ACIDS Taste sour vinegar lemons Electrolytes in solution



![p. H: a summary worksheet p. OH [OH-] [H+] p. H 11 p. H: a summary worksheet p. OH [OH-] [H+] p. H 11](https://slidetodoc.com/presentation_image_h2/07da6d9b3f0bc1051a340944c9fbb04c/image-11.jpg)
![p. OH 14 [OH-] 13 12 11 10 10 -14 10 -13 10 -12 p. OH 14 [OH-] 13 12 11 10 10 -14 10 -13 10 -12](https://slidetodoc.com/presentation_image_h2/07da6d9b3f0bc1051a340944c9fbb04c/image-12.jpg)
![p. H = -log p. OH = -log + [H ] [OH ] p. p. H = -log p. OH = -log + [H ] [OH ] p.](https://slidetodoc.com/presentation_image_h2/07da6d9b3f0bc1051a340944c9fbb04c/image-13.jpg)

![HYDROGEN IONS & ACIDITY Water is a neutral solution because [H+] = [OH-] H HYDROGEN IONS & ACIDITY Water is a neutral solution because [H+] = [OH-] H](https://slidetodoc.com/presentation_image_h2/07da6d9b3f0bc1051a340944c9fbb04c/image-20.jpg)
- Slides: 24


ACIDS ~Taste sour (vinegar, lemons) ~Electrolytes in solution ~React with some metals to produce H 2(g) Zn + 2 HCl Zn. Cl 2 + H 2 (SR reaction) BASES ~Taste bitter, feels slippery ~Electrolyte in solution ~React with acids to produce salt and H 2 O HCl + Na. OH Na. Cl + H 2 O

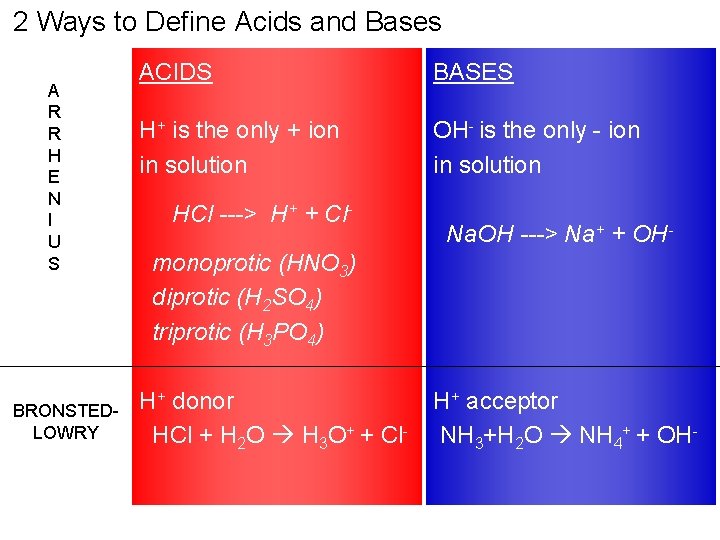
2 Ways to Define Acids and Bases A R R H E N I U S BRONSTEDLOWRY ACIDS BASES H+ is the only + ion in solution OH- is the only - ion in solution HCl ---> H+ + Cl- Na. OH ---> Na+ + OH- monoprotic (HNO 3) diprotic (H 2 SO 4) triprotic (H 3 PO 4) H+ donor HCl + H 2 O H 3 O+ + Cl- H+ acceptor NH 3+H 2 O NH 4+ + OH-

