ACIP HPV Vaccine Recommendations for Males Eileen F






- Slides: 26

ACIP HPV Vaccine Recommendations for Males Eileen F. Dunne MD, MPH CDC/NCHHSTP National Immunization Conference March 26, 2012 National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of STD Prevention

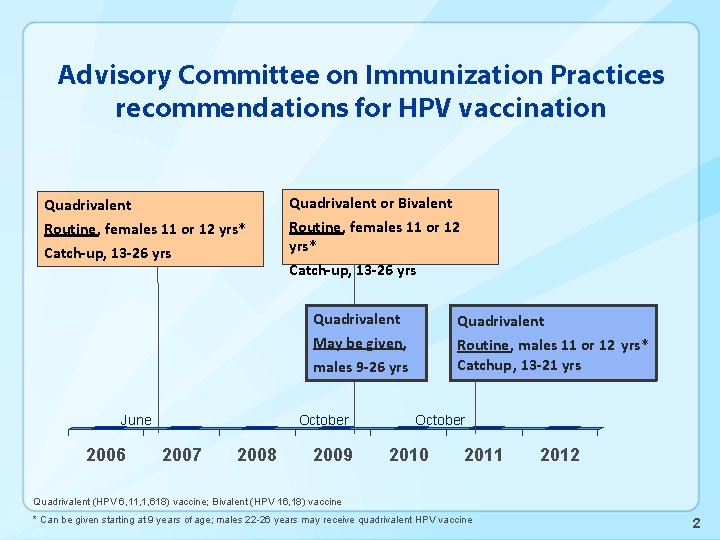
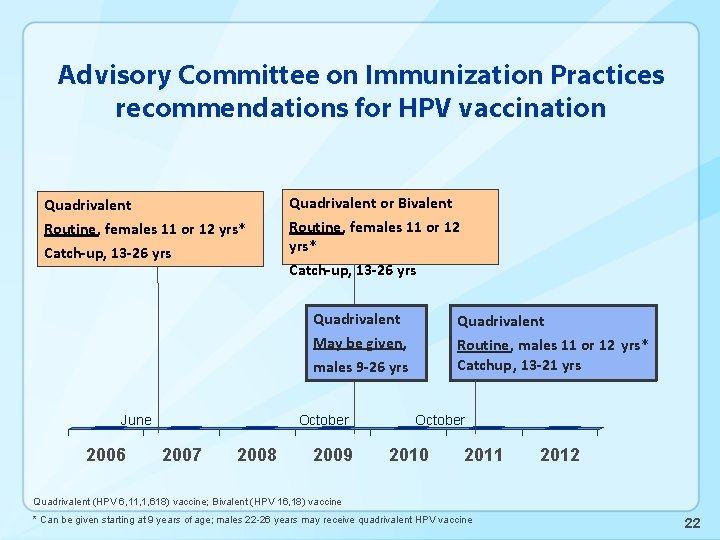
Advisory Committee on Immunization Practices recommendations for HPV vaccination Quadrivalent or Bivalent Routine, females 11 or 12 yrs* Catch-up, 13 -26 yrs Quadrivalent Routine, males 11 or 12 yrs* Catchup, 13 -21 yrs May be given, males 9 -26 yrs June 2006 October 2007 2008 2009 October 2010 2011 2012 Quadrivalent (HPV 6, 11, 1, 618) vaccine; Bivalent (HPV 16, 18) vaccine * Can be given starting at 9 years of age; males 22 -26 years may receive quadrivalent HPV vaccine 2

HPV infection in males • Common sexually acquired infection in both females and males • As in females, first infection occurs soon after sexual debut • Most infections clear; persistent infection most important risk factor for cancer outcomes • Some unknowns about natural history • Persistence and clearance may differ in males and females 3

