Acids vs Bases Acids Turn blue litmus red
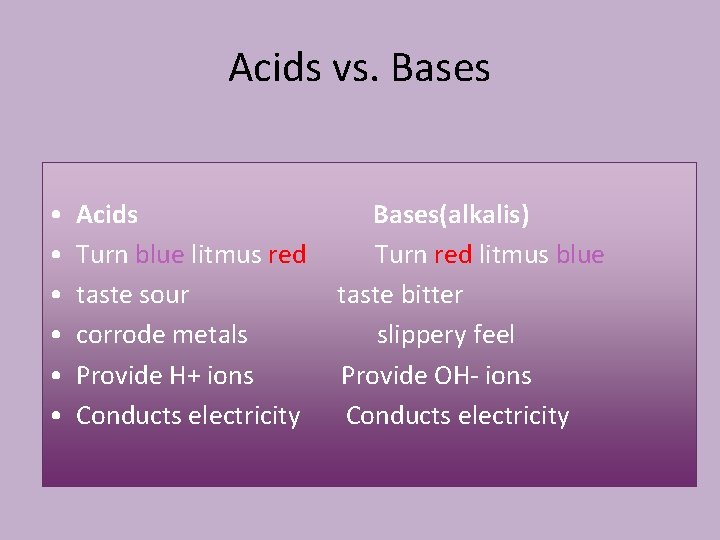
Acids vs. Bases • • • Acids Turn blue litmus red taste sour corrode metals Provide H+ ions Conducts electricity Bases(alkalis) Turn red litmus blue taste bitter slippery feel Provide OH- ions Conducts electricity
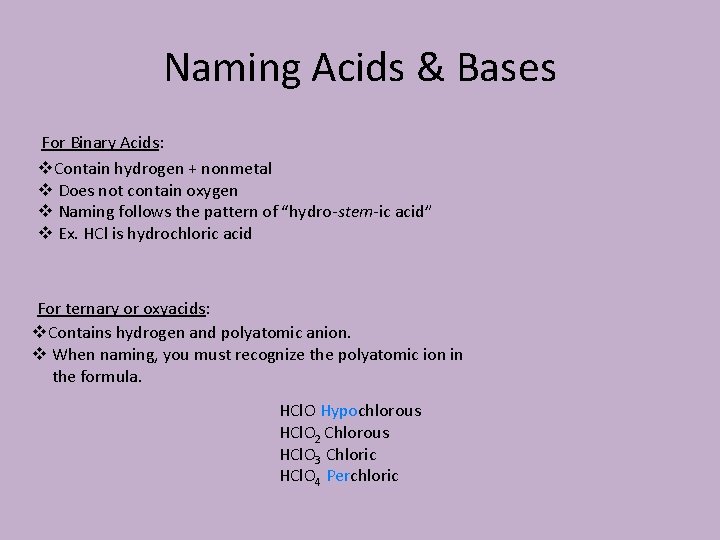
Naming Acids & Bases For Binary Acids: v. Contain hydrogen + nonmetal v Does not contain oxygen v Naming follows the pattern of “hydro-stem-ic acid” v Ex. HCl is hydrochloric acid For ternary or oxyacids: v. Contains hydrogen and polyatomic anion. v When naming, you must recognize the polyatomic ion in the formula. HCl. O Hypochlorous HCl. O 2 Chlorous HCl. O 3 Chloric HCl. O 4 Perchloric

Arrhenius Definition Acids release hydrogen ions in water: HCl H+ + Cl. Bases release hydroxide ions in water: Na. OH Na+ + OH-

Bronsted-Lowry Definition • Acids are PROTON DONORS • Bases are PROTON ACCEPTORS • When HCN Dissolves in water a reaction occurs: • HCN + H 2 O ----> H 3 O+ + CN • HCN is a Bronsted Acid • Water is acting as a Bronsted Base.
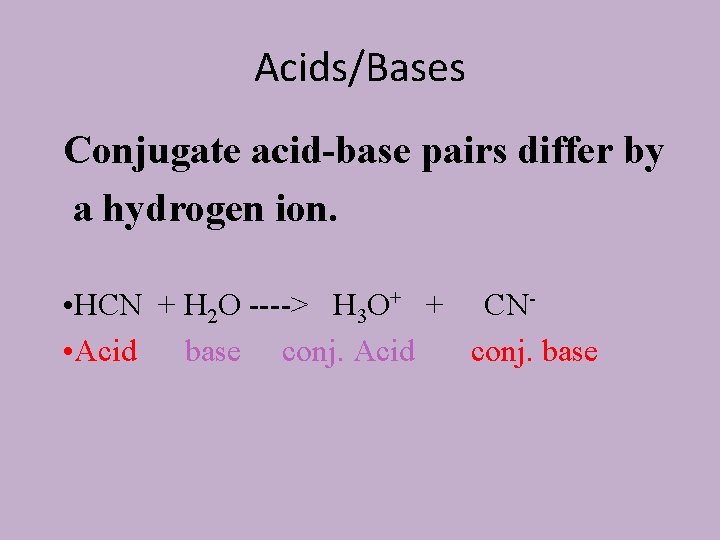
Acids/Bases Conjugate acid-base pairs differ by a hydrogen ion. • HCN + H 2 O ----> H 3 O+ + CN • Acid base conj. Acid conj. base
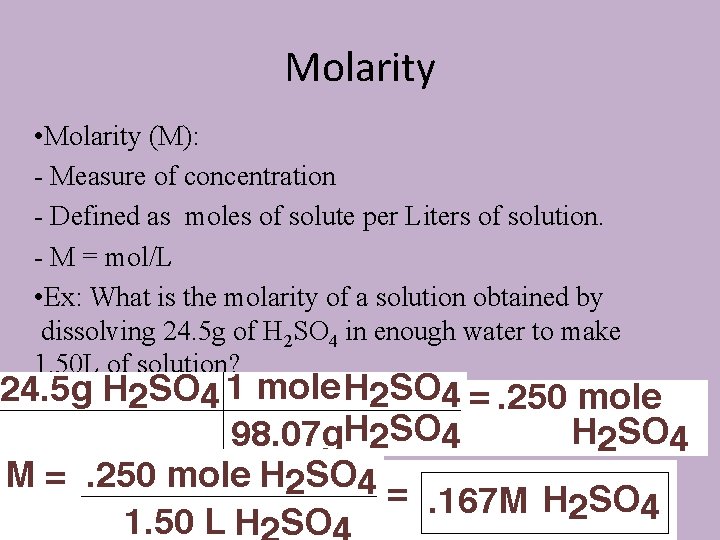
Molarity • Molarity (M): - Measure of concentration - Defined as moles of solute per Liters of solution. - M = mol/L • Ex: What is the molarity of a solution obtained by dissolving 24. 5 g of H 2 SO 4 in enough water to make 1. 50 L of solution?

