ACTIONS OF LTHYROXINE T 4 AND NANODIAMINOTETRAC NDAT








- Slides: 17

ACTIONS OF L-THYROXINE (T 4) AND NANO-DIAMINO-TETRAC (NDAT, NANOTETRAC) ON PD-L 1 IN CANCER CELLS Paul J. Davis, Hung-Yun Lin, Shaker A. Mousa Albany Medical College, Albany, NY, USA; Pharmaceutical Research Institute, Albany College of Pharmacy and Health Sciences; Taipei Medical University, Taipei, Taiwan

The PD-1 (programmed death 1)/PD-L 1 (PD-ligand 1) checkpoint is a critical regulator of activated T cell-cancer cell interactions, serving to defend tumor cells against (T cell -mediated) immune destruction. Pharmaceutical interest is high in PD-L 1 antibody use in solid tumor chemo-therapy to render cancer cells susceptible to host killer T cell action. We have developed a nonimmunological strategy for downregulation of PD-L 1 gene expression and PD-L 1 protein content in tumor cells.

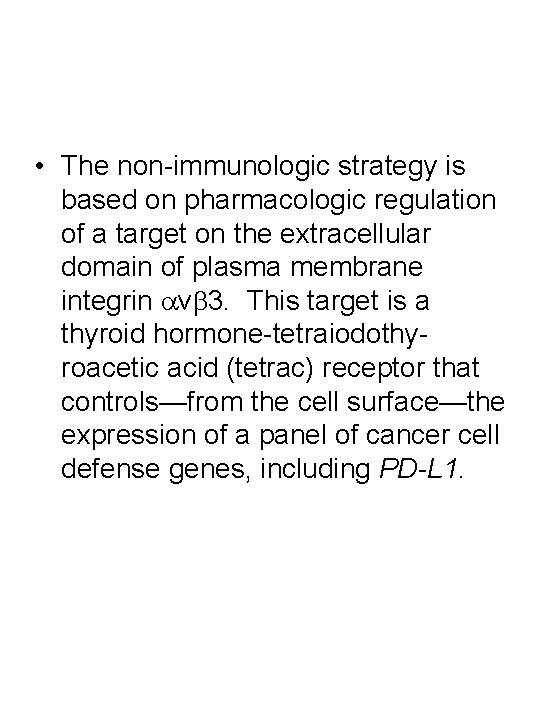
• The non-immunologic strategy is based on pharmacologic regulation of a target on the extracellular domain of plasma membrane integrin avb 3. This target is a thyroid hormone-tetraiodothyroacetic acid (tetrac) receptor that controls—from the cell surface—the expression of a panel of cancer cell defense genes, including PD-L 1.

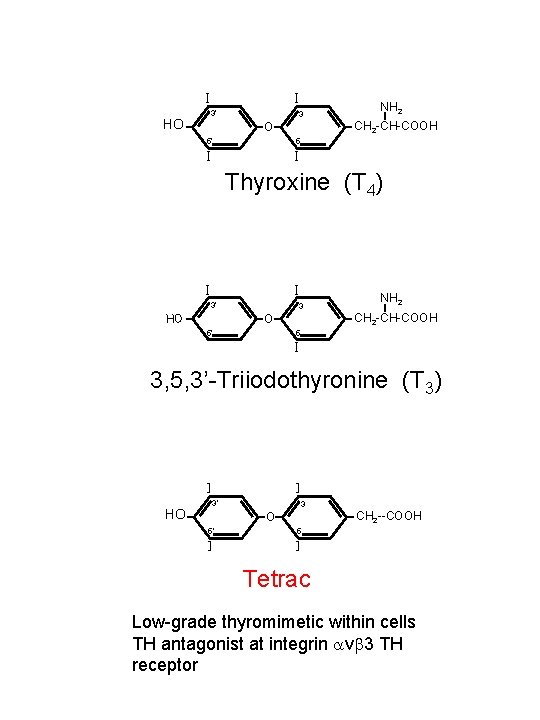
-I - -I 3’ HO 3 CH 2 -CH-COOH O 5’ NH 2 5 -I - Thyroxine (T 4) -I -I 3’ 3 CH 2 -CH-COOH O HO 5’ NH 2 5 -I 3, 5, 3’-Triiodothyronine (T 3) -I - HO -I 3’ 3 CH 2 --COOH O 5’ -I - 5 -I - Tetrac Low-grade thyromimetic within cells TH antagonist at integrin avb 3 TH receptor