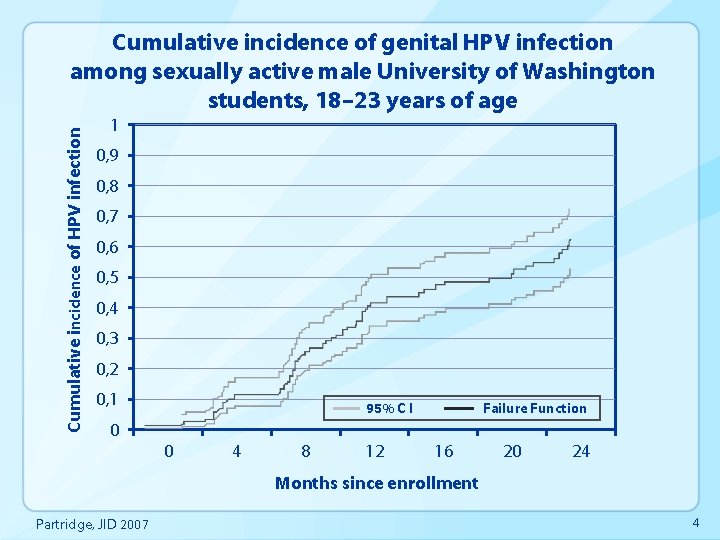
Cumulative incidence of HPV infection Cumulative incidence of genital HPV infection among sexually active male University of Washington students, 18– 23 years of age 1 0, 9 0, 8 0, 7 0, 6 0, 5 0, 4 0, 3 0, 2 0, 1 95% C I Failure Function 0 0 4 8 12 16 20 24 Months since enrollment Partridge, JID 2007 4

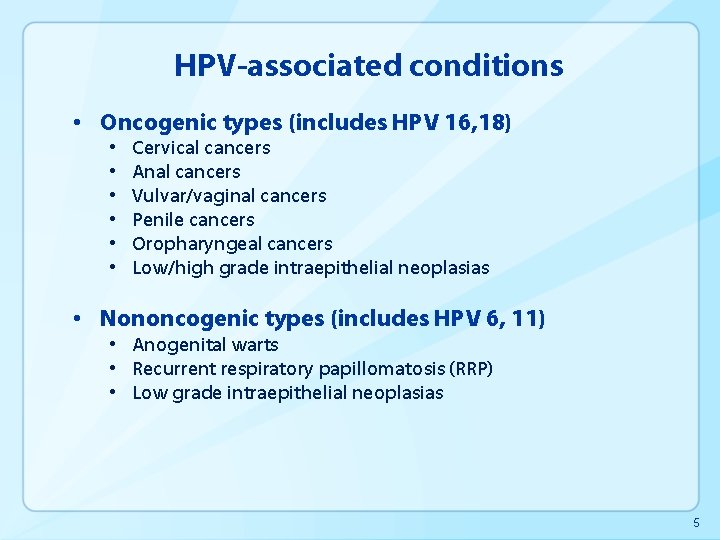
HPV-associated conditions • Oncogenic types (includes HPV 16, 18) • • • Cervical cancers Anal cancers Vulvar/vaginal cancers Penile cancers Oropharyngeal cancers Low/high grade intraepithelial neoplasias • Nononcogenic types (includes HPV 6, 11) • Anogenital warts • Recurrent respiratory papillomatosis (RRP) • Low grade intraepithelial neoplasias 5

