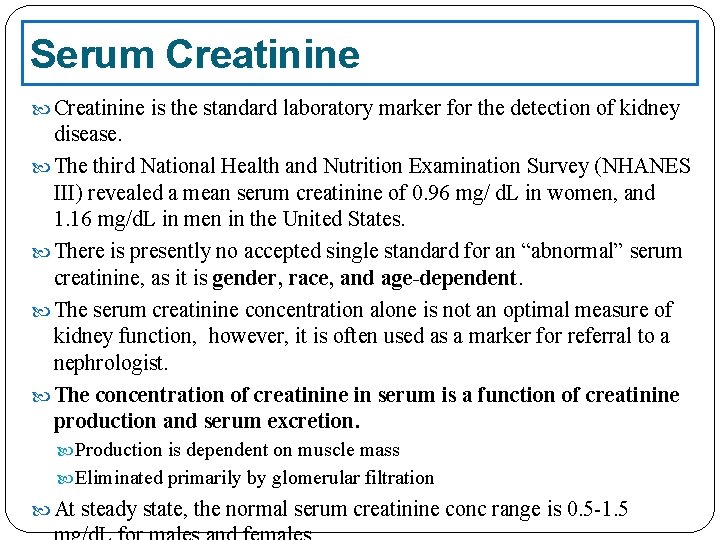
Serum Creatinine is the standard laboratory marker for the detection of kidney disease. The third National Health and Nutrition Examination Survey (NHANES III) revealed a mean serum creatinine of 0. 96 mg/ d. L in women, and 1. 16 mg/d. L in men in the United States. There is presently no accepted single standard for an “abnormal” serum creatinine, as it is gender, race, and age-dependent. The serum creatinine concentration alone is not an optimal measure of kidney function, however, it is often used as a marker for referral to a nephrologist. The concentration of creatinine in serum is a function of creatinine production and serum excretion. Production is dependent on muscle mass Eliminated primarily by glomerular filtration At steady state, the normal serum creatinine conc range is 0. 5 -1. 5

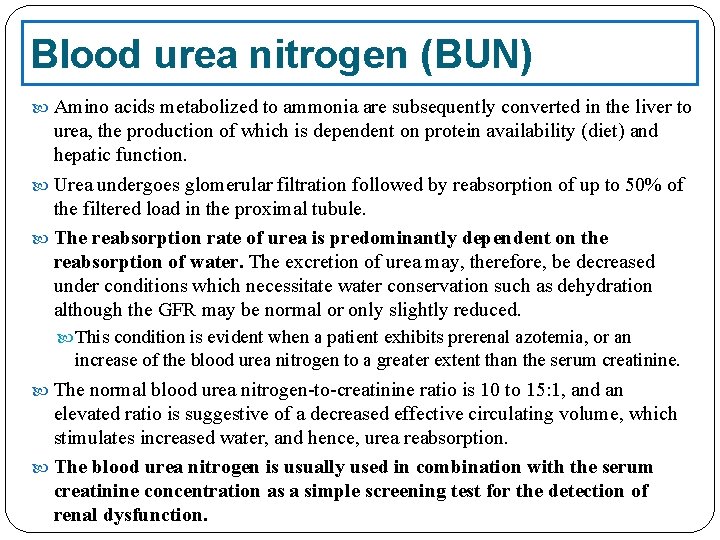
Blood urea nitrogen (BUN) Amino acids metabolized to ammonia are subsequently converted in the liver to urea, the production of which is dependent on protein availability (diet) and hepatic function. Urea undergoes glomerular filtration followed by reabsorption of up to 50% of the filtered load in the proximal tubule. The reabsorption rate of urea is predominantly dependent on the reabsorption of water. The excretion of urea may, therefore, be decreased under conditions which necessitate water conservation such as dehydration although the GFR may be normal or only slightly reduced. This condition is evident when a patient exhibits prerenal azotemia, or an increase of the blood urea nitrogen to a greater extent than the serum creatinine. The normal blood urea nitrogen-to-creatinine ratio is 10 to 15: 1, and an elevated ratio is suggestive of a decreased effective circulating volume, which stimulates increased water, and hence, urea reabsorption. The blood urea nitrogen is usually used in combination with the serum creatinine concentration as a simple screening test for the detection of renal dysfunction.

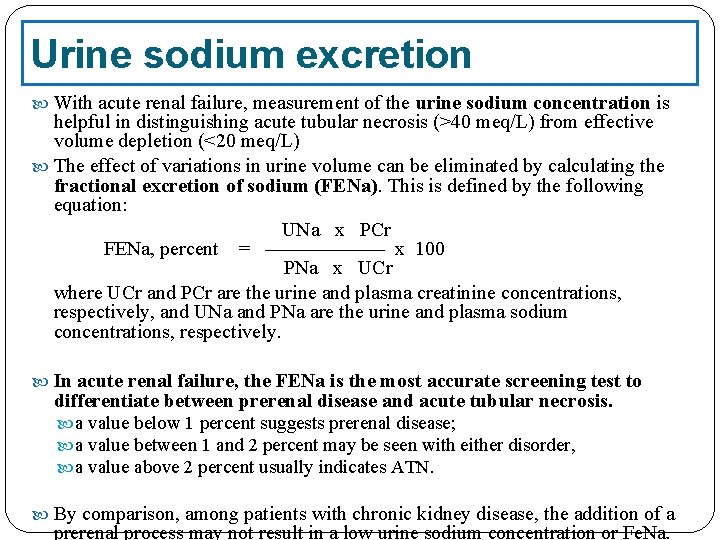
Urine sodium excretion With acute renal failure, measurement of the urine sodium concentration is helpful in distinguishing acute tubular necrosis (>40 meq/L) from effective volume depletion (<20 meq/L) The effect of variations in urine volume can be eliminated by calculating the fractional excretion of sodium (FENa). This is defined by the following equation: UNa x PCr FENa, percent = —————— x 100 PNa x UCr where UCr and PCr are the urine and plasma creatinine concentrations, respectively, and UNa and PNa are the urine and plasma sodium concentrations, respectively. In acute renal failure, the FENa is the most accurate screening test to differentiate between prerenal disease and acute tubular necrosis. a value below 1 percent suggests prerenal disease; a value between 1 and 2 percent may be seen with either disorder, a value above 2 percent usually indicates ATN. By comparison, among patients with chronic kidney disease, the addition of a prerenal process may not result in a low urine sodium concentration or Fe. Na.

Urinalysis The urinalysis is the most important noninvasive test in the diagnostic evaluation since characteristic findings strongly suggest certain diagnoses. It can be used to detect and monitor the progression of diseases such as DM, glomerulonephritis and chronic UTI. Urinalysis involves: Visual observation (volume and color) Microscopic examination of the urinary sediment to determine formed elements: erythrocytes, leukocytes, casts and crytals. Dipstick testing for protein, p. H, concentration, glucose, ketones, hematuria and pyuria.

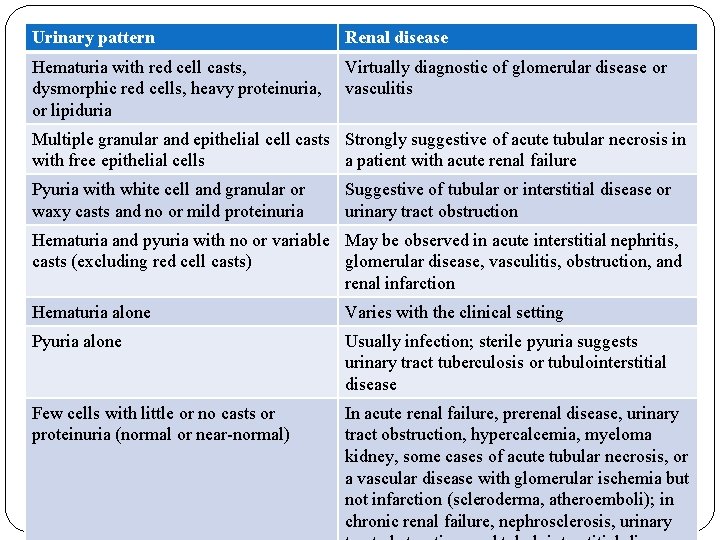
Urinary pattern Renal disease Hematuria with red cell casts, dysmorphic red cells, heavy proteinuria, or lipiduria Virtually diagnostic of glomerular disease or vasculitis Multiple granular and epithelial cell casts Strongly suggestive of acute tubular necrosis in with free epithelial cells a patient with acute renal failure Pyuria with white cell and granular or waxy casts and no or mild proteinuria Suggestive of tubular or interstitial disease or urinary tract obstruction Hematuria and pyuria with no or variable May be observed in acute interstitial nephritis, casts (excluding red cell casts) glomerular disease, vasculitis, obstruction, and renal infarction Hematuria alone Varies with the clinical setting Pyuria alone Usually infection; sterile pyuria suggests urinary tract tuberculosis or tubulointerstitial disease Few cells with little or no casts or proteinuria (normal or near-normal) In acute renal failure, prerenal disease, urinary tract obstruction, hypercalcemia, myeloma kidney, some cases of acute tubular necrosis, or a vascular disease with glomerular ischemia but not infarction (scleroderma, atheroemboli); in chronic renal failure, nephrosclerosis, urinary