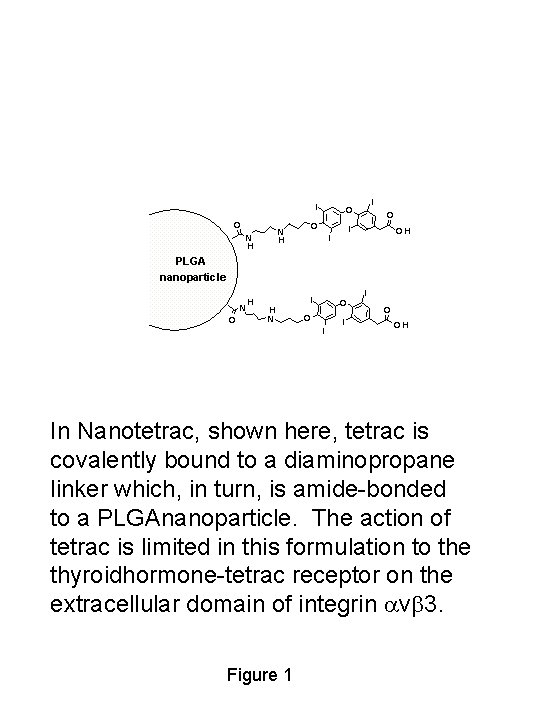
I O O N H I O O I I OH PLGA nanoparticle O N H H N I O O I I I O OH In Nanotetrac, shown here, tetrac is covalently bound to a diaminopropane linker which, in turn, is amide-bonded to a PLGAnanoparticle. The action of tetrac is limited in this formulation to the thyroidhormone-tetrac receptor on the extracellular domain of integrin avb 3. Figure 1

• Human triple-negative breast cancer (MDA-MB-231) cells and human colon cancer (HCT 116. HT 29) cells were cultured in DMEM (breast) or RPMI-1640 (colon), each with 10% FBS. Two days prior to study of cells, 0. 25% charcoal-stripped serum replaced 10% FBS. • Cells were treated with L-thyroxine (T 4, 10 -7 M total hormone, 10 -10 M free), NDAT (10 -7 M tetrac equivalent) or both for 24 h.

• Tumor cell RNA was harvested and PD-L 1 m. RNA quantitated by q. PCR. • PD-L 1 protein was measured by western blotting.

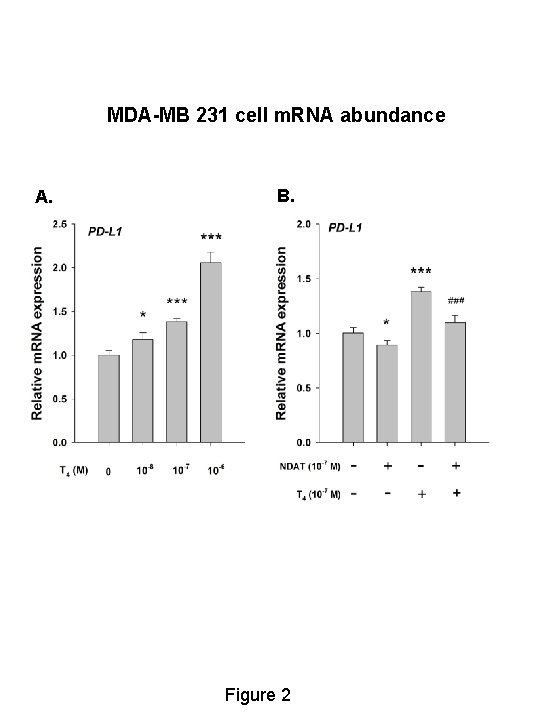
MDA-MB 231 cell m. RNA abundance A. B. Figure 2