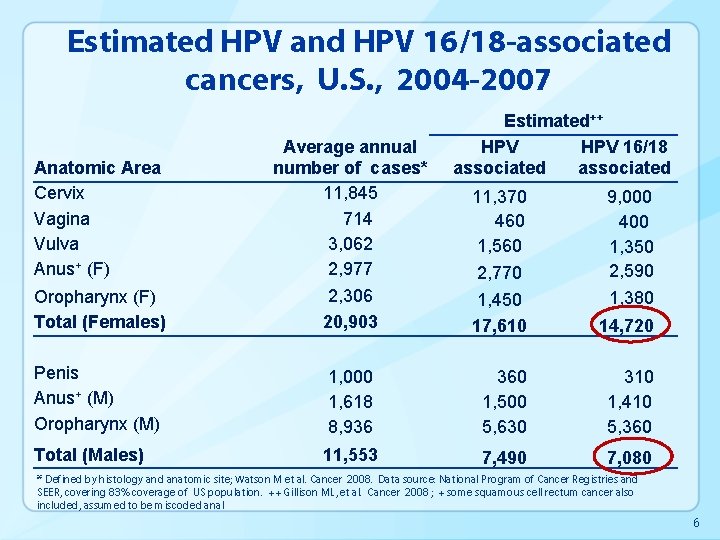
Estimated HPV and HPV 16/18 -associated cancers, U. S. , 2004 -2007 Oropharynx (F) Total (Females) Average annual number of cases* 11, 845 714 3, 062 2, 977 2, 306 20, 903 Penis Anus+ (M) Oropharynx (M) Total (Males) Anatomic Area Cervix Vagina Vulva Anus+ (F) Estimated++ HPV 16/18 associated 11, 370 460 1, 560 2, 770 9, 000 400 1, 350 2, 590 1, 380 1, 450 17, 610 14, 720 1, 000 1, 618 8, 936 360 1, 500 5, 630 310 1, 410 5, 360 11, 553 7, 490 7, 080 * Defined by histology and anatomic site; Watson M et al. Cancer 2008. Data source: National Program of Cancer Registries and SEER, covering 83% coverage of US population. ++ Gillison ML, et al. Cancer 2008 ; + some squamous cell rectum cancer also included, assumed to be miscoded anal 6

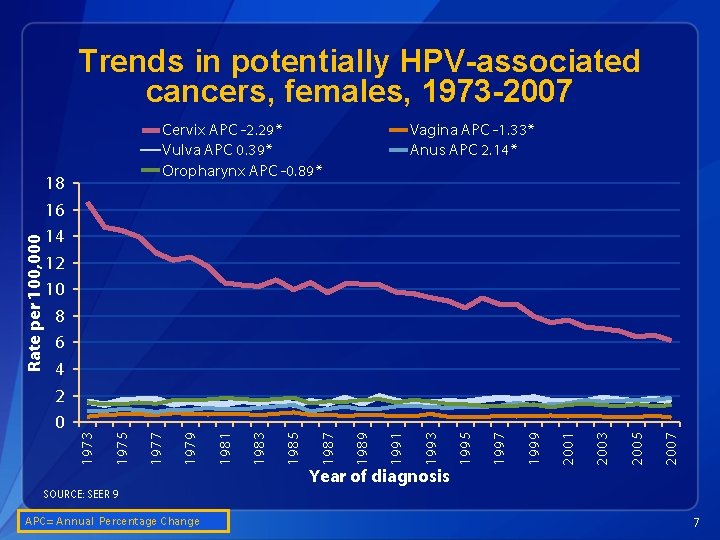
2007 2005 2003 2001 1999 1997 1995 1993 1991 Vagina APC -1. 33* Anus APC 2. 14* 1989 1987 1985 1983 1981 1979 1977 1975 18 16 14 12 10 8 6 4 2 0 Cervix APC -2. 29* Vulva APC 0. 39* Oropharynx APC -0. 89* 1973 Rate per 100, 000 Trends in potentially HPV-associated cancers, females, 1973 -2007 Year of diagnosis SOURCE: SEER 9 A APC= Annual Percentage Change 7

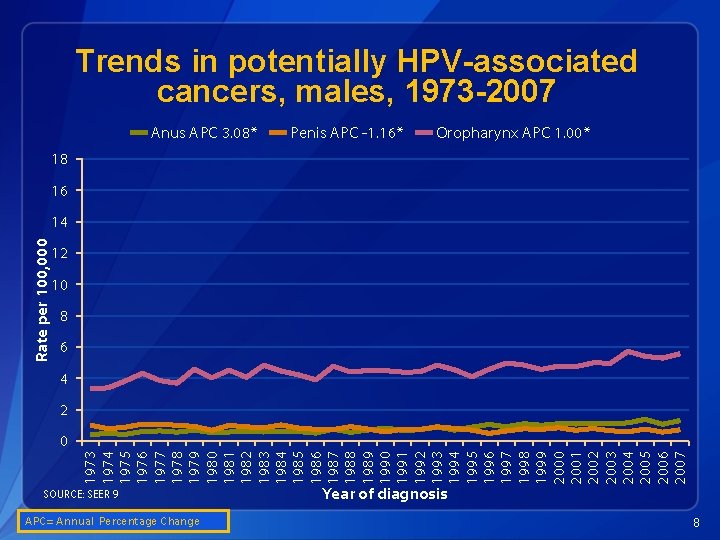
Trends in potentially HPV-associated cancers, males, 1973 -2007 Anus APC 3. 08* Penis APC -1. 16* Oropharynx APC 1. 00* 18 16 Rate per 100, 000 14 12 10 8 6 4 2 1973 1974 1975 1976 1977 1978 1979 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 0 SOURCE: SEER 9 APC= Annual Percentage Change Year of diagnosis 8