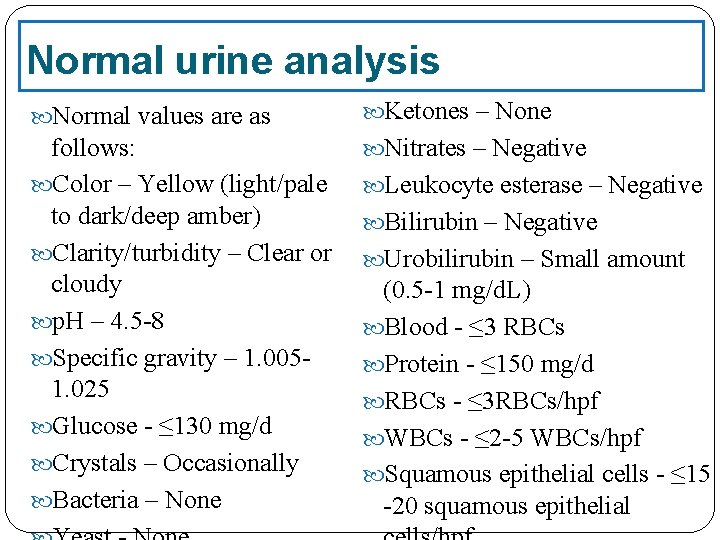
Normal urine analysis Normal values are as Ketones – None follows: Color – Yellow (light/pale to dark/deep amber) Clarity/turbidity – Clear or cloudy p. H – 4. 5 -8 Specific gravity – 1. 0051. 025 Glucose - ≤ 130 mg/d Crystals – Occasionally Bacteria – None Nitrates – Negative Leukocyte esterase – Negative Bilirubin – Negative Urobilirubin – Small amount (0. 5 -1 mg/d. L) Blood - ≤ 3 RBCs Protein - ≤ 150 mg/d RBCs - ≤ 3 RBCs/hpf WBCs - ≤ 2 -5 WBCs/hpf Squamous epithelial cells - ≤ 15 -20 squamous epithelial

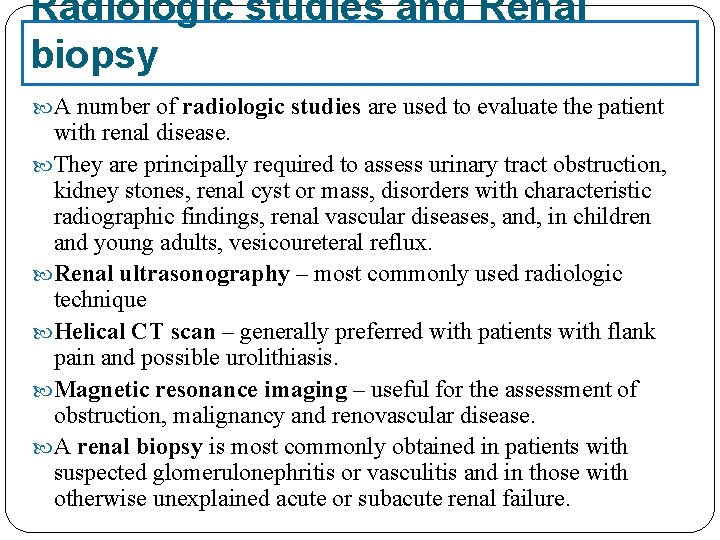
Radiologic studies and Renal biopsy A number of radiologic studies are used to evaluate the patient with renal disease. They are principally required to assess urinary tract obstruction, kidney stones, renal cyst or mass, disorders with characteristic radiographic findings, renal vascular diseases, and, in children and young adults, vesicoureteral reflux. Renal ultrasonography – most commonly used radiologic technique Helical CT scan – generally preferred with patients with flank pain and possible urolithiasis. Magnetic resonance imaging – useful for the assessment of obstruction, malignancy and renovascular disease. A renal biopsy is most commonly obtained in patients with suspected glomerulonephritis or vasculitis and in those with otherwise unexplained acute or subacute renal failure.

Acute Kidney Injury 21

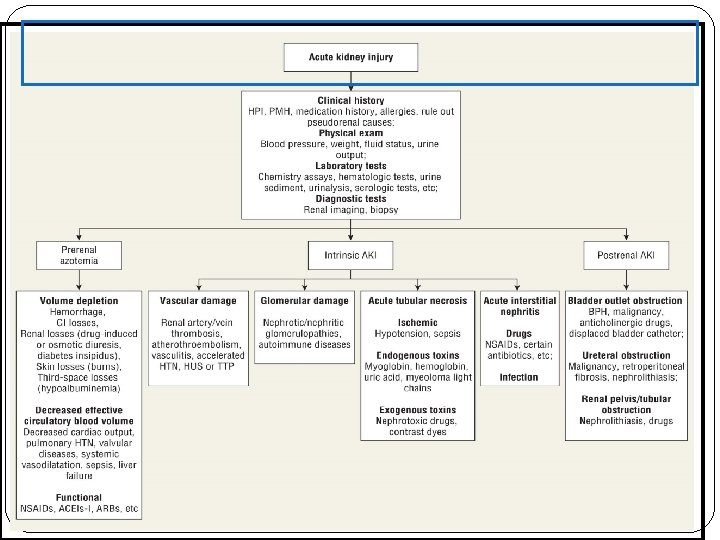
Definition (ARF or AKI) Acute renal failure (ARF) is characterized clinically by an abrupt decrease in renal function over a period of hours to days, and sometimes over weeks, resulting in the accumulation of nitrogenous waste products (azotemia) and the inability to maintain and regulate fluid, electrolyte, and acid–base balance. A decrease in urine output is often observed but not required for ARF. Patients with ARF are often categorized as being: Anuric UO <50 ml/day Oliguric UO <500 ml/day Non-oliguric UO >500 ml/day

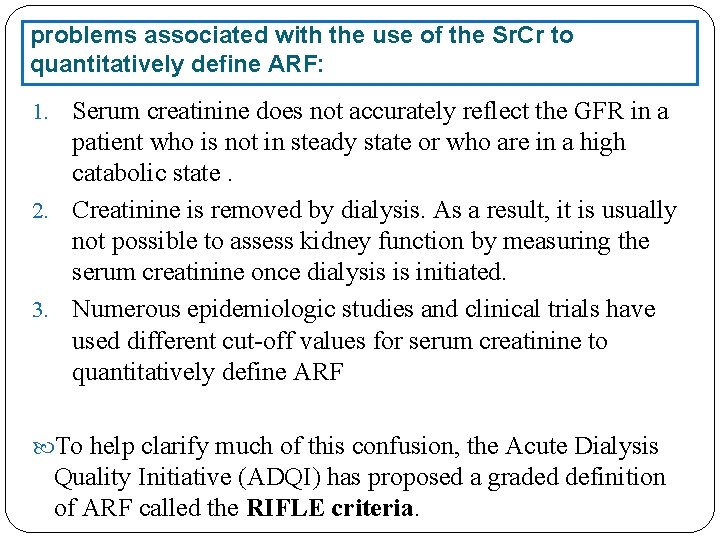
problems associated with the use of the Sr. Cr to quantitatively define ARF: Serum creatinine does not accurately reflect the GFR in a patient who is not in steady state or who are in a high catabolic state. 2. Creatinine is removed by dialysis. As a result, it is usually not possible to assess kidney function by measuring the serum creatinine once dialysis is initiated. 3. Numerous epidemiologic studies and clinical trials have used different cut-off values for serum creatinine to quantitatively define ARF 1. To help clarify much of this confusion, the Acute Dialysis Quality Initiative (ADQI) has proposed a graded definition of ARF called the RIFLE criteria.