Neutralization • An Acid/ base reaction is called a neutralization reaction. • Ex: Na. OH + HCl --> Na. Cl +HOH • base + acid --> a salt + water

Titration Equation: (Moles A) MBVB = (Moles B) MAVA Dilution Equation: M 1 V 1 = M 2 V 2
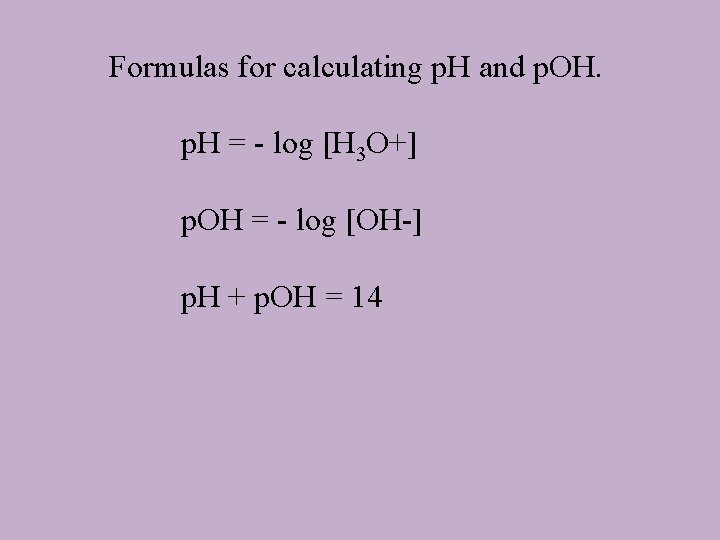
Formulas for calculating p. H and p. OH. p. H = - log [H 3 O+] p. OH = - log [OH-] p. H + p. OH = 14
![Formulas for calculating concentration. [H 3 O+] = 10 -p. H [OH-] = 10 Formulas for calculating concentration. [H 3 O+] = 10 -p. H [OH-] = 10](http://slidetodoc.com/presentation_image_h2/af4b43849281531f3caf5d37ef91785b/image-10.jpg)
Formulas for calculating concentration. [H 3 O+] = 10 -p. H [OH-] = 10 –p. OH [H 3 O+][OH-] = 10 -14
![p. H Practice 1. What is the p. H of a solution having [OH-] p. H Practice 1. What is the p. H of a solution having [OH-]](http://slidetodoc.com/presentation_image_h2/af4b43849281531f3caf5d37ef91785b/image-11.jpg)
p. H Practice 1. What is the p. H of a solution having [OH-] = 1 x 10 -3? p. OH = 3 so 14 -3=11, p. H = 11 2. What is the concentration of a solution with a p. H = 3. 5? a. b. c. d. 3. 5 mol/L 5. 0 x 10 -3 mol/L 3. 2 x 10 -11 mol/L 3. 2 x 10 -4 mol/L Try without a calculator D is the correct answer

Hydrolysis • Are each of the following salts acidic, basic or neutral? • Al(NO 3)3 • Ca. SO 4
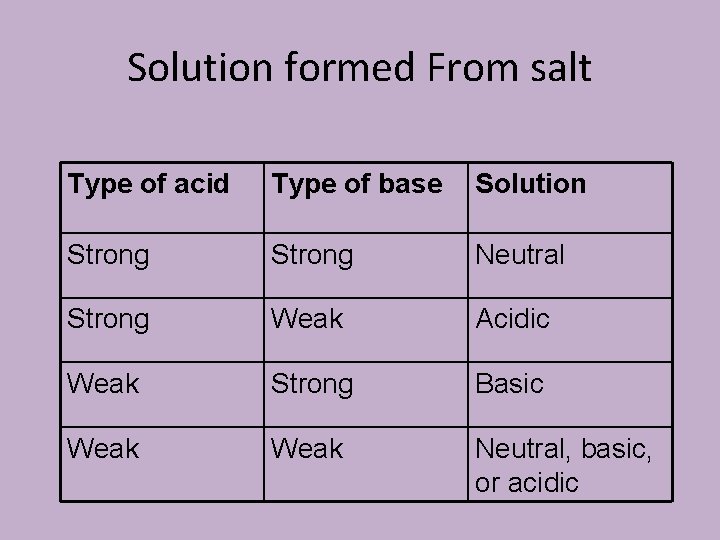
Solution formed From salt Type of acid Type of base Solution Strong Neutral Strong Weak Acidic Weak Strong Basic Weak Neutral, basic, or acidic
- Slides: 13