Summary Burden of HPV-associated cancer, U. S. • Cancers associated with HPV include cervical, vaginal, vulvar, anal, penile and oropharyngeal cancers • Approximately 25, 000 HPV-associated cancers; 22, 000 HPV 16/18 associated cancers annually (~15, 000 in women and ~7, 000 in men) • Cervical cancer rates are decreasing due to screening • Oropharyngeal cancer rates increasing in men • Increases in HPV-associated oropharyngeal cancers • Anal cancers rates increasing in men and women • Anal cancer incidence is highest among MSM, particularly HIVinfected MSM 9

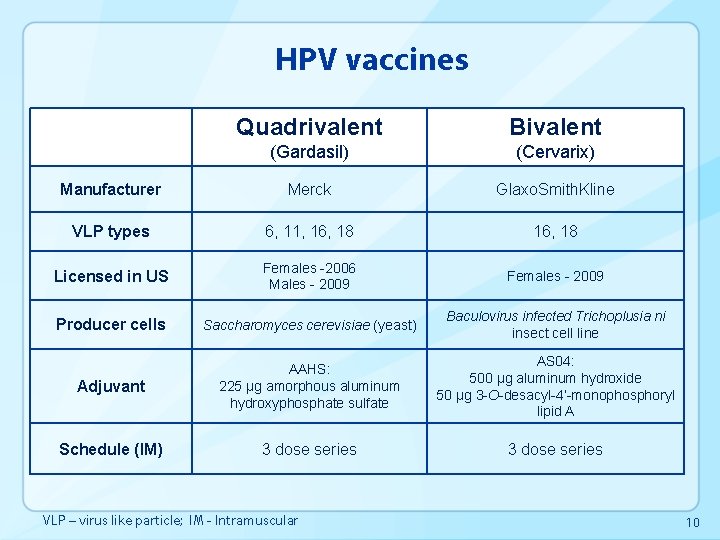
HPV vaccines Quadrivalent Bivalent (Gardasil) (Cervarix) Manufacturer Merck Glaxo. Smith. Kline VLP types 6, 11, 16, 18 Licensed in US Females -2006 Males - 2009 Females - 2009 Producer cells Saccharomyces cerevisiae (yeast) Baculovirus infected Trichoplusia ni insect cell line Adjuvant AAHS: 225 µg amorphous aluminum hydroxyphosphate sulfate AS 04: 500 µg aluminum hydroxide 50 µg 3 -O-desacyl-4’-monophosphoryl lipid A Schedule (IM) 3 dose series VLP – virus like particle; IM - Intramuscular 10

