Acids Lesson 3 Acid and Base Reactions Conductivity

Acids Lesson 3 Acid and Base Reactions
Conductivity The conductivity of an acid is determined by the number of ions generated in a solution and is therefore a combination of both the strength and concentration of the acid. 1. 0 M HCl is a better conductor than 0. 10 M HCl 1. 0 M HI is a better conductor than 1. 0 M HF has the same conductivity as 0. 02 M HCl.
Amphiprotic Chemical Species Amphiprotic chemical species can act as acids and donate protons or bases and accept protons. They are listed both on the left side of the table as an acid and the right side as a base. Acid, Base, or Amphiprotic PO 43
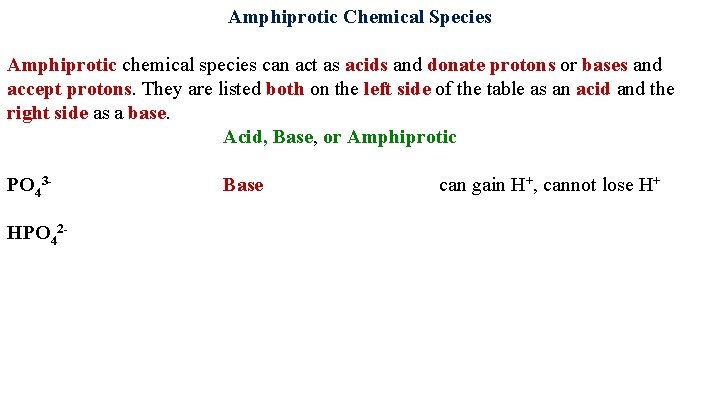
Amphiprotic Chemical Species Amphiprotic chemical species can act as acids and donate protons or bases and accept protons. They are listed both on the left side of the table as an acid and the right side as a base. Acid, Base, or Amphiprotic PO 43 Base can gain H+, cannot lose H+ HPO 42 -