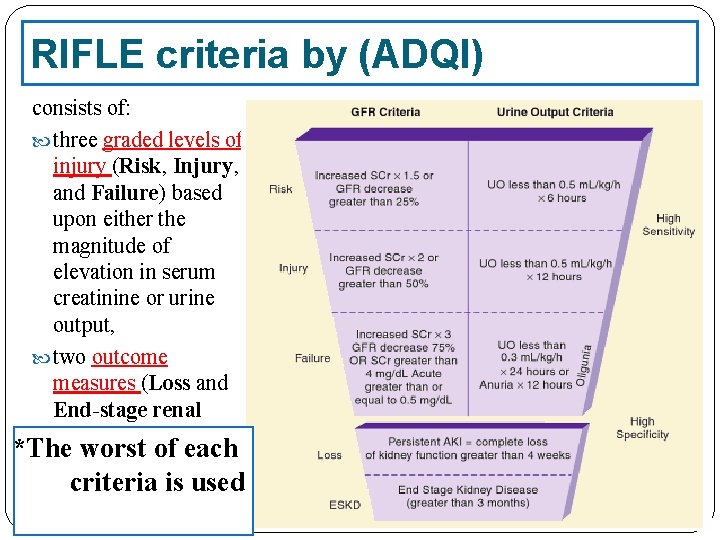
RIFLE criteria by (ADQI) consists of: three graded levels of injury (Risk, Injury, and Failure) based upon either the magnitude of elevation in serum creatinine or urine output, two outcome measures (Loss and End-stage renal disease) *The worst of each criteria is used

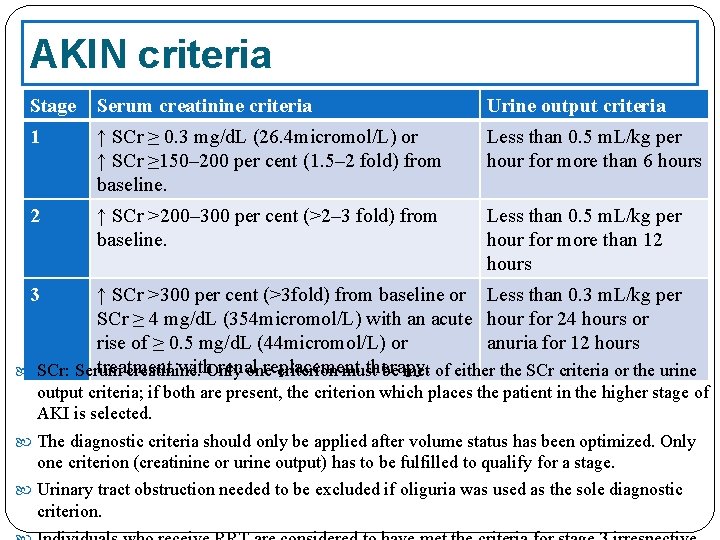
AKIN criteria The Acute Kidney Injury Network (AKIN) modified the RIFLE criteria in order to: include less severe ARF, impose a time constraint of 48 hours, allow for correction of volume status and obstructive causes of ARF prior to classification. The AKIN proposed the term acute kidney injury (AKI) to represent the entire spectrum of acute renal failure, recognizing that an acute decline in kidney function is often secondary to an injury that causes functional or structural changes in the kidneys and that the injury can have important consequences for the patient even if it does not lead to organ failure and a requirement for renal replacement therapy

The AKIN defined AKI as “an abrupt (within 48 hours) reduction in kidney function, currently defined as an absolute increase in serum creatinine level of more than or equal to 0. 3 mg/d. L (26. 4 μmol/L), a percentage increase in serum creatinine of more than or equal to 50% (1. 5 -fold from baseline), or a reduction in urine output (documented oliguria of less than 0. 5 m. L/kg/hr for more than 6 hours). ”

AKIN criteria Stage Serum creatinine criteria Urine output criteria 1 ↑ SCr ≥ 0. 3 mg/d. L (26. 4 micromol/L) or ↑ SCr ≥ 150– 200 per cent (1. 5– 2 fold) from baseline. Less than 0. 5 m. L/kg per hour for more than 6 hours 2 ↑ SCr >200– 300 per cent (>2– 3 fold) from baseline. Less than 0. 5 m. L/kg per hour for more than 12 hours 3 ↑ SCr >300 per cent (>3 fold) from baseline or Less than 0. 3 m. L/kg per SCr ≥ 4 mg/d. L (354 micromol/L) with an acute hour for 24 hours or rise of ≥ 0. 5 mg/d. L (44 micromol/L) or anuria for 12 hours treatment with. Only renal replacement therapy. SCr: Serum creatinine. one criterion must be met of either the SCr criteria or the urine output criteria; if both are present, the criterion which places the patient in the higher stage of AKI is selected. The diagnostic criteria should only be applied after volume status has been optimized. Only one criterion (creatinine or urine output) has to be fulfilled to qualify for a stage. Urinary tract obstruction needed to be excluded if oliguria was used as the sole diagnostic criterion.

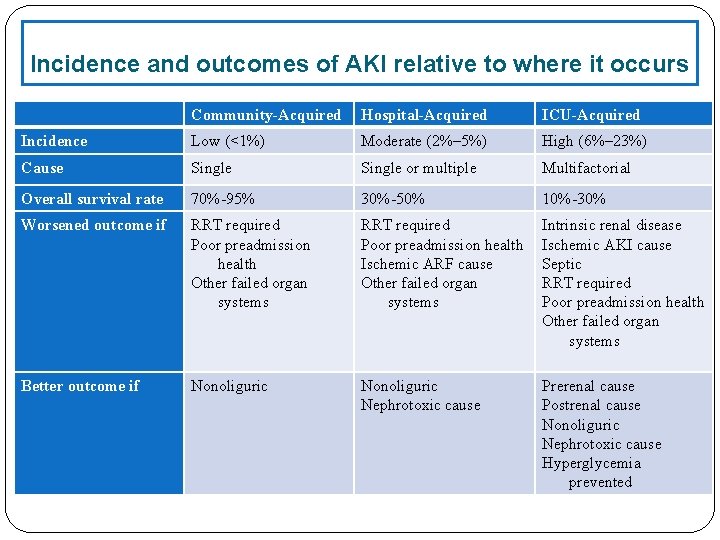
Incidence and outcomes of AKI relative to where it occurs Community-Acquired Hospital-Acquired ICU-Acquired Incidence Low (<1%) Moderate (2%– 5%) High (6%– 23%) Cause Single or multiple Multifactorial Overall survival rate 70%-95% 30%-50% 10%-30% Worsened outcome if RRT required Poor preadmission health Other failed organ systems RRT required Poor preadmission health Ischemic ARF cause Other failed organ systems Intrinsic renal disease Ischemic AKI cause Septic RRT required Poor preadmission health Other failed organ systems Better outcome if Nonoliguric Nephrotoxic cause Prerenal cause Postrenal cause Nonoliguric Nephrotoxic cause Hyperglycemia prevented

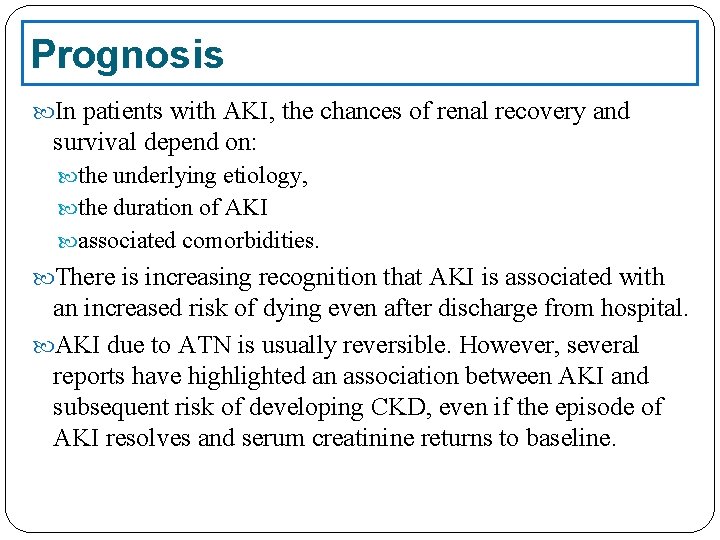
Prognosis In patients with AKI, the chances of renal recovery and survival depend on: the underlying etiology, the duration of AKI associated comorbidities. There is increasing recognition that AKI is associated with an increased risk of dying even after discharge from hospital. AKI due to ATN is usually reversible. However, several reports have highlighted an association between AKI and subsequent risk of developing CKD, even if the episode of AKI resolves and serum creatinine returns to baseline.

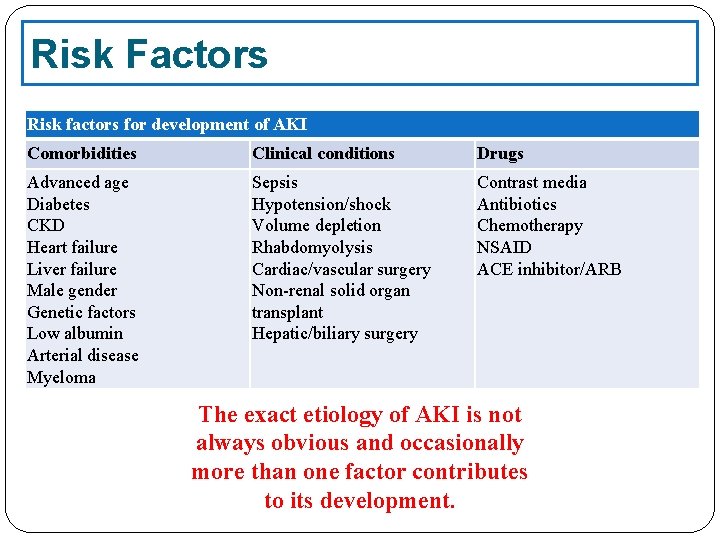
Risk Factors Risk factors for development of AKI Comorbidities Clinical conditions Drugs Advanced age Diabetes CKD Heart failure Liver failure Male gender Genetic factors Low albumin Arterial disease Myeloma Sepsis Hypotension/shock Volume depletion Rhabdomyolysis Cardiac/vascular surgery Non-renal solid organ transplant Hepatic/biliary surgery Contrast media Antibiotics Chemotherapy NSAID ACE inhibitor/ARB The exact etiology of AKI is not always obvious and occasionally more than one factor contributes to its development.

