Activation Energy Lesson Outline Reaction Rate 5 Factors






- Slides: 12

Activation Energy

Lesson Outline

Reaction Rate 5 Factors that Affect Reaction Rate 1. 2. 3. 4. 5. Surface Area Temperature Concentration Aggregation Addition of a catalyst

Collison Reaction Theory Describes, explains and predicts the characteristics of a chemical reaction. Entities are in constant motion at various speeds and rebound elastically from collisions w each other. (Kinetic energy is conserved) Chemical reactions involve collisions of reactants Most Collisions do not result in reactions

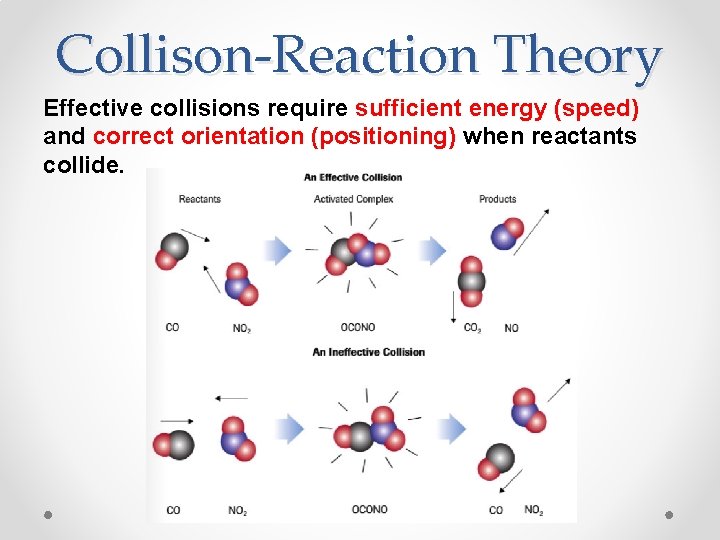
Collison-Reaction Theory Effective collisions require sufficient energy (speed) and correct orientation (positioning) when reactants collide.

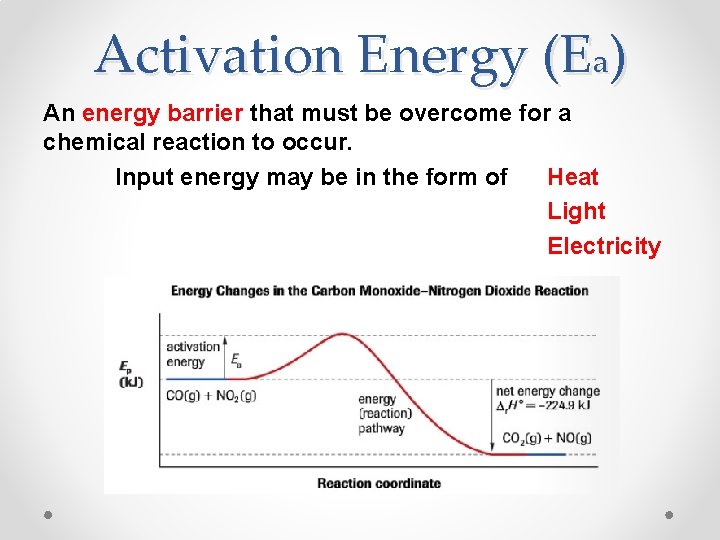
Activation Energy (Ea) An energy barrier that must be overcome for a chemical reaction to occur. Input energy may be in the form of Heat Light Electricity

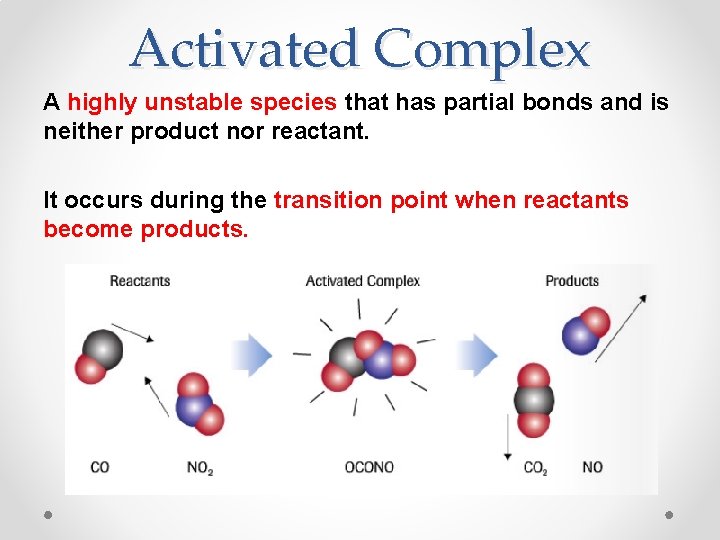
Activated Complex A highly unstable species that has partial bonds and is neither product nor reactant. It occurs during the transition point when reactants become products.

Potential Energy Diagrams (w Activation energy) Draw energy pathway diagrams for a general endothermic and exothermic reaction. Label the reactants, products, enthalpy change, activation energy and activation complex

Bond Energy Is the energy required to break a chemical bond. It is also the energy released when bonds are formed. ΔH= energy released – energy required Bond Making Bond Breaking Exothermic: Making > BreakingΔH= Negative Endothermic: Making < Breaking ΔH= Positive

Catalyst and Reaction Rates A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Examples: Ions - Mn, Cu, Fe, Cl Solids- Pt, Cu, Fe, Ni H ions from acids Enzymes- Biological catalysts (amylase)

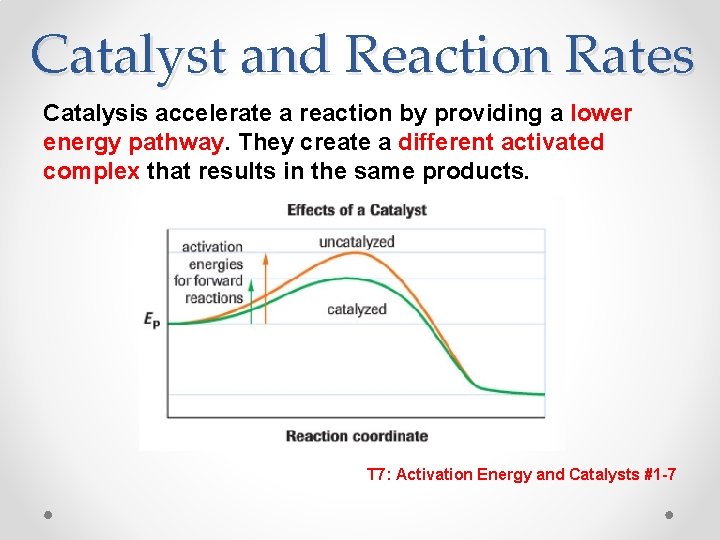
Catalyst and Reaction Rates Catalysis accelerate a reaction by providing a lower energy pathway. They create a different activated complex that results in the same products. T 7: Activation Energy and Catalysts #1 -7

Looking Forward Tuesday: Catalyst Laboratory Wednesday: Thermochemistry Review (T 8) Thursday: Thermochemistry Unit Test