Amphiprotic Chemical Species Amphiprotic chemical species can act as acids and donate protons or bases and accept protons. They are listed both on the left side of the table as an acid and the right side as a base. Acid, Base, or Amphiprotic PO 43 Base can gain H+, cannot lose H+ HPO 42 H 2 PO 4 - Amphiprotic can gain H+and lose H+

Amphiprotic Chemical Species Amphiprotic chemical species can act as acids and donate protons or bases and accept protons. They are listed both on the left side of the table as an acid and the right side as a base. Acid, Base, or Amphiprotic PO 43 Base can gain H+, cannot lose H+ HPO 42 - Amphiprotic can gain H+and lose H+ H 2 PO 4 - Amphiprotic can gain H+and lose H+ H 3 PO 4

Amphiprotic Chemical Species Amphiprotic chemical species can act as acids and donate protons or bases and accept protons. They are listed both on the left side of the table as an acid and the right side as a base. Acid, Base, or Amphiprotic PO 43 Base can gain H+, cannot lose H+ HPO 42 - Amphiprotic can gain H+and lose H+ H 2 PO 4 - Amphiprotic can gain H+and lose H+ H 3 PO 4 Acid cannot gain H+, can lose H+

Amphiprotic Chemical Species Amphiprotic chemical species can act as acids and donate protons or bases and accept protons. They are listed both on the left side of the table as an acid and the right side as a base. Acid, Base, or Amphiprotic CO 32
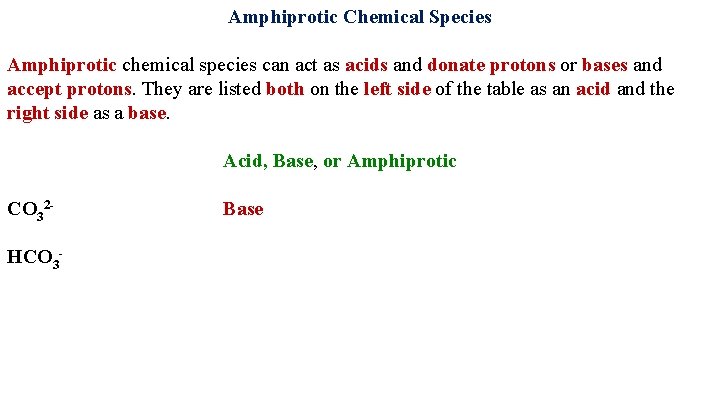
Amphiprotic Chemical Species Amphiprotic chemical species can act as acids and donate protons or bases and accept protons. They are listed both on the left side of the table as an acid and the right side as a base. Acid, Base, or Amphiprotic CO 32 Base HCO 3 -
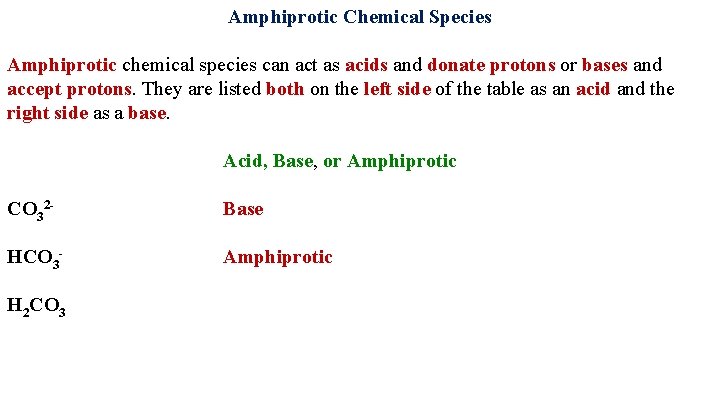
Amphiprotic Chemical Species Amphiprotic chemical species can act as acids and donate protons or bases and accept protons. They are listed both on the left side of the table as an acid and the right side as a base. Acid, Base, or Amphiprotic CO 32 Base HCO 3 H 2 CO 3 Amphiprotic