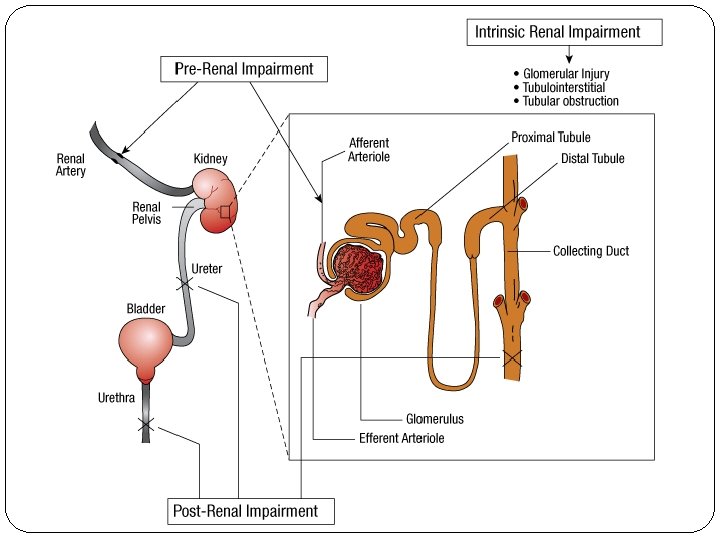
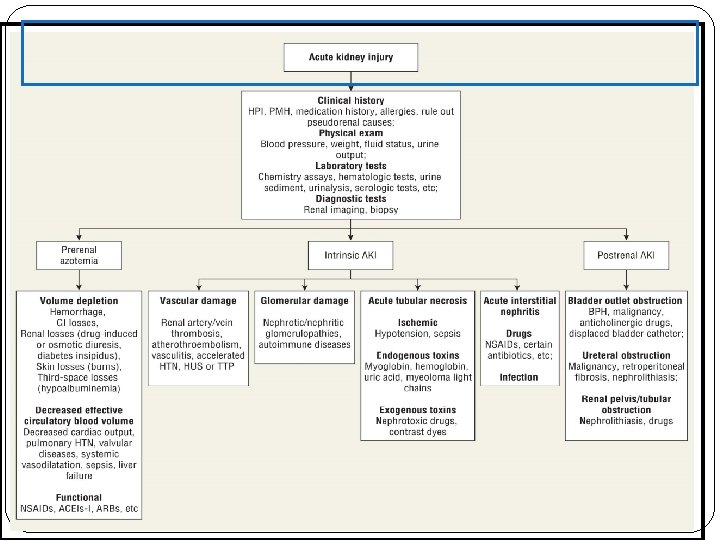
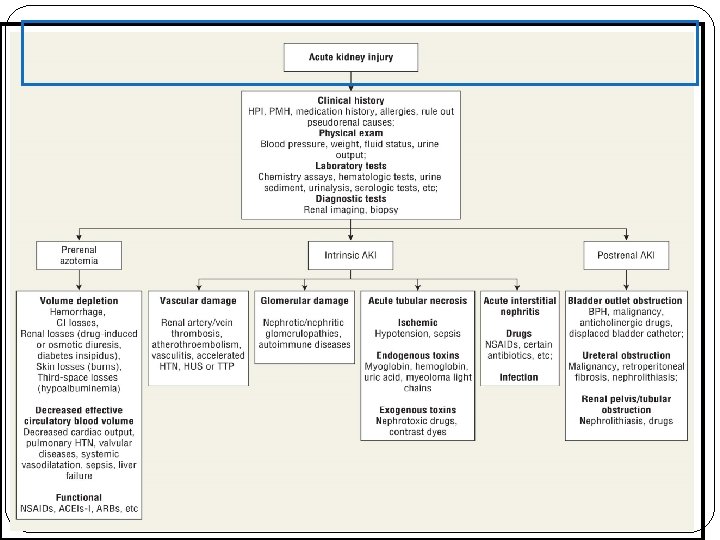
Pathophysiology There are typically three categories of AKI: Prerenal AKI Intrensic AKI Postrenal AKI The pathophysiologic mechanisms differ for each of the categories.



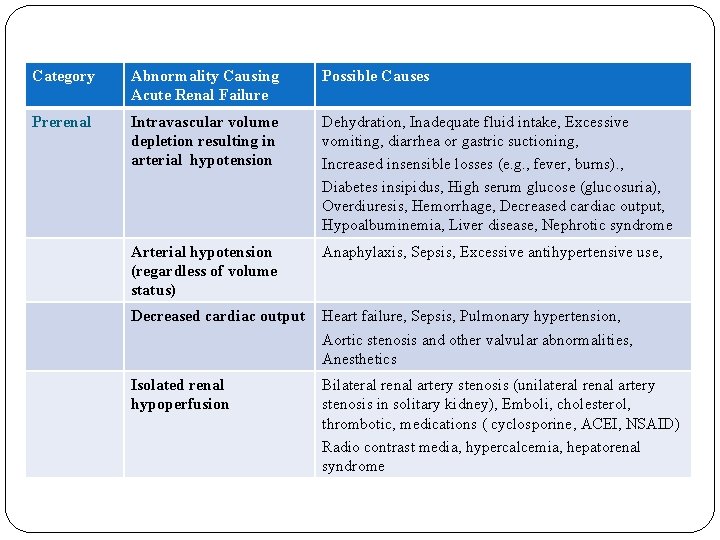
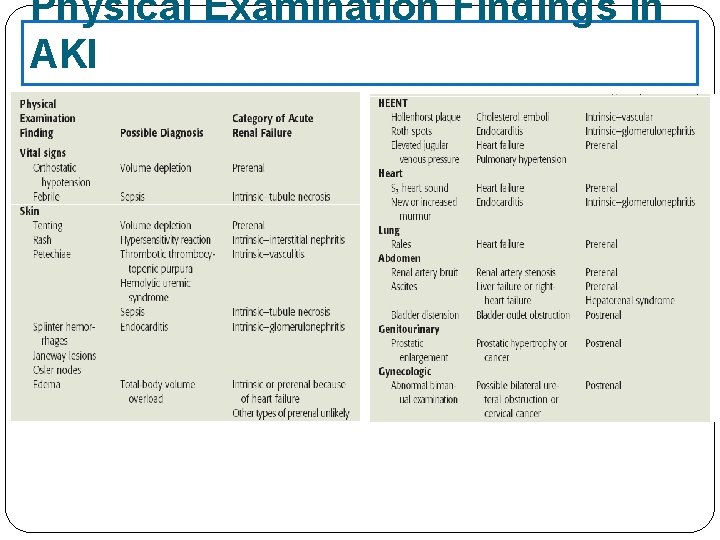
Category Abnormality Causing Acute Renal Failure Possible Causes Prerenal Intravascular volume depletion resulting in arterial hypotension Dehydration, Inadequate fluid intake, Excessive vomiting, diarrhea or gastric suctioning, Increased insensible losses (e. g. , fever, burns). , Diabetes insipidus, High serum glucose (glucosuria), Overdiuresis, Hemorrhage, Decreased cardiac output, Hypoalbuminemia, Liver disease, Nephrotic syndrome Arterial hypotension (regardless of volume status) Anaphylaxis, Sepsis, Excessive antihypertensive use, Decreased cardiac output Heart failure, Sepsis, Pulmonary hypertension, Aortic stenosis and other valvular abnormalities, Anesthetics Isolated renal hypoperfusion Bilateral renal artery stenosis (unilateral renal artery stenosis in solitary kidney), Emboli, cholesterol, thrombotic, medications ( cyclosporine, ACEI, NSAID) Radio contrast media, hypercalcemia, hepatorenal syndrome

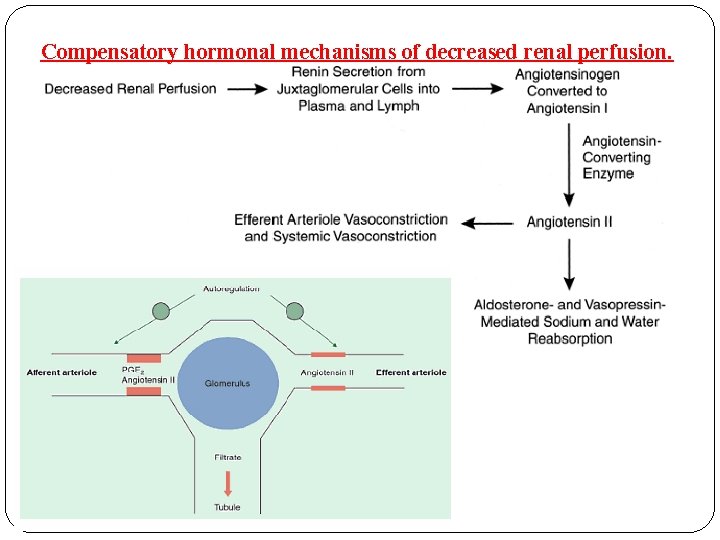
Compensatory hormonal mechanisms of decreased renal perfusion.