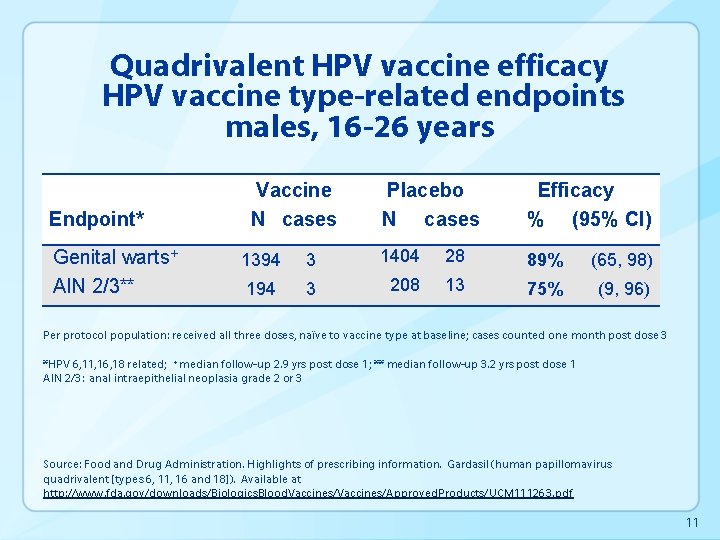
Quadrivalent HPV vaccine efficacy HPV vaccine type-related endpoints males, 16 -26 years Endpoint* Genital warts+ AIN 2/3** Vaccine N cases Placebo N cases Efficacy % (95% CI) 1394 3 1404 28 89% (65, 98) 194 3 208 13 75% (9, 96) Per protocol population: received all three doses, naïve to vaccine type at baseline; cases counted one month post dose 3 *HPV 6, 11, 16, 18 related; +median follow-up 2. 9 yrs post dose 1; ** median follow-up 3. 2 yrs post dose 1 AIN 2/3: anal intraepithelial neoplasia grade 2 or 3 Source: Food and Drug Administration. Highlights of prescribing information. Gardasil (human papillomavirus quadrivalent [types 6, 11, 16 and 18]). Available at http: //www. fda. gov/downloads/Biologics. Blood. Vaccines/Approved. Products/UCM 111263. pdf 11

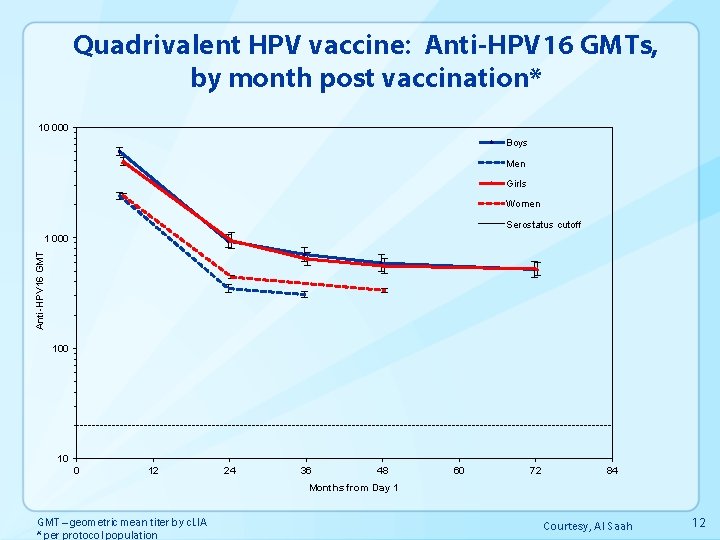
Quadrivalent HPV vaccine: Anti-HPV 16 GMTs, by month post vaccination* 10 000 Boys Men Girls Women Serostatus cutoff Anti-HPV 16 GMT 1 000 10 0 12 24 36 48 60 72 84 Months from Day 1 GMT – geometric mean titer by c. LIA * per protocol population Courtesy, Al Saah 12

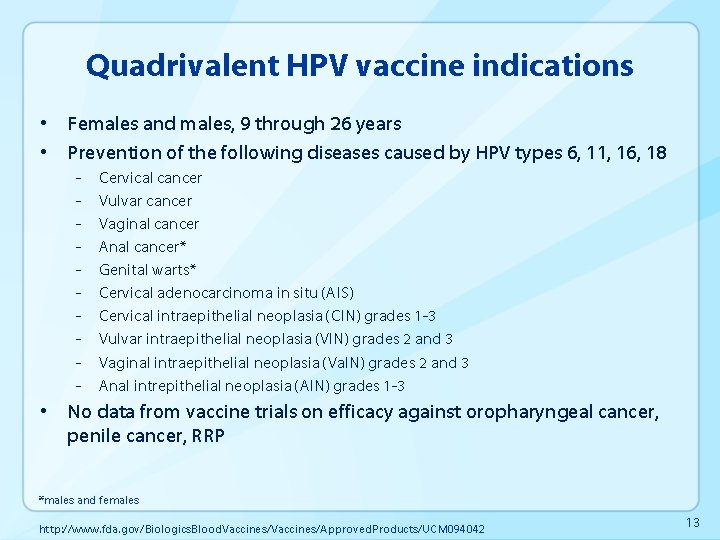
Quadrivalent HPV vaccine indications • Females and males, 9 through 26 years • Prevention of the following diseases caused by HPV types 6, 11, 16, 18 - Cervical cancer Vulvar cancer Vaginal cancer Anal cancer* Genital warts* Cervical adenocarcinoma in situ (AIS) Cervical intraepithelial neoplasia (CIN) grades 1 -3 Vulvar intraepithelial neoplasia (VIN) grades 2 and 3 Vaginal intraepithelial neoplasia (Va. IN) grades 2 and 3 Anal intrepithelial neoplasia (AIN) grades 1 -3 • No data from vaccine trials on efficacy against oropharyngeal cancer, penile cancer, RRP *males and females http: //www. fda. gov/Biologics. Blood. Vaccines/Approved. Products/UCM 094042 13

