Activity 25 Solubility With your table read and

Activity #25 - Solubility With your table, read and take Cornell Notes on the following slides. Make sure you include information about the following vocabulary words: Solubility Solutions Solute Solvent Examples of Solutions Concentrated Solution Dilute Solution Insoluble Mixture Pay attention to what is in YELLOW!

Solubility describes the ability of one substance to dissolve in another.
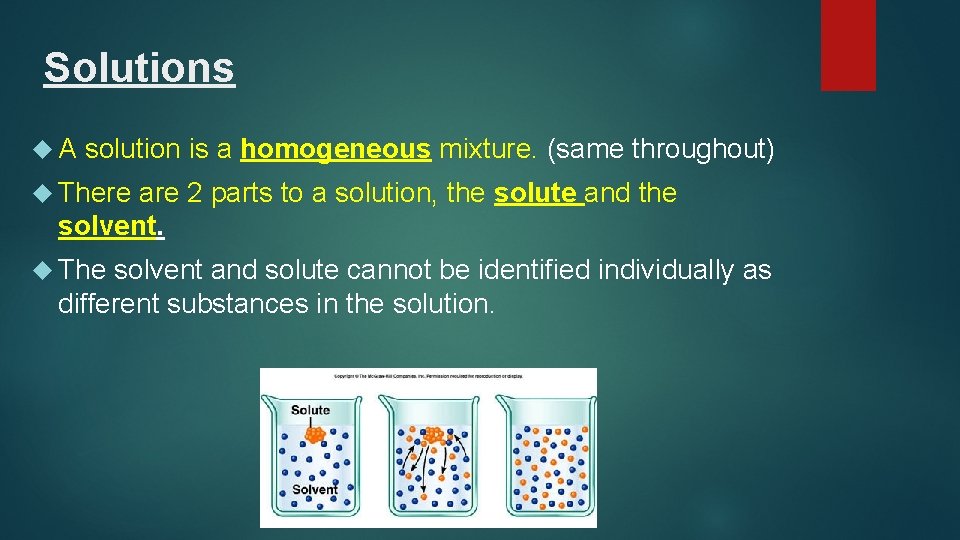
Solutions A solution is a homogeneous mixture. (same throughout) There are 2 parts to a solution, the solute and the solvent. The solvent and solute cannot be identified individually as different substances in the solution.

Solute Solvent The solute is the substance that is dissolved. The solvent dissolves the solute. Water is the most common and important solvent. Also known as the “UNIVERSAL SOLVENT”.

Examples of Solutions

Solutions can be concentrated or dilute. Concentrated solution- contains a large amount of solute Dilute Adding water to concentrated OJ helps to dilute it!! solutioncontains a small amount of solute

Insoluble Mixtures When a solute does not dissolve in the solvent the mixture is insoluble. Example - oil and water do not mix.

Chromatography Experiment Chromatography is the Solvent = process of using a Water solvent to dissolve and separate the dyes Solute = Ink in inks. We will use a coffee filter, water, and washable markers.
- Slides: 8