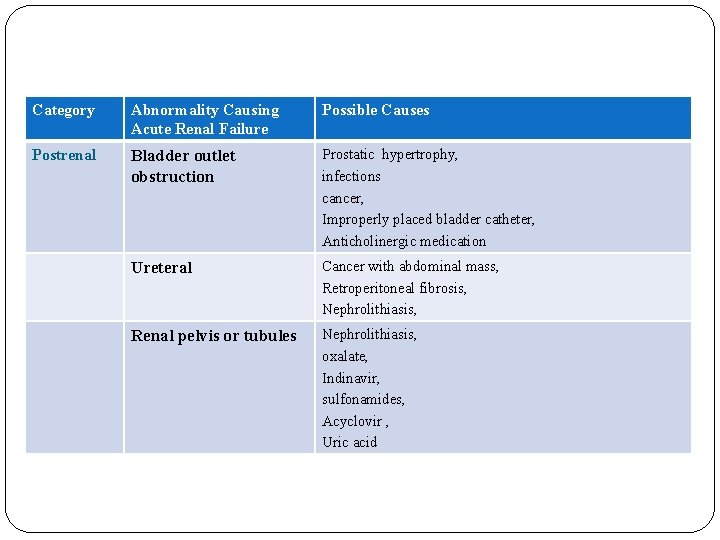
Category Abnormality Causing Acute Renal Failure Possible Causes Postrenal Bladder outlet obstruction Prostatic hypertrophy, infections cancer, Improperly placed bladder catheter, Anticholinergic medication Ureteral Cancer with abdominal mass, Retroperitoneal fibrosis, Nephrolithiasis, Renal pelvis or tubules Nephrolithiasis, oxalate, Indinavir, sulfonamides, Acyclovir , Uric acid


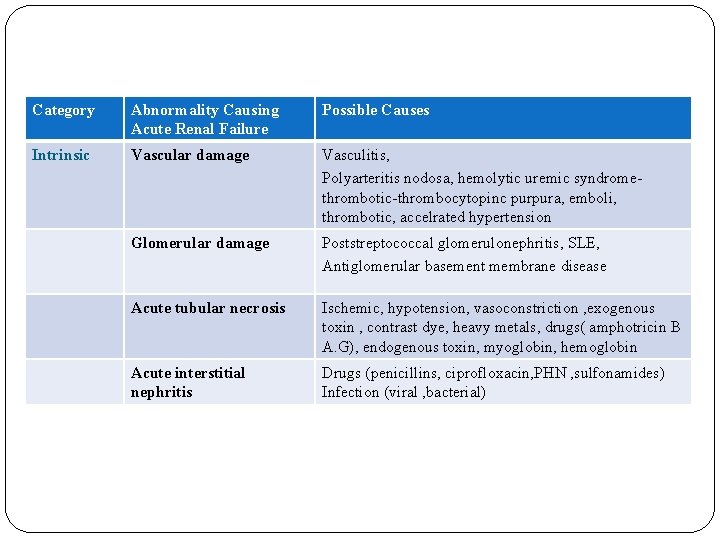
Category Abnormality Causing Acute Renal Failure Possible Causes Intrinsic Vascular damage Vasculitis, Polyarteritis nodosa, hemolytic uremic syndromethrombotic-thrombocytopinc purpura, emboli, thrombotic, accelrated hypertension Glomerular damage Poststreptococcal glomerulonephritis, SLE, Antiglomerular basement membrane disease Acute tubular necrosis Ischemic, hypotension, vasoconstriction , exogenous toxin , contrast dye, heavy metals, drugs( amphotricin B A. G), endogenous toxin, myoglobin, hemoglobin Acute interstitial nephritis Drugs (penicillins, ciprofloxacin, PHN , sulfonamides) Infection (viral , bacterial)

Intrarenal AKI = Intrinsic renal failure Caused by diseases that can affect the integrity of the tubules, glomerulus, interstitium, or blood vessels. Damage is within the kidney; changes in kidney structure can be seen on microscopy. The most common cause of intrinsic renal failure is Acute Tubular Necrosis (ATN) and it accounts for approximately 50% of all cases of AKI. ATN represents a pathophysiologic condition that results from toxic (aminoglycosides, contrast agents, or amphotericin B) or ischemic insult to the kidney. ATN results in necrosis of the proximal tubule epithelium and basement membrane, decreased glomerular capillary permeability, and backleak of glomerular filtrate into the venous circulation. Maintenance of ATN is mediated by intrarenal vasoconstriction. Glomerular, interstitial, and blood vessel diseases may also lead to intrinsic AKI, but occur with a much lower incidence. Examples include glomerulonephritis, systemic lupus erythematosus, interstitial nephritis, and vasculitis. In addition, prerenal AKI can progress to intrinsic AKI if the underlying condition is not promptly corrected.

Drug Induced AKI - Homework Discuss the mechanism of nephrotoxicity of the following drugs: Aminoglycosides Amphotericin B Radiocontrast media Cyclosporine and Tacrolimus Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Non-steroidal Anti-Inflammatory Drugs

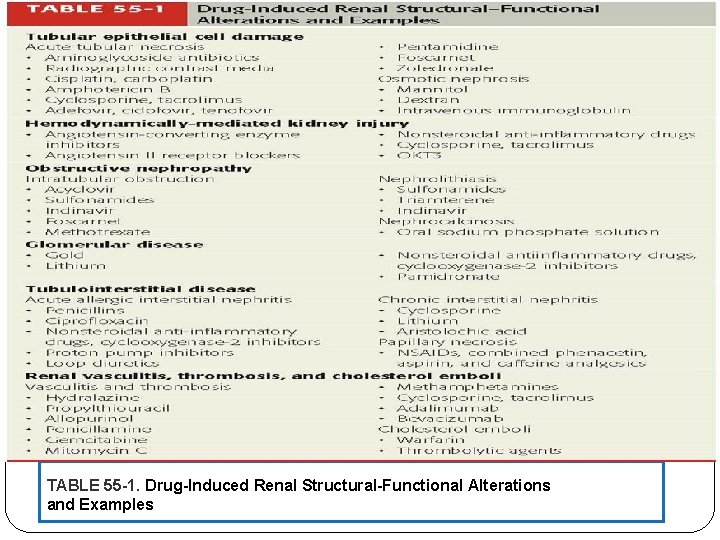
TABLE 55 -1. Drug-Induced Renal Structural-Functional Alterations and Examples

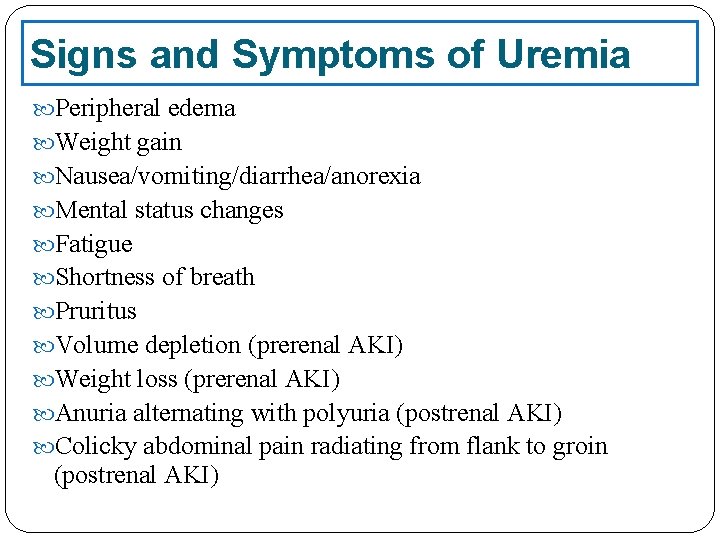
Signs and Symptoms of Uremia Peripheral edema Weight gain Nausea/vomiting/diarrhea/anorexia Mental status changes Fatigue Shortness of breath Pruritus Volume depletion (prerenal AKI) Weight loss (prerenal AKI) Anuria alternating with polyuria (postrenal AKI) Colicky abdominal pain radiating from flank to groin (postrenal AKI)