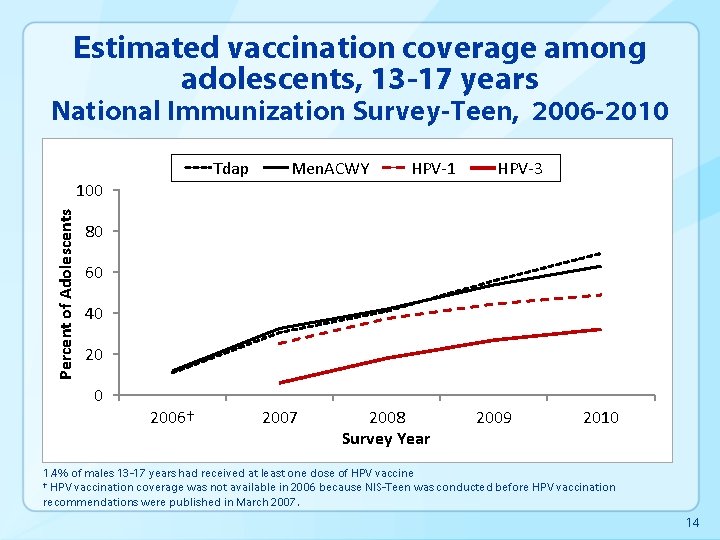
Estimated vaccination coverage among adolescents, 13 -17 years National Immunization Survey-Teen, 2006 -2010 Tdap Percent of Adolescents 100 Men. ACWY HPV-1 HPV-3 80 60 40 2006† 2007 2008 Survey Year 2009 2010 1. 4% of males 13 -17 years had received at least one dose of HPV vaccine † HPV vaccination coverage was not available in 2006 because NIS-Teen was conducted before HPV vaccination recommendations were published in March 2007. 14

Considered for Male HPV Vaccine Recommendations • • HPV vaccine efficacy, safety, immunogenicity Burden of diseases/cancers in males Status of HPV vaccination program Cost-effectiveness Acceptability and values Programmatic issues Equity 15

Cost-effectiveness of HPV vaccination Males • Routine male HPV vaccination at 11 or 12 years most costeffective when female coverage low (<50%) – $24, 000 to $62, 000 per QALY in published studies – With increasing coverage in females, males are protected through herd immunity – Male HPV vaccination cost-effectiveness increases with increasing at age vaccination (estimates for male 19 -26 years are $150, 00 ->400, 000/ QALY) *$24, 000 per QALY is from Merck model (Elbasha & Dasbach, 2010) with effective coverage (all 3 doses) by age 18 of ≈40% and ≈25% for females and males, respectively. $62, 000 per QALY is from Kim et al. (2009) with 50% 3 -dose coverage of girls and boys by age 12. 16