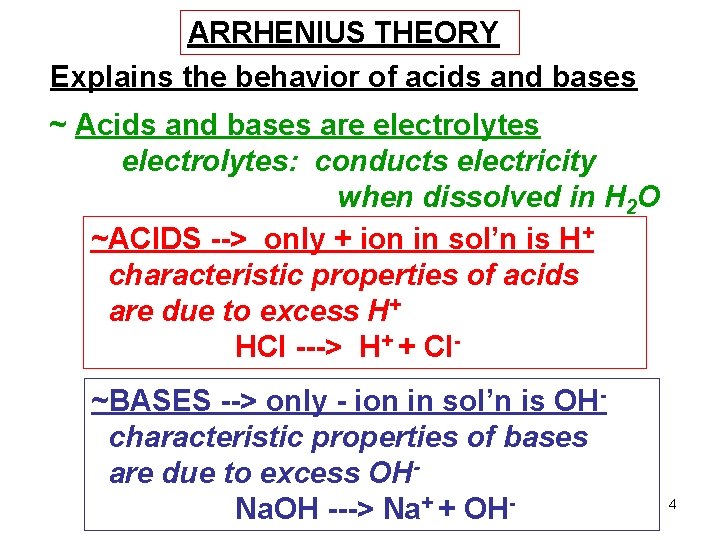
ARRHENIUS THEORY Explains the behavior of acids and bases ~ Acids and bases are electrolytes: conducts electricity when dissolved in H 2 O ~ACIDS --> only + ion in sol’n is H+ characteristic properties of acids are due to excess H+ HCl ---> H+ + Cl~BASES --> only - ion in sol’n is OHcharacteristic properties of bases are due to excess OHNa. OH ---> Na+ + OH- 4

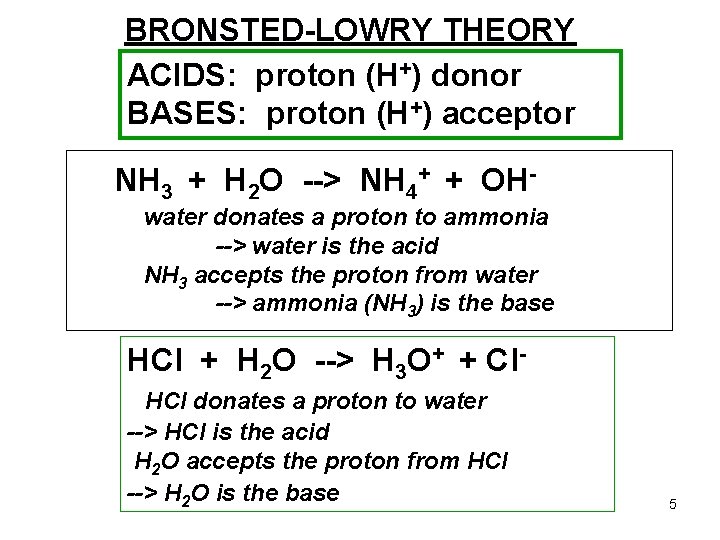
BRONSTED-LOWRY THEORY ACIDS: proton (H+) donor BASES: proton (H+) acceptor NH 3 + H 2 O --> NH 4+ + OHwater donates a proton to ammonia --> water is the acid NH 3 accepts the proton from water --> ammonia (NH 3) is the base HCl + H 2 O --> H 3 O+ + Cl. HCl donates a proton to water --> HCl is the acid H 2 O accepts the proton from HCl --> H 2 O is the base 5

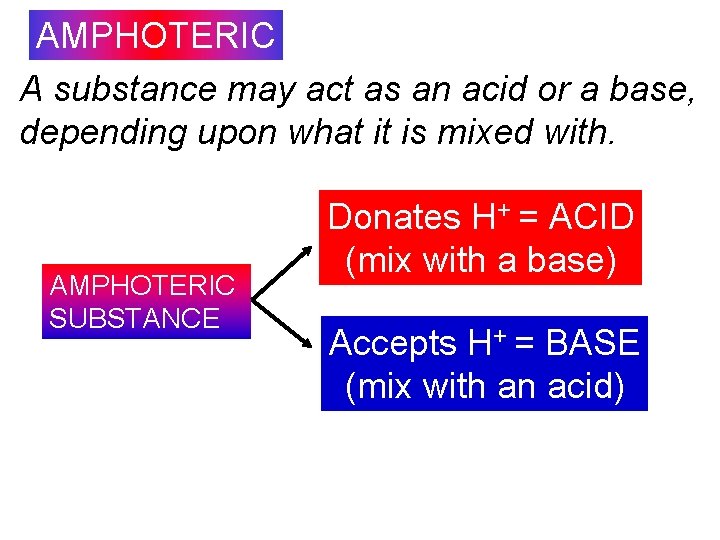
AMPHOTERIC or AMPHIPROTIC Substances that can act as either an acid or a base, depending upon who they are mixed with (environment). If in the presence of a strong base, act as an acid. If in the presence of a strong acid, act as a base. Examples: water, HSO 4 -, HS-, HPO 4 -2 6