Physical Examination Findings Hypertension Jugular venous distention Pulmonary edema Rales Asterixis Pericardial or pleural friction rub Hypotension/orthostatic hypotension (prerenal AKI) Rash (acute interstitial nephritis) Bladder distention (postrenal bladder outlet obstruction) Prostatic enlargement (postrenal AKI)

Physical Examination Findings in AKI

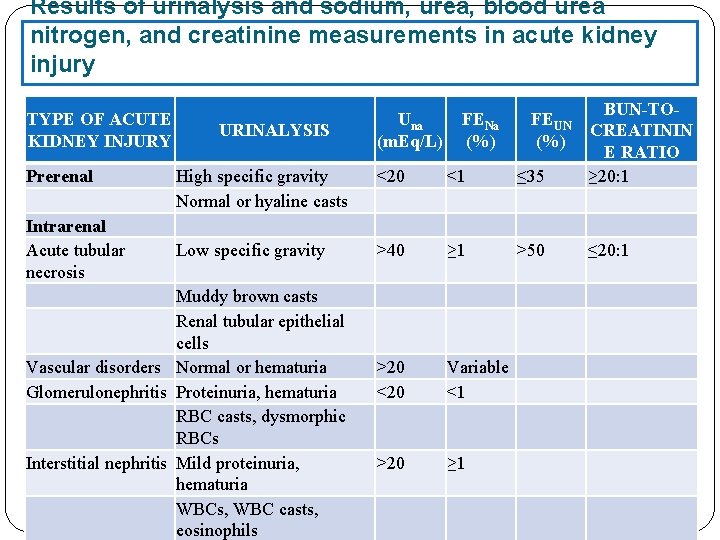
Results of urinalysis and sodium, urea, blood urea nitrogen, and creatinine measurements in acute kidney injury TYPE OF ACUTE KIDNEY INJURY Prerenal Intrarenal Acute tubular necrosis High specific gravity Normal or hyaline casts <20 <1 ≤ 35 BUN-TOCREATININ E RATIO ≥ 20: 1 Low specific gravity >40 ≥ 1 >50 ≤ 20: 1 >20 <20 Variable <1 >20 ≥ 1 URINALYSIS Muddy brown casts Renal tubular epithelial cells Vascular disorders Normal or hematuria Glomerulonephritis Proteinuria, hematuria RBC casts, dysmorphic RBCs Interstitial nephritis Mild proteinuria, hematuria WBCs, WBC casts, eosinophils Una (m. Eq/L) FENa (%) FEUN (%)

Treatment of AKI 77

Desired Outcomes and Goals A primary goal of therapy is ameliorating any identifiable underlying causes of AKI such as hypovolemia, nephrotoxic drug administration, or ureter obstruction. Prerenal and postrenal AKI can be reversed if the underlying problem is promptly identified and corrected, while treatment of intrinsic renal failure is more supportive in nature. There is no evidence that drug therapy hastens patient recovery in AKI, decreases length of hospitalization, or improves survival.

The evaluation and initial management of patients with acute kidney injury (AKI) should include: 1) an assessment of the contributing causes of the kidney injury, 2) an assessment of the clinical course including comorbidities, 3) a careful assessment of volume status, 4) the institution of appropriate therapeutic measures designed to reverse or prevent worsening of functional or structural kidney abnormalities.

Things you will be asked about or will need to watch for in practice: • How can ARF be prevented? • Is the ARF drug-induced? • How should ARF be treated? • How should drugs be dosed in ARF?

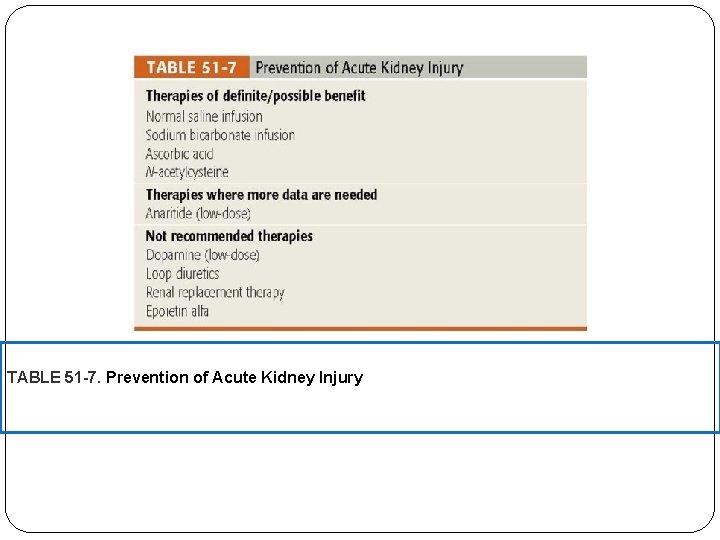
Prevention of AKI The best preventive measure for AKI, especially in individuals at high risk, is to avoid medications that are known to precipitate AKI. Nephrotoxicity is a significant side effect of aminoglycosides, angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists, amphotericin B, nonsteroidal anti-inflammatory drugs, cyclosporine, tacrolimus, and radiographic contrast agents. Unfortunately, an effective, non-nephrotoxic alternative may not always be appropriate for a given patient and the risks and benefits of selecting a drug with nephrotoxic potential must be considered. For example, serious gram-negative infections may require double antibiotic coverage, and based on culture and sensitivity reports, aminoglycoside therapy may be necessary. In cases such as this, other measures to reduce the risk of AKI should be instituted. Thus, identifying patients at high risk for development of AKI and implementing preventive methods to decrease its occurrence or

TABLE 51 -7. Prevention of Acute Kidney Injury

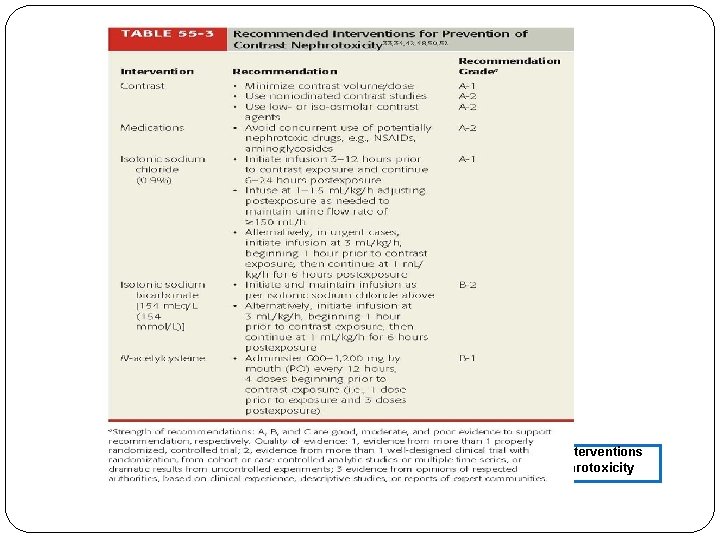
FIGURE 55 -3. Recommended Interventions for Prevention of Contrast Nephrotoxicity

Is the ARF drug-induced? Drug list: if drug-induced, remember to remove offending agent until urine flow is re-established.

How should ARF be treated? options are limited to: 1. prevention of adverse drug reactions by discontinuing nephrotoxic drugs/treat cause 2. adjustment of drug dosages based on the patient’s renal function is desired. 3. supportive therapy, such as fluid, 4. electrolyte, and nutritional support, 5. renal replacement therapy (RRT), 6. treatment of non-renal complications such as sepsis and gastrointestinal bleeding while regeneration of the renal epithelium occurs.

How should ARF be treated? What to check: . Volume status • BP. hydration is key when BP low; avoid diuretics until BP normalized. Symptomatic orthostasis is a systolic drop of 30 or a diastolic drop of 10 and indicates moderate to severe volume depletion in patients not on a rate controller. Patients on a rate controller can be orthostatic with smaller volume loss. • HR: an increase in heart rate with little change in blood pressure can indicate mild dehydration. Symptomatic tachycardia will indicate at least moderate dehydration. HR inc 30 • Weight. In mild volume depletion, 2 -3% body weight lost. In moderate-severe volume loss, ≥ 5 % body weight lost. BUN: Cr ratio. A BUN: serum creatinine ratio of ≥ 20: 1 indicates at least moderate volume depletion

Volume depletion • oral rehydration. Mild volume depletion can usually be treated with oral rehydration: juice or sports drink if only dehydration • intravenous rehydration. Patients with moderate volume loss, can be treated with NS at 80 -150 m. L/hr (usually 1 -2 Liters are enough) until BP, urine flow re-establishes , HR normal, then D 5 1/2 NS at 75 or 80 m. L/hr) until output balances input. For severe volume depletion (e. g. , pt shocky) NS @ 200 ml/hr until urine flow begins to reestablish (target 0. 5 ml/kg/hr), then match input to output. Only switch to D 5 1/2 NS when BP and HR normal. Monitor BP, HR, wt, urine output, edema, chem. 7.