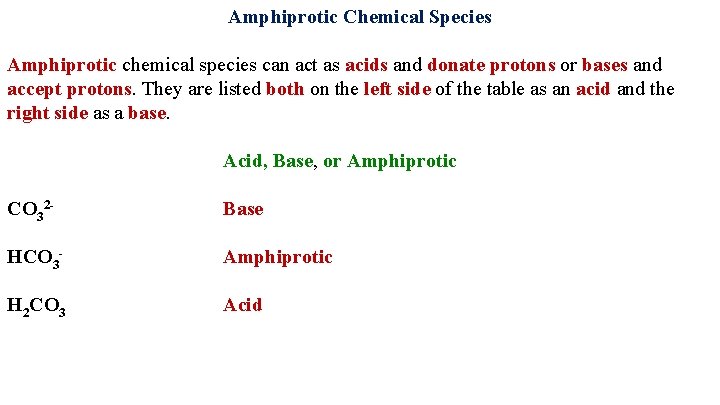
Amphiprotic Chemical Species Amphiprotic chemical species can act as acids and donate protons or bases and accept protons. They are listed both on the left side of the table as an acid and the right side as a base. Acid, Base, or Amphiprotic CO 32 Base HCO 3 - Amphiprotic H 2 CO 3 Acid

In Chemistry 11 H 2 SO 4 + 2 KOH
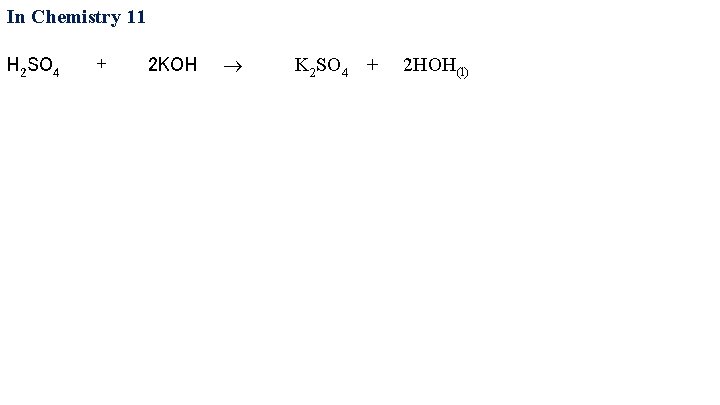
In Chemistry 11 H 2 SO 4 + 2 KOH K 2 SO 4 + 2 HOH(l)
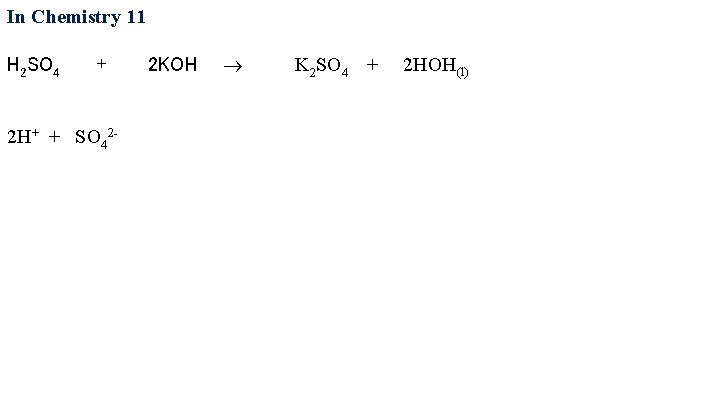
In Chemistry 11 H 2 SO 4 + 2 KOH 2 H+ + SO 42 - K 2 SO 4 + 2 HOH(l)
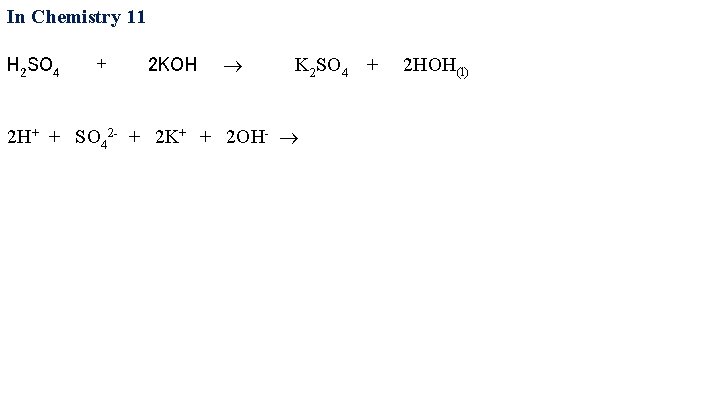
In Chemistry 11 H 2 SO 4 + 2 KOH K 2 SO 4 + 2 HOH(l) 2 H+ + SO 42 - + 2 K+ + 2 OH-
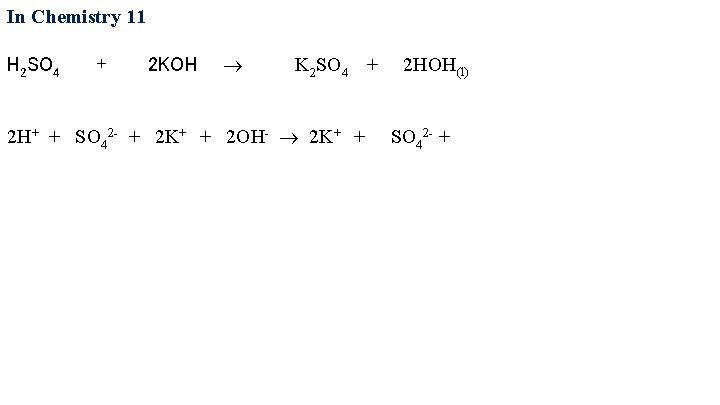
In Chemistry 11 H 2 SO 4 + 2 KOH K 2 SO 4 + 2 HOH(l) 2 H+ + SO 42 - + 2 K+ + 2 OH- 2 K+ + SO 42 - +
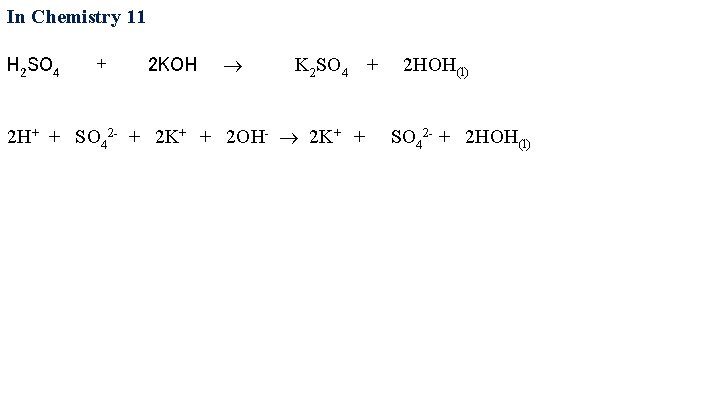
In Chemistry 11 H 2 SO 4 + 2 KOH K 2 SO 4 + 2 HOH(l) 2 H+ + SO 42 - + 2 K+ + 2 OH- 2 K+ + SO 42 - + 2 HOH(l)