AMPHOTERIC A substance may act as an acid or a base, depending upon what it is mixed with. AMPHOTERIC SUBSTANCE Donates H+ = ACID (mix with a base) Accepts H+ = BASE (mix with an acid)

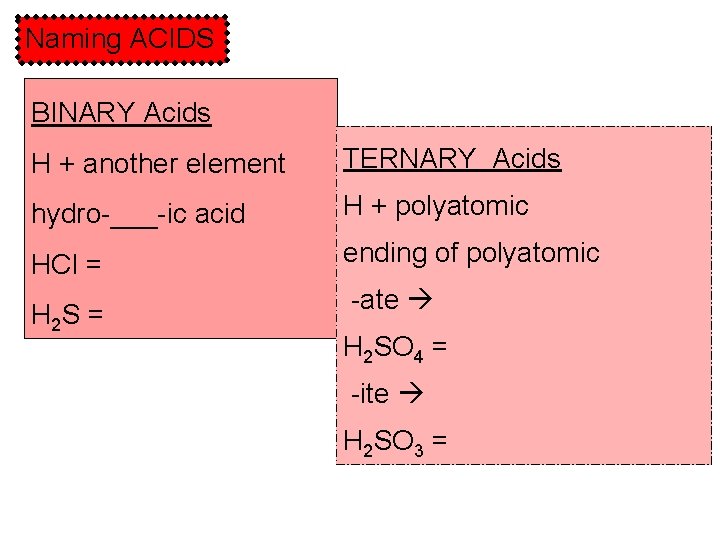
Naming ACIDS BINARY Acids H + another element TERNARY Acids hydro-___-ic acid H + polyatomic HCl = ending of polyatomic H 2 S = -ate H 2 SO 4 = -ite H 2 SO 3 =

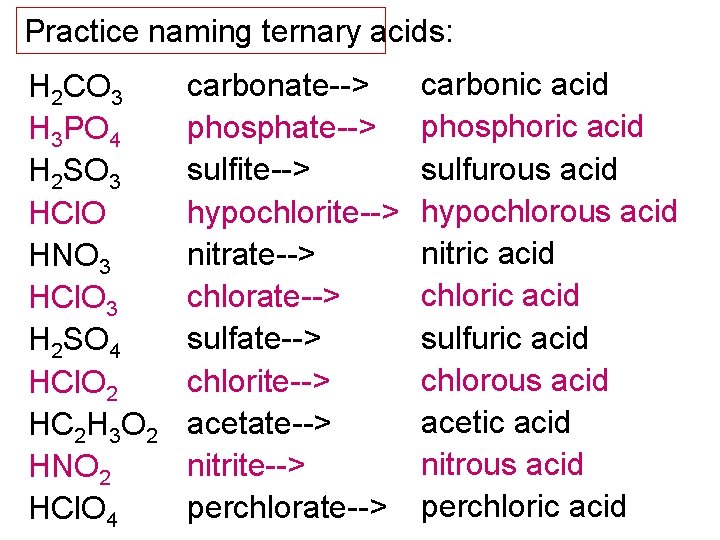
Practice naming ternary acids: H 2 CO 3 H 3 PO 4 H 2 SO 3 HCl. O HNO 3 HCl. O 3 H 2 SO 4 HCl. O 2 HC 2 H 3 O 2 HNO 2 HCl. O 4 carbonate--> phosphate--> sulfite--> hypochlorite--> nitrate--> chlorate--> sulfate--> chlorite--> acetate--> nitrite--> perchlorate--> carbonic acid phosphoric acid sulfurous acid hypochlorous acid nitric acid chloric acid sulfuric acid chlorous acid acetic acid nitrous acid perchloric acid

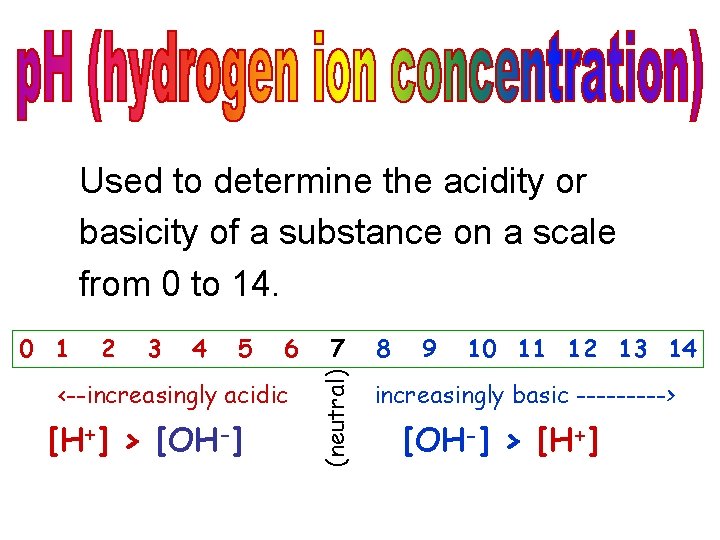
Used to determine the acidity or basicity of a substance on a scale from 0 to 14. 2 3 4 5 6 <--increasingly acidic [H+] > [OH-] 7 (neutral) 0 1 8 9 10 11 12 13 14 increasingly basic -----> [OH-] > [H+]
![p H a summary worksheet p OH OH H p H 11 p. H: a summary worksheet p. OH [OH-] [H+] p. H 11](https://slidetodoc.com/presentation_image_h2/07da6d9b3f0bc1051a340944c9fbb04c/image-11.jpg)
p. H: a summary worksheet p. OH [OH-] [H+] p. H 11
![p OH 14 OH 13 12 11 10 10 14 10 13 10 12 p. OH 14 [OH-] 13 12 11 10 10 -14 10 -13 10 -12](https://slidetodoc.com/presentation_image_h2/07da6d9b3f0bc1051a340944c9fbb04c/image-12.jpg)
p. OH 14 [OH-] 13 12 11 10 10 -14 10 -13 10 -12 10 -11 10 -10 9 10 -9 8 7 6 5 4 3 10 -8 10 -7 10 -6 10 -5 10 -4 10 -3 2 1 0 10 -2 10 -1 1 [OH-] [H+] 10 -1 10 -2 1 2 10 -3 10 -4 10 -5 3 4 5 10 -6 10 -7 10 -8 6 <--increasingly acidic [H+] > [OH-] 7 (neutral) [H+] 1 p. H 0 8 10 -9 10 -10 9 10 10 -11 10 -12 10 -13 10 -14 11 12 13 14 increasingly basic -----> [OH-] > [H+] 12
![p H log p OH log H OH p p. H = -log p. OH = -log + [H ] [OH ] p.](https://slidetodoc.com/presentation_image_h2/07da6d9b3f0bc1051a340944c9fbb04c/image-13.jpg)
p. H = -log p. OH = -log + [H ] [OH ] p. H + p. OH = 14 + [H ] [OH ] =1 x -14 10

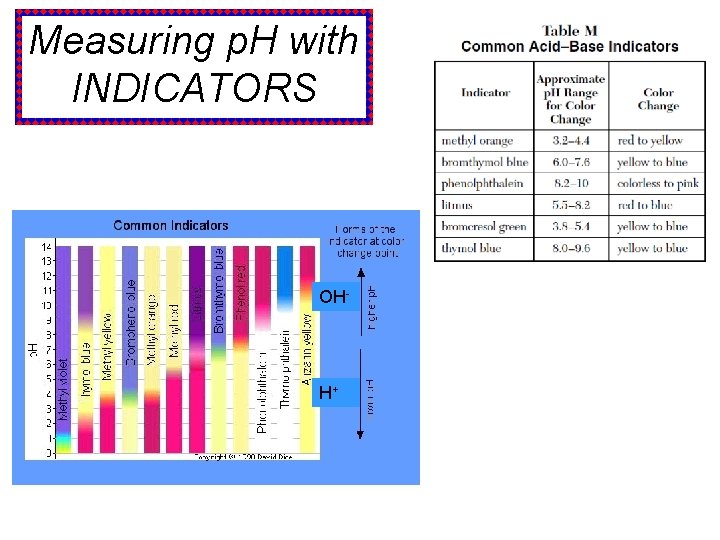
Measuring p. H with INDICATORS OH- H+

ACID-BASE TITRATIONS 1) Used to determine an unknown concentration of either an acid or a base, or the p. H. TITRATION- process used to perform a neutralization reaction 2) Must determine the volumes of acid and base involved. 3) Standard solution: the acid or base of known molarity (concentration is known) 4) End point: use an indicator to know when to stop Titration Calculations MAv. A = MBv. B MA = molarity of acid v. A = volume of acid MB = molarity of base v. B = volume of base

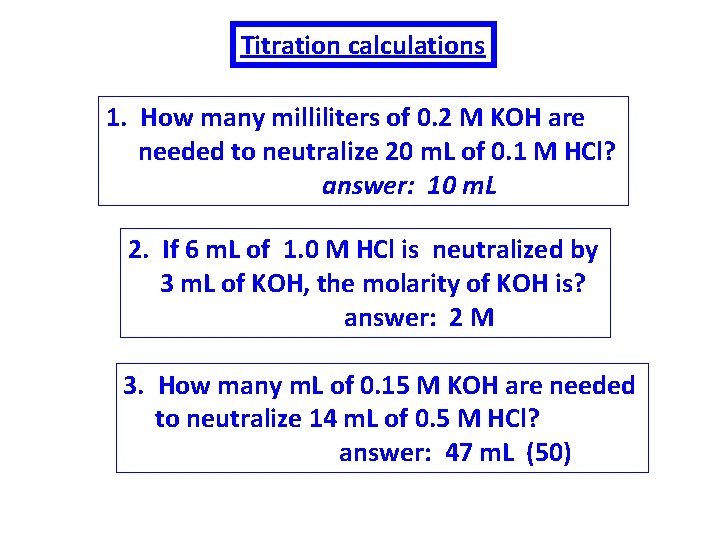
Titration calculations 1. How many milliliters of 0. 2 M KOH are needed to neutralize 20 m. L of 0. 1 M HCl? answer: 10 m. L 2. If 6 m. L of 1. 0 M HCl is neutralized by 3 m. L of KOH, the molarity of KOH is? answer: 2 M 3. How many m. L of 0. 15 M KOH are needed to neutralize 14 m. L of 0. 5 M HCl? answer: 47 m. L (50)

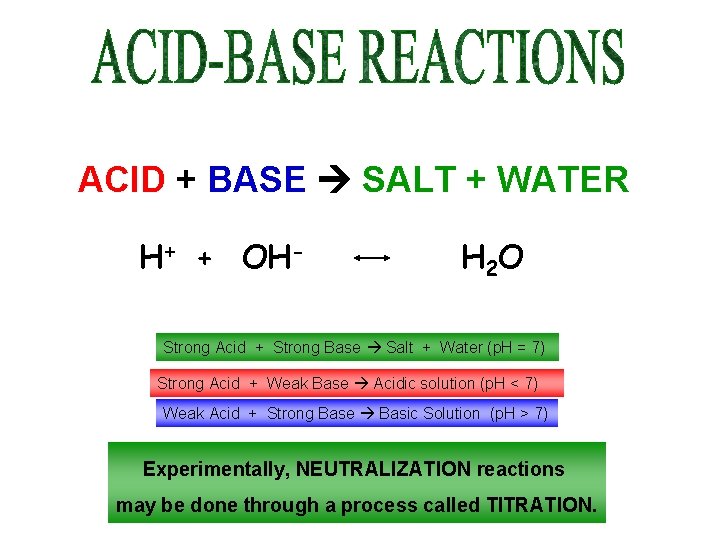
ACID + BASE SALT + WATER H+ + OH- H 2 O Strong Acid + Strong Base Salt + Water (p. H = 7) Strong Acid + Weak Base Acidic solution (p. H < 7) Weak Acid + Strong Base Basic Solution (p. H > 7) Experimentally, NEUTRALIZATION reactions may be done through a process called TITRATION.

Write the eqtn for the neutralization of each: 1. hydrochloric acid by sodium hydroxide HCl + Na. OH --> Na. Cl + H 2 O 2. nitric acid by potassium hydroxide HNO 3 + KOH --> KNO 3 + H 2 O 3. sulfuric acid by calcium hydroxide H 2 SO 4 + Ca(OH)2 --> Ca. SO 4 + 2 H 2 O 4. phosphoric acid by sodium hydroxide H 3 PO 4 + 3 Na. OH --> Na 3 PO 4 + 3 H 2 O 5. carbonic acid by calcium hydroxide H 2 CO 3 + Ca(OH)2 --> Ca. CO 3 + 2 H 2 O 6. ethanoic acid by potassium hydroxide HC 2 H 3 O 2 + KOH --> KC 2 H 3 O 2 + H 2 O 7. acetic acid by sodium hydroxide HC 2 H 3 O 2 + Na. OH --> Na. C 2 H 3 O 2 + H 2 O

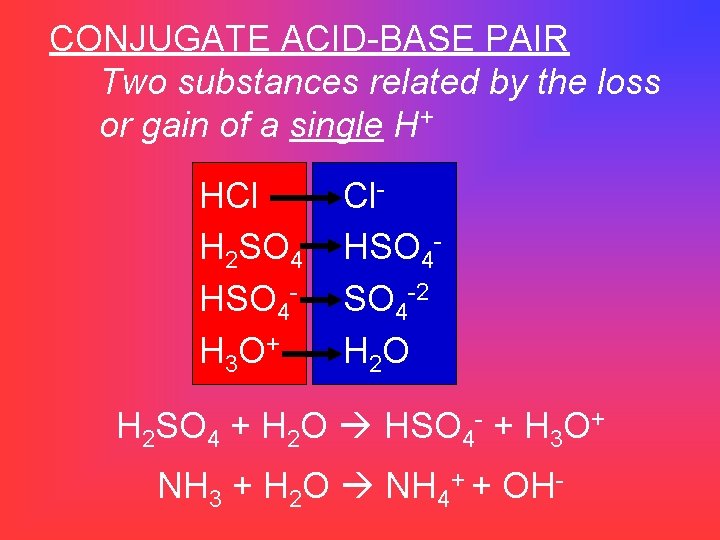
CONJUGATE ACID-BASE PAIR Two substances related by the loss or gain of a single H+ HCl H 2 SO 4 HSO 4 H 3 O + Cl. HSO 4 -2 H 2 O H 2 SO 4 + H 2 O HSO 4 - + H 3 O+ NH 3 + H 2 O NH 4+ + OH-
![HYDROGEN IONS ACIDITY Water is a neutral solution because H OH H HYDROGEN IONS & ACIDITY Water is a neutral solution because [H+] = [OH-] H](https://slidetodoc.com/presentation_image_h2/07da6d9b3f0bc1051a340944c9fbb04c/image-20.jpg)
HYDROGEN IONS & ACIDITY Water is a neutral solution because [H+] = [OH-] H 2 O + H 2 O H 3 O+ + OHSelf-ionization of water occurs to a small extent H+ is transferred from one water mc to the other

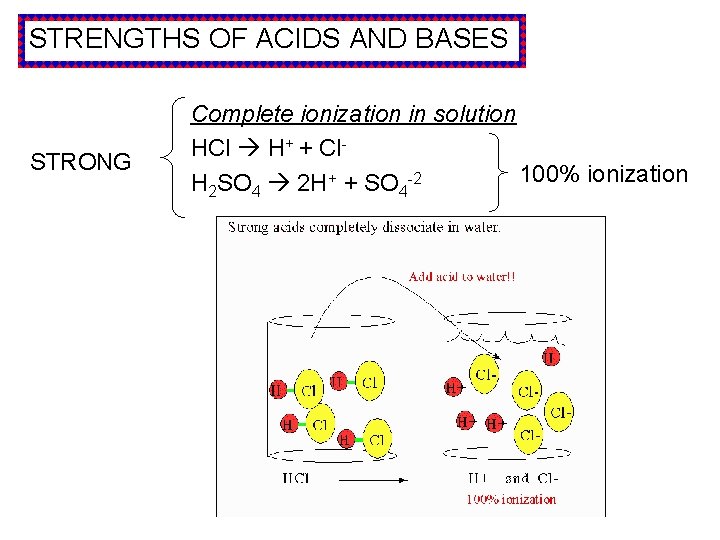
STRENGTHS OF ACIDS AND BASES STRONG Complete ionization in solution HCl H+ + Cl 100% ionization H 2 SO 4 2 H+ + SO 4 -2

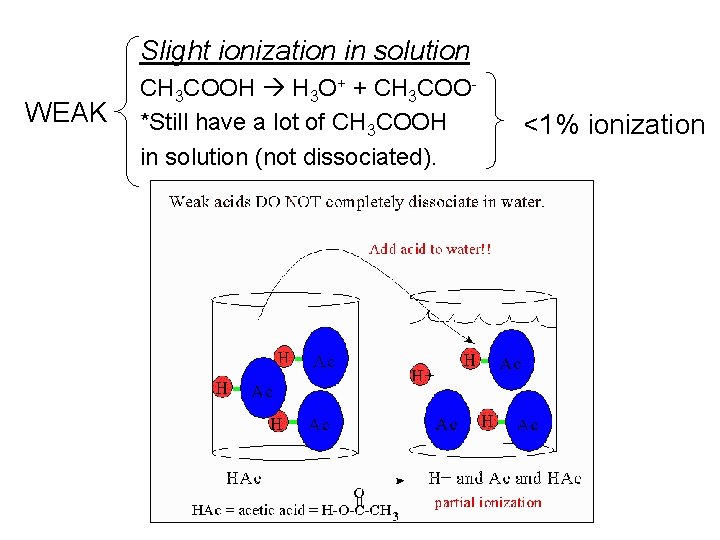
Slight ionization in solution WEAK CH 3 COOH H 3 O+ + CH 3 COO*Still have a lot of CH 3 COOH in solution (not dissociated). <1% ionization

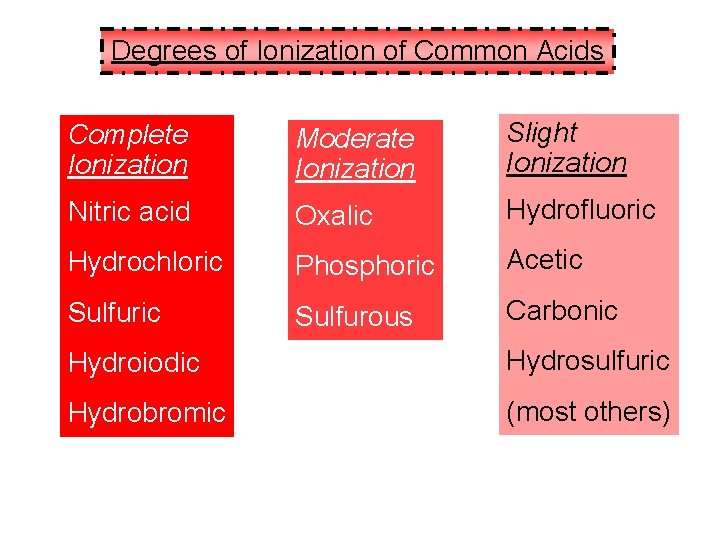
Degrees of Ionization of Common Acids Complete Ionization Moderate Ionization Slight Ionization Nitric acid Oxalic Hydrofluoric Hydrochloric Phosphoric Acetic Sulfurous Carbonic Hydroiodic Hydrosulfuric Hydrobromic (most others)

Strength vs. Concentration Strong and weak Extent of ionization Concentrated and dilute Describes how much is dissolved (# of moles in the volume, or Molarity (M))