Other Therapies Loop diuretics Dopamine Fenoldopam

Loop diuretics There is significant controversy over the role of loop diuretics in the treatment of AKI. Theoretical benefits in hastening recovery of renal function include: decreased metabolic oxygen requirements of the kidney, increased resistance to ischemia, increased urine flow rates that reduce intraluminal obstruction and filtrate backleak, renal vasodilation. Theoretically, these effects could lead to: increased urine output, decreased need for dialysis, improved renal recovery, increased survival. Most studies demonstrate an improvement in urine output, but with no effect on survival or need for dialysis. There are some reports that loop diuretics may worsen renal function. This may be due in part to excessive preload reduction that results in renal vasoconstriction. Thus, loop diuretics are limited to instances of volume overload and edema and are not intended to hasten renal recovery or improve survival.

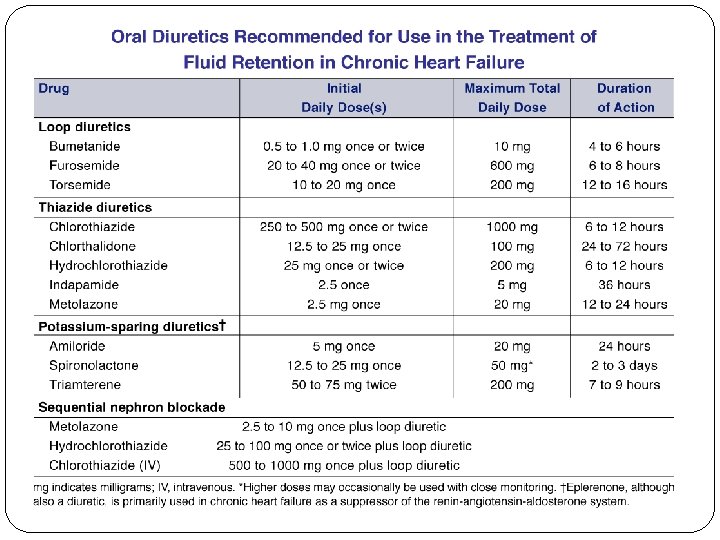
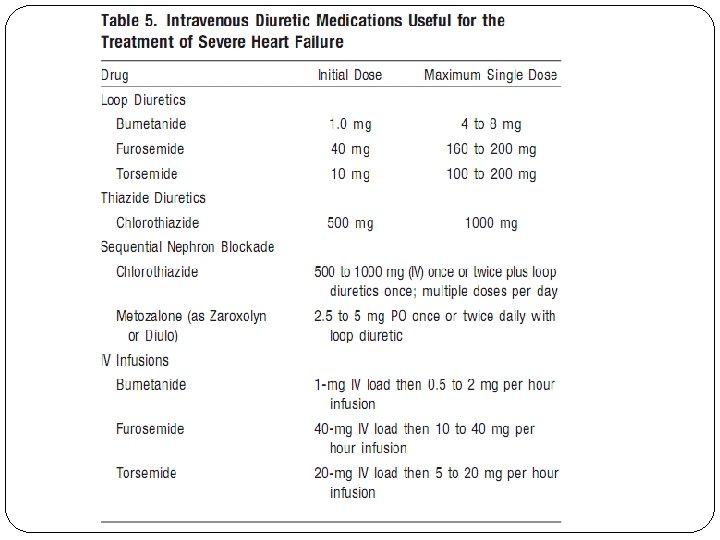
Loop diuretics – the agents furosemide, bumetanide, torsemide, and ethacrynic acid all equally effective when given in equivalent doses Patients will not benefit from switching from one loop diuretic to another (similar MOA) A usual starting dose of IV furosemide for the treatment of AKI is 40 mg. Reasonable starting doses for bumetanide and torsemide are 1 mg and 20 mg, respectively. selection is based on the side-effect profile, cost, and pharmacokinetics of the agents. Cost: Furosemide and bumetanide are both available in generic formulations and are generally less expensive than torsemide. Side effects: The incidence of ototoxicity is significantly higher with ethacrynic acid compared to the other loop diuretics; therefore, its use is limited to patients who are allergic to the sulfa component in the other loop diuretics. While ototoxicity is a well-established side effect of furosemide, its incidence is greater when administered by the intravenous route at a rate exceeding 4 mg per minute. Torsemide has not been reported to cause ototoxicity.

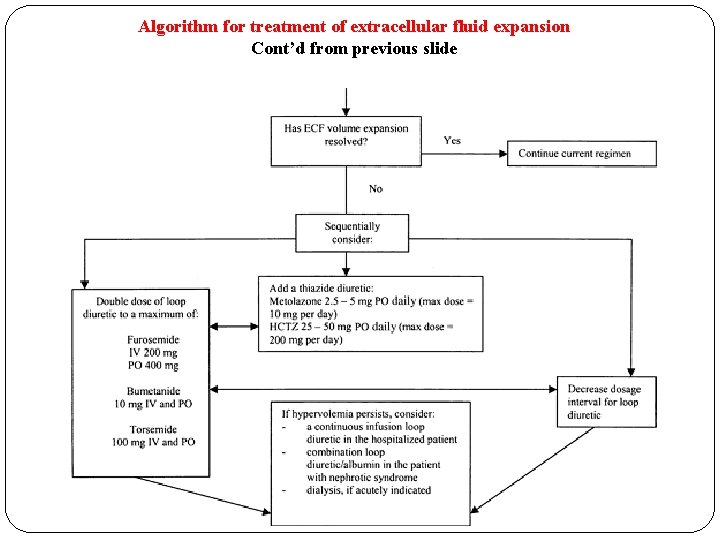
Loop diuretics – Monitoring Efficacy of diuretic administration can be determined by comparison of a patient’s hourly fluid balance. Other methods to minimize volume overload, such as fluid restriction and concentration of IV medications, should be initiated as needed. If urine output does not increase to about 1 m. L/kg per hour, the dosage can be increased to a maximum of 160 to 200 mg of furosemide or its equivalent. Other methods to improve diuresis can be initiated sequentially, such as: (1) shortening the dosage interval; (2) adding hydrochlorothiazide or metolazone; (3) switching to a continuous infusion loop diuretic. A loading dose should be administered prior to both initiating a continuous infusion and increasing the infusion rate. When high doses of loop diuretics are administered, especially in combination with distal convoluted tubule diuretics, the hemodynamic and fluid status of the patient should be monitored every shift, and the electrolyte status of the patient should be monitored at least daily to prevent profound diuresis and electrolyte abnormalities, such as

Algorithm for treatment of extracellular fluid expansion Cont’d from previous slide



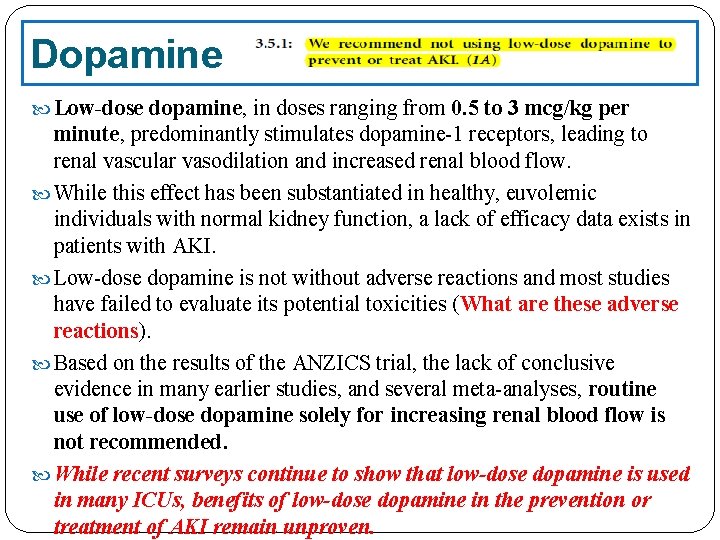
Dopamine Low-dose dopamine, in doses ranging from 0. 5 to 3 mcg/kg per minute, predominantly stimulates dopamine-1 receptors, leading to renal vascular vasodilation and increased renal blood flow. While this effect has been substantiated in healthy, euvolemic individuals with normal kidney function, a lack of efficacy data exists in patients with AKI. Low-dose dopamine is not without adverse reactions and most studies have failed to evaluate its potential toxicities (What are these adverse reactions). Based on the results of the ANZICS trial, the lack of conclusive evidence in many earlier studies, and several meta-analyses, routine use of low-dose dopamine solely for increasing renal blood flow is not recommended. While recent surveys continue to show that low-dose dopamine is used in many ICUs, benefits of low-dose dopamine in the prevention or treatment of AKI remain unproven.

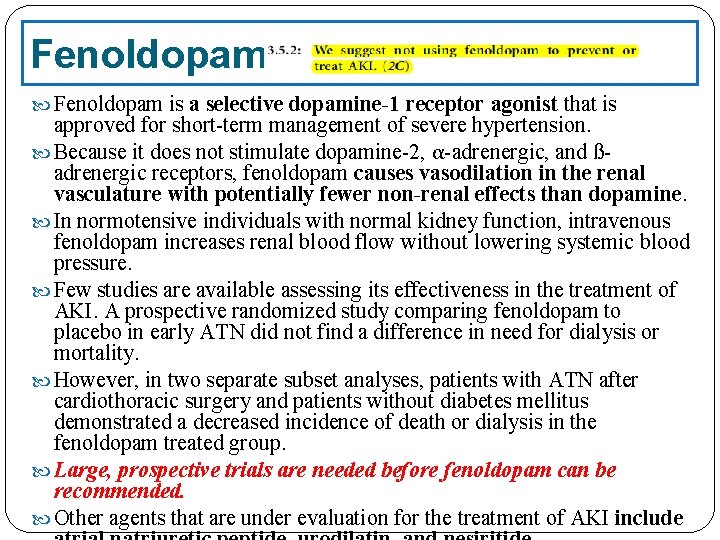
Fenoldopam is a selective dopamine-1 receptor agonist that is approved for short-term management of severe hypertension. Because it does not stimulate dopamine-2, α-adrenergic, and ßadrenergic receptors, fenoldopam causes vasodilation in the renal vasculature with potentially fewer non-renal effects than dopamine. In normotensive individuals with normal kidney function, intravenous fenoldopam increases renal blood flow without lowering systemic blood pressure. Few studies are available assessing its effectiveness in the treatment of AKI. A prospective randomized study comparing fenoldopam to placebo in early ATN did not find a difference in need for dialysis or mortality. However, in two separate subset analyses, patients with ATN after cardiothoracic surgery and patients without diabetes mellitus demonstrated a decreased incidence of death or dialysis in the fenoldopam treated group. Large, prospective trials are needed before fenoldopam can be recommended. Other agents that are under evaluation for the treatment of AKI include

Supportive therapy – includes: adequate nutrition, correction of electrolyte and acid-base abnormalities (particularly hyperkalemia and metabolic acidosis), fluid management, correction of any hematologic abnormalities. because AKI is often associated with multiorgan failure, treatment includes the medical management of infections, cardiovascular and gastrointestinal conditions, and respiratory failure. all drugs should be reviewed, and dosage adjustments made based on an estimate of the patient’s glomerular filtration rate.

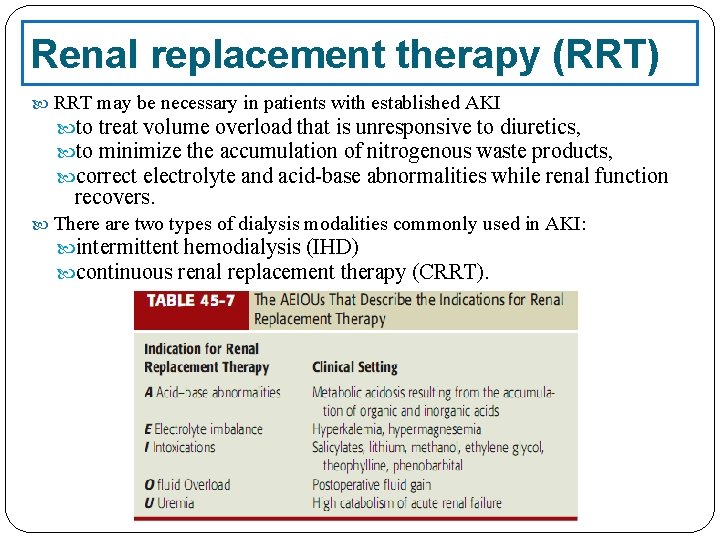
Renal replacement therapy (RRT) RRT may be necessary in patients with established AKI to treat volume overload that is unresponsive to diuretics, to minimize the accumulation of nitrogenous waste products, correct electrolyte and acid-base abnormalities while renal function recovers. There are two types of dialysis modalities commonly used in AKI: intermittent hemodialysis (IHD) continuous renal replacement therapy (CRRT).

Dosing Drugs in Acute Renal Disease • there are no published guidelines on what to do • most important: fix reason for renal function change • can hold every other dose of some drugs if renal function rapidly deteriorating • for vital drugs, you may need to adjust dose based on drug serum concentrations— • Can use Bennett’s tables and pharmacokinetic calculations for narrow therapeutic range drugs, but be aware that renal function will be changing rapidly, so q. Od or even qd monitoring of SCr, UO, and weight will be necessary. Avoid the most potent nephrotoxins if possible. Consider metabolicallycleared drugs with inactive metabolites at these times.


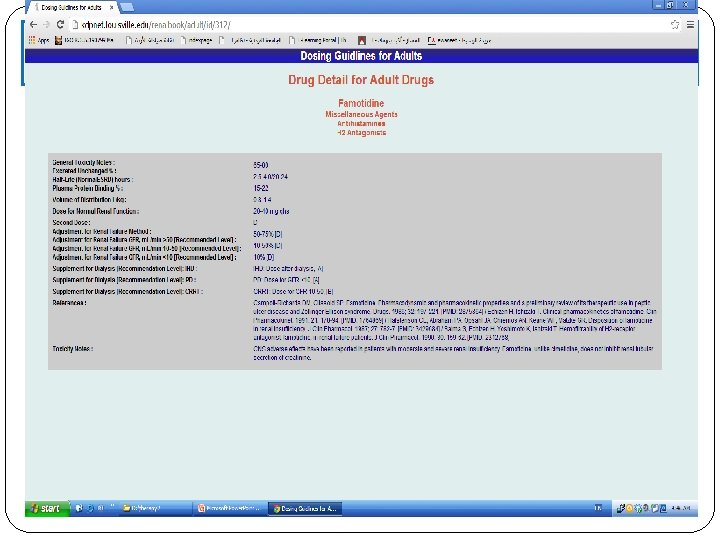
Bennett’s tables http: //www. kdp-baptist. louisville. edu/renalbook/

Outcome Evaluation Goals of therapy are: to maintain a state of euvolemia with good urine output (at least 1 ml/kg per hour), to return serum creatinine and BUN to baseline, to correct electrolyte and acid-base abnormalities. Vital signs, weight, fluid intake, urine output, BUN, creatinine, and electrolytes should be assessed daily in the unstable patient.

Patient Care and Monitoring Assess kidney function by evaluating a patient’s signs and symptoms, laboratory test results, and urinary indices. Calculate a patient’s creatinine clearance to evaluate the severity of kidney disease. Obtain a thorough and accurate drug history, including the use of non-prescription drugs such as NSAIDs. Evaluate a patient’s current drug regimen to: 1. 2. 3. 4. Determine if drug therapy may be contributing to AKI. Consider not only drugs that can directly cause AKI (e. g. , aminoglycosides, amphotericin B, NSAIDs, cyclosporine, tacrolimus, ACE inhibitors, and ARBs), but also drugs that can predispose a patient to nephrotoxicity or prerenal AKI (i. e. , diuretics and antihypertensive agents). Determine if any drugs need to be discontinued, or alternate drugs selected, to prevent worsening of renal function. Adjust drug dosages based on the patient’s creatinine clearance or evidence of adverse drug reactions or interactions. Develop a plan to provide symptomatic care of complications associated with AKI, such as diuretic therapy to treat volume overload. Monitor the patient’s weight, urine output, electrolytes (such as potassium), and blood pressure to assess efficacy of the

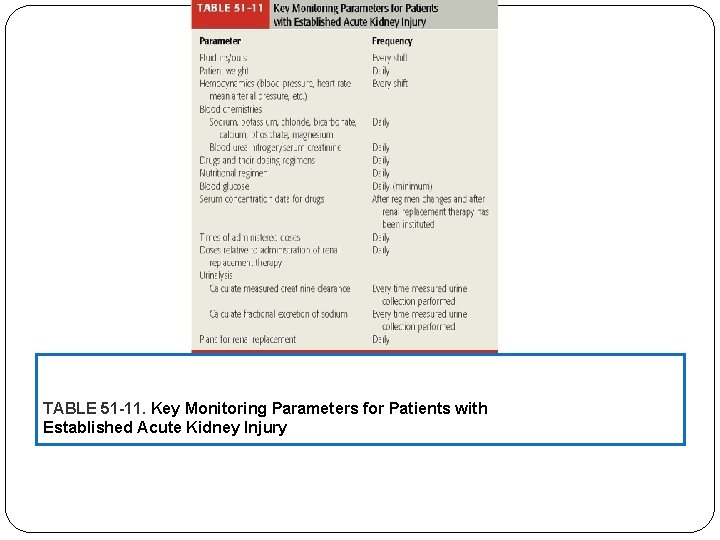
TABLE 51 -11. Key Monitoring Parameters for Patients with Established Acute Kidney Injury

Case