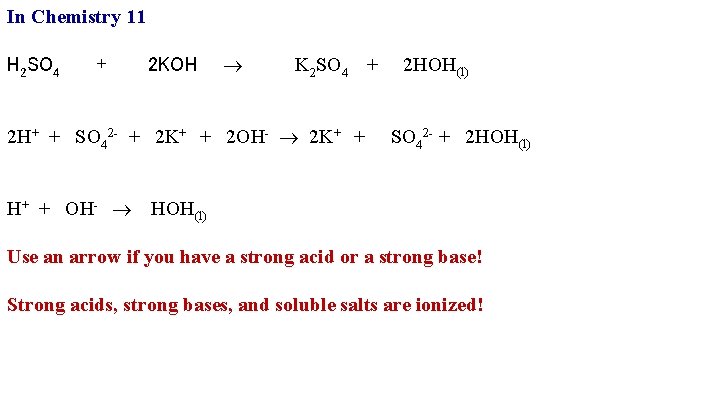
In Chemistry 11 H 2 SO 4 + 2 KOH K 2 SO 4 + 2 HOH(l) 2 H+ + SO 42 - + 2 K+ + 2 OH- 2 K+ + SO 42 - + 2 HOH(l) H+ + OH- HOH(l) Use an arrow if you have a strong acid or a strong base! Strong acids, strong bases, and soluble salts are ionized!
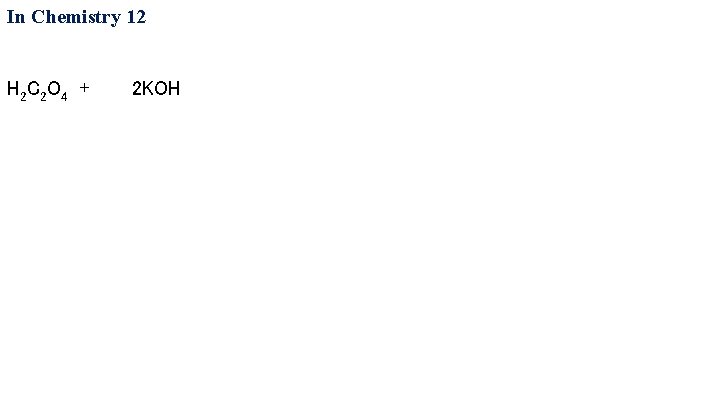
In Chemistry 12 H 2 C 2 O 4 + 2 KOH
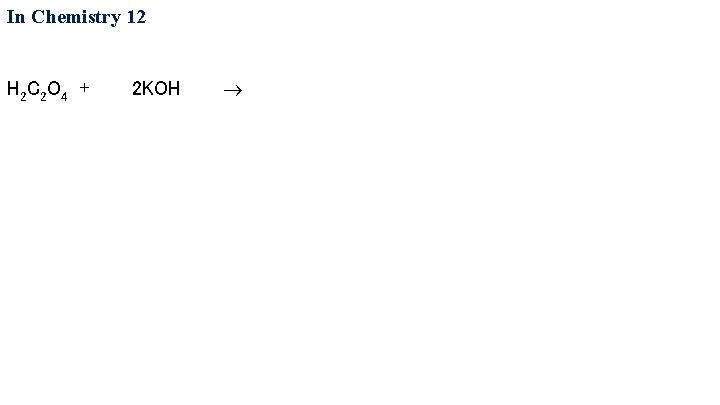
In Chemistry 12 H 2 C 2 O 4 + 2 KOH
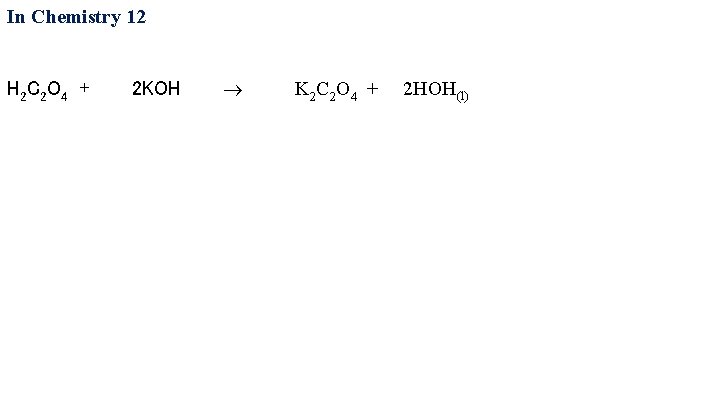
In Chemistry 12 H 2 C 2 O 4 + 2 KOH K 2 C 2 O 4 + 2 HOH(l)
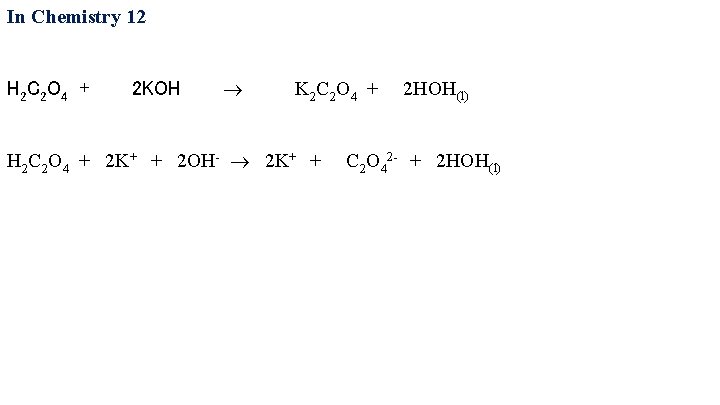
In Chemistry 12 H 2 C 2 O 4 + 2 KOH K 2 C 2 O 4 + 2 HOH(l) H 2 C 2 O 4 + 2 K+ + 2 OH- 2 K+ + C 2 O 42 - + 2 HOH(l)
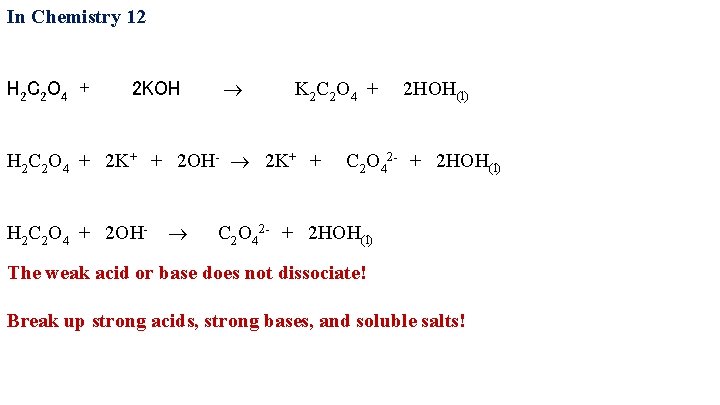
In Chemistry 12 H 2 C 2 O 4 + 2 KOH K 2 C 2 O 4 + 2 HOH(l) H 2 C 2 O 4 + 2 K+ + 2 OH- 2 K+ + C 2 O 42 - + 2 HOH(l) H 2 C 2 O 4 + 2 OH- C 2 O 42 - + 2 HOH(l) The weak acid or base does not dissociate! Break up strong acids, strong bases, and soluble salts!