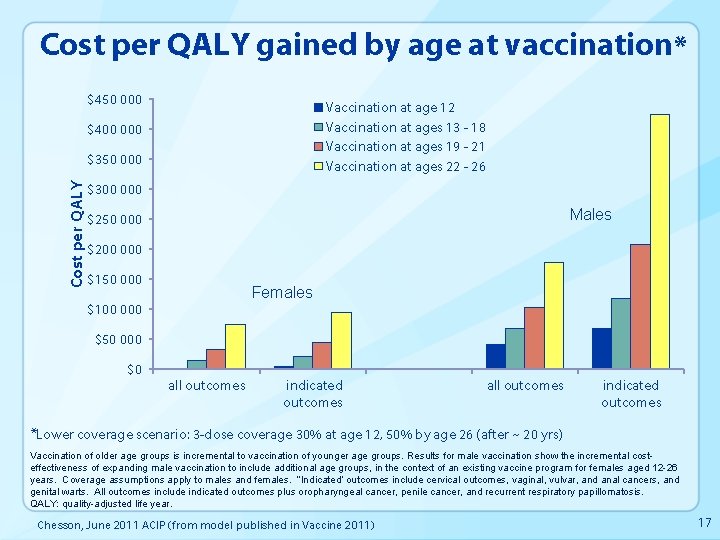
Cost per QALY gained by age at vaccination* $450 000 Vaccination at age 12 Vaccination at ages 13 - 18 Vaccination at ages 19 - 21 Vaccination at ages 22 - 26 $400 000 Cost per QALY $350 000 $300 000 Males $250 000 $200 000 $150 000 Females $100 000 $50 000 $0 all outcomes indicated outcomes *Lower coverage scenario: 3 -dose coverage 30% at age 12, 50% by age 26 (after ~ 20 yrs) Vaccination of older age groups is incremental to vaccination of younger age groups. Results for male vaccination show the incremental costeffectiveness of expanding male vaccination to include additional age groups, in the context of an existing vaccine program for females aged 12 -26 years. Coverage assumptions apply to males and females. “Indicated” outcomes include cervical outcomes, vaginal, vulvar, and anal cancers, and genital warts. All outcomes include indicated outcomes plus oropharyngeal cancer, penile cancer, and recurrent respiratory papillomatosis. QALY: quality-adjusted life year. Chesson, June 2011 ACIP (from model published in Vaccine 2011) 17

ACIP deliberations: HPV vaccination of males • Options considered: routine recommendation for males – Recommend routine vaccination of 11 -12 year old males, or – Retain current permissive recommendation for males 9 -26 years • Considerations: – – – – Quadrivalent HPV is safe and effective in males Burden of disease in males justifies routine vaccination Likely benefit against all HPV vaccine type associated disease Males as well as females should be protected against HPV for equity reasons Vaccination of adolescent males is cost effective at current female coverage Historically, risk based strategies not successful In addition to protecting heterosexual males and their female sex partners, for protection of MSM, routine vaccination is the best way to reach men with this sexual orientation at an age when they could most benefit 18

ACIP Recommendations for Male Vaccination ACIP recommends routine vaccination of males aged 11 or 12 years with HPV 4 administered as a 3 -dose series. The vaccination series can be started beginning at age 9 years. (recommendation category: A, evidence type: 2) Vaccination with HPV 4 is recommended for males aged 13 through 21 years who have not been vaccinated previously or who have not completed the 3 -dose series. Males aged 22 through 26 years may be vaccinated. Evidence tables: summarize the benefits & harms, strengths and limitations of the body of evidence. http: //www. cdc. gov/vaccines/recs/acip/GRADE/table-refs. htm MMWR 2011; 60: 1705 -8 19

ACIP Recommendations for Special Populations HPV 4 is not a live vaccine and can be administered to persons who are immunocompromised as a result of infection (including HIV), disease, or medications. The immune response and vaccine efficacy might be less than that in immunocompetent persons. For immunocompromised males, ACIP recommends routine vaccination with HPV 4 as for all males, and vaccination through age 26 years for those who have not been vaccinated previously or who have not completed the 3 -dose series. MMWR 2011; 60: 1705 -8 20

ACIP Recommendations for Special Populations MSM may especially benefit from vaccination to prevent condyloma and anal cancer. For MSM, ACIP recommends routine vaccination with HPV 4 as for all males, and vaccination through age 26 years for those who have not been vaccinated previously or who have not completed the 3 -dose series. MMWR 2011; 60: 1705 -8 21