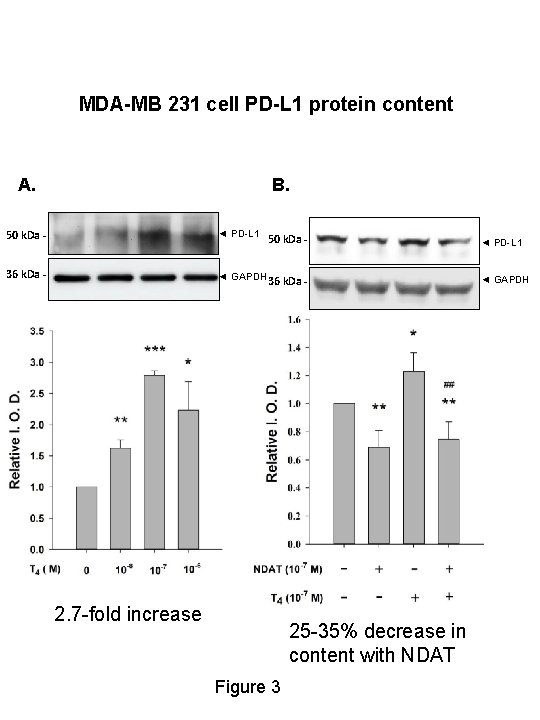
MDA-MB 231 cell PD-L 1 protein content A. B. 50 k. Da - ◄ PD-L 1 36 k. Da - ◄ GAPDH 36 k. Da - 50 k. Da - 2. 7 -fold increase 25 -35% decrease in content with NDAT Figure 3 ◄ PD-L 1 ◄ GAPDH

HCT 116 cell m. RNA A. B. Figure 4

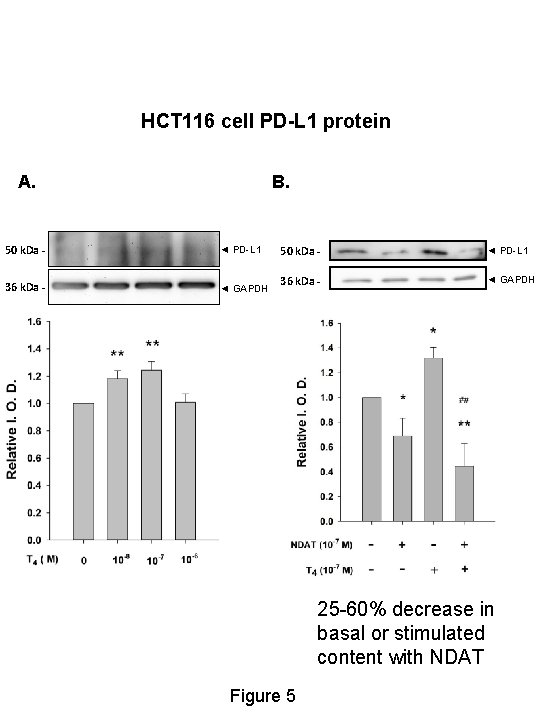
HCT 116 cell PD-L 1 protein A. B. 50 k. Da - ◄ PD-L 1 36 k. Da - ◄ GAPDH 25 -60% decrease in basal or stimulated content with NDAT Figure 5

HT-29 cell m. RNA A. B. Figure 6

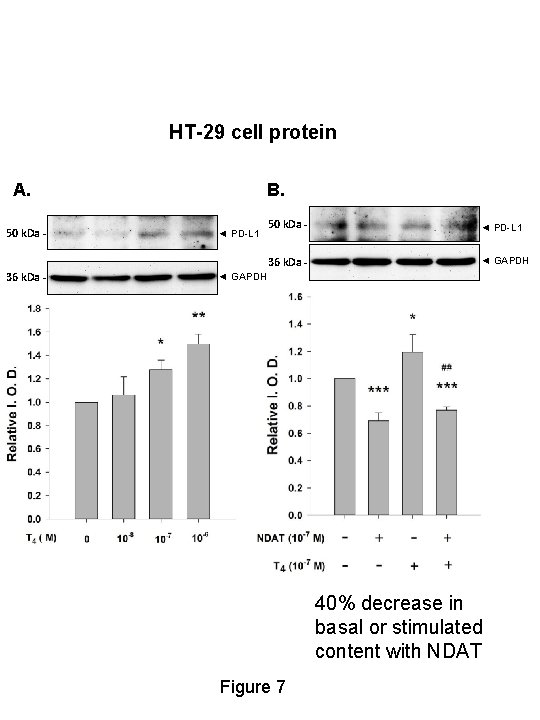
HT-29 cell protein A. 50 k. Da - 36 k. Da - B. ◄ PD-L 1 50 k. Da - ◄ PD-L 1 36 k. Da - ◄ GAPDH 40% decrease in basal or stimulated content with NDAT Figure 7

