Activity and Activity Coefficient In a solution diferent




- Slides: 7

Activity and Activity Coefficient • In a solution, diferent charged ions (cations and anions) have electrostatic interaction. This interaction in the neutral solutions do not have between inons. • When ions in a solution have high concentrations, cations tend to be surrounded by nearby anions and anions tend to be surrounded by nearby cations. This effect is significant at ion concentrations of 0. 01 M and greater.

Activity and Activity Coefficient • When ions in a solution have high concentrations, cations tend to be surrounded by nearby anions and anions tend to be surrounded by nearby cations. This effect is significant at ion concentrations of 0. 01 M and greater.

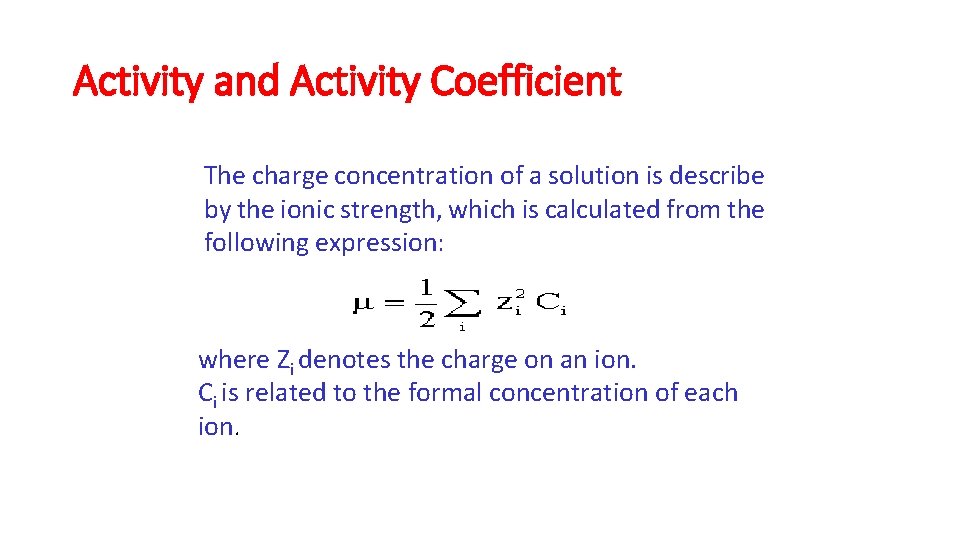
Activity and Activity Coefficient The charge concentration of a solution is describe by the ionic strength, which is calculated from the following expression: where Zi denotes the charge on an ion. Ci is related to the formal concentration of each ion.

Activity and Activity Coefficient

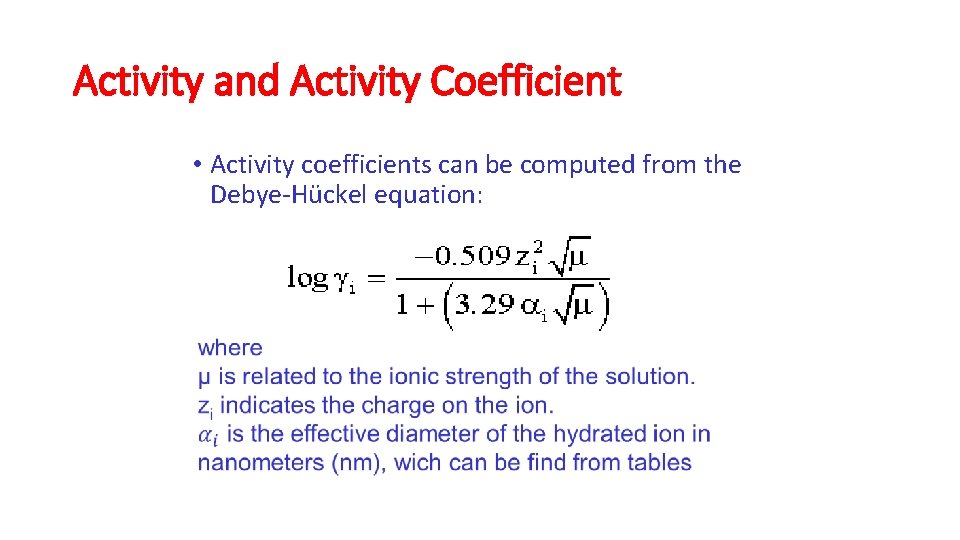
Activity and Activity Coefficient Based on these activity coefficients, the equilibrium constants of ideal solutions can be used to determine equilibria in non-ideal solutions. Activity coefficients are unitless numbers that are computed from the Debye. Hückel equation:

Activity and Activity Coefficient • Activity coefficients can be computed from the Debye-Hückel equation:

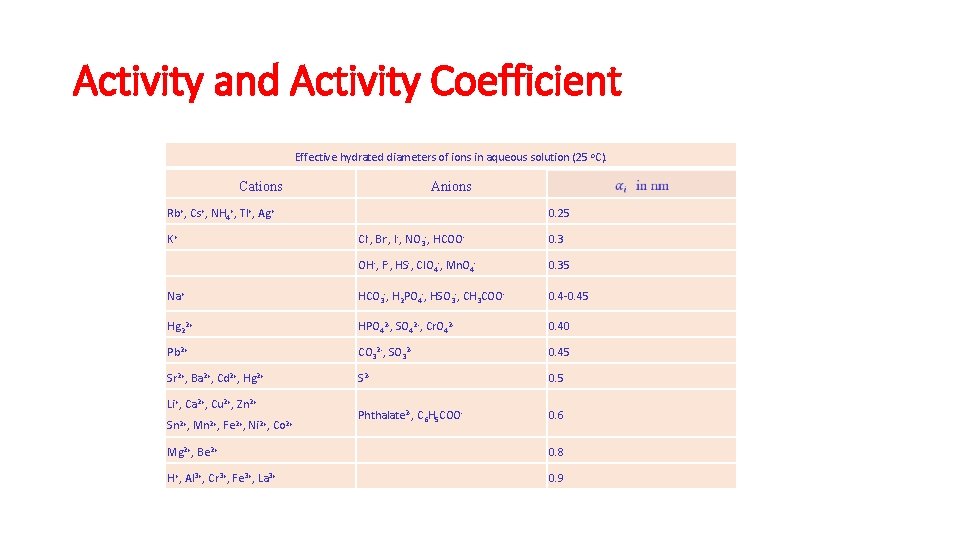
Activity and Activity Coefficient Effective hydrated diameters of ions in aqueous solution (25 o. C). Cations Anions Rb+, Cs+, NH 4+, Tl+, Ag+ K+ 0. 25 Cl-, Br-, I-, NO 3 -, HCOO- 0. 3 OH-, F-, HS-, Cl. O 4 -, Mn. O 4 - 0. 35 Na+ HCO 3 -, H 2 PO 4 -, HSO 3 -, CH 3 COO- 0. 4 -0. 45 Hg 22+ HPO 42 -, SO 42 -, Cr. O 42 - 0. 40 Pb 2+ CO 32 -, SO 32 - 0. 45 Sr 2+, Ba 2+, Cd 2+, Hg 2+ S 2 - 0. 5 Phthalate 2 -, C 6 H 5 COO- 0. 6 Li+, Ca 2+, Cu 2+, Zn 2+ Sn 2+, Mn 2+, Fe 2+, Ni 2+, Co 2+ Mg 2+, Be 2+ 0. 8 H+, Al 3+, Cr 3+, Fe 3+, La 3+ 0. 9