Advisory Committee on Immunization Practices recommendations for HPV vaccination Quadrivalent or Bivalent Routine, females 11 or 12 yrs* Catch-up, 13 -26 yrs Quadrivalent Routine, males 11 or 12 yrs* Catchup, 13 -21 yrs May be given, males 9 -26 yrs June 2006 October 2007 2008 2009 October 2010 2011 2012 Quadrivalent (HPV 6, 11, 1, 618) vaccine; Bivalent (HPV 16, 18) vaccine * Can be given starting at 9 years of age; males 22 -26 years may receive quadrivalent HPV vaccine 22


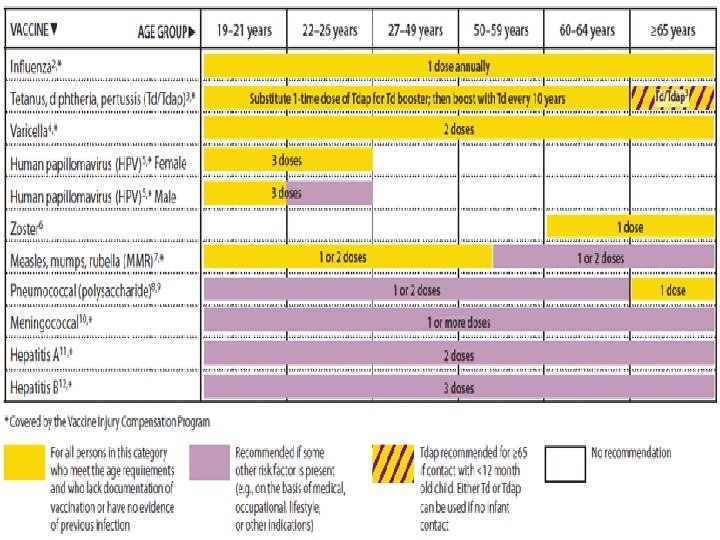
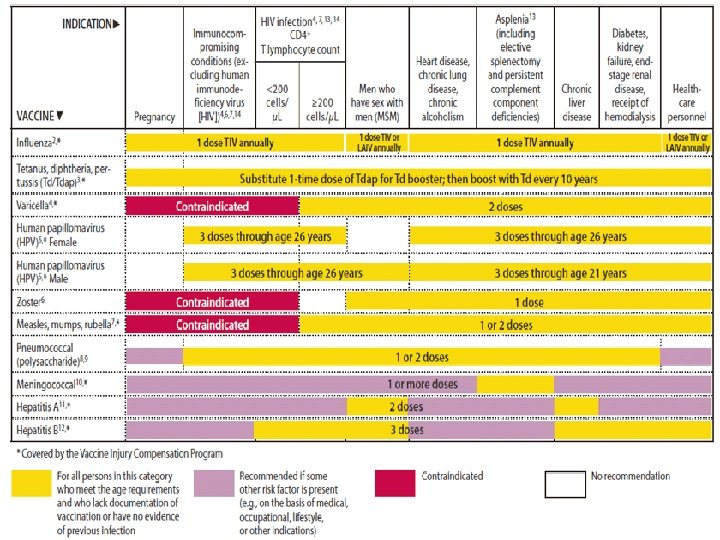
FIGURE 2. Vaccines that might be indicated for adults, based on medical and other indications 1 — United States, 2012 24

Why are the male recommendations different from the female recommendations? q A number of issues considered for male and female recommendations § Although HPV is a sexually transmitted infection that is common in men and women, most of the health effects are in women making direct vaccination of women most cost-effective § HPV vaccine is most cost-effective for younger ages—this is especially so for males as cost-effectiveness increases with increasing age § Emphasis on younger ages provides focus on this group for largest population benefit § Specific risk populations (MSM and HIV-infected) are recommended to receive vaccine through age 26 years

Why is just one vaccine recommended for males and two for females? q Quadrivalent HPV vaccine was studied in males and is licensed for males aged 9 -26 years § Bivalent vaccine has not been licensed in men q Both vaccines prevent types of HPV most likely to cause cancers in men § Only quadrivalent HPV vaccine has been demonstrated to reduce genital warts and anal precancers in men