Dependence on MAPK of induction by T 4 of PD-L 1 in cultured HCT 116 cells NDAT (10 -7 M) T 4 (10 -7 M) - - PD 98059 + - - + + + - + PD 98059 + - - + + + 50 k. Da - ◄ PD-L 1 36 k. Da - ◄ GAPDH Figure 8

SUMMARY • In MDA-MB-231 breast cancer cells, T 4 significantly stimulated PDL 1 gene expression by 40% and increased PD-L 1 protein 2. 7 -fold; these effects were blocked by NDAT. • In HCT 116 and HT-29 colon carcinoma cells, T 4 significantly increased PD-L 1 gene expression by 20 -60% and protein abundance by 25 -65%; these effects were blocked by NDAT. • Basal levels of m. RNA and protein were also reduced by NDAT. • MAPK mediates the T 4 effects.

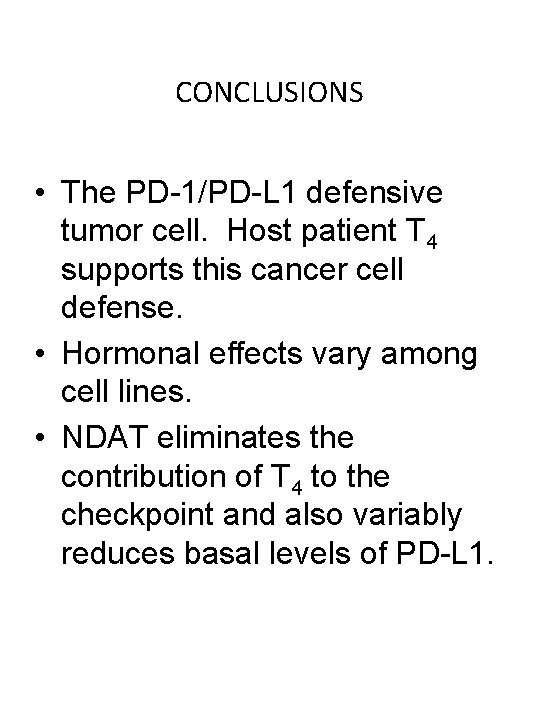
CONCLUSIONS • The PD-1/PD-L 1 defensive tumor cell. Host patient T 4 supports this cancer cell defense. • Hormonal effects vary among cell lines. • NDAT eliminates the contribution of T 4 to the checkpoint and also variably reduces basal levels of PD-L 1.

CONCLUSIONS 2 • Immunologic reduction in tumor cell production of PD-L 1 and attendant side effects can be obviated by hormonal/chemical strategies.
 Group polarization vs groupthink
Group polarization vs groupthink Claim value
Claim value Actions to address risks and opportunities
Actions to address risks and opportunities Japanese militarists aggressive actions
Japanese militarists aggressive actions Pps emergency signals and actions
Pps emergency signals and actions The methods and actions taken to accomplish strategies
The methods and actions taken to accomplish strategies Mastering self management
Mastering self management Motivated blindness
Motivated blindness Future plans and finished future actions
Future plans and finished future actions Cause and effect examples
Cause and effect examples Wisdom will be proved right by her actions
Wisdom will be proved right by her actions Infraspinatus actions
Infraspinatus actions Sacramentals examples
Sacramentals examples The form of past continuous
The form of past continuous Proximate norm meaning
Proximate norm meaning Ankle flexion muscles
Ankle flexion muscles Andy sometimes read comics
Andy sometimes read comics Principles to actions
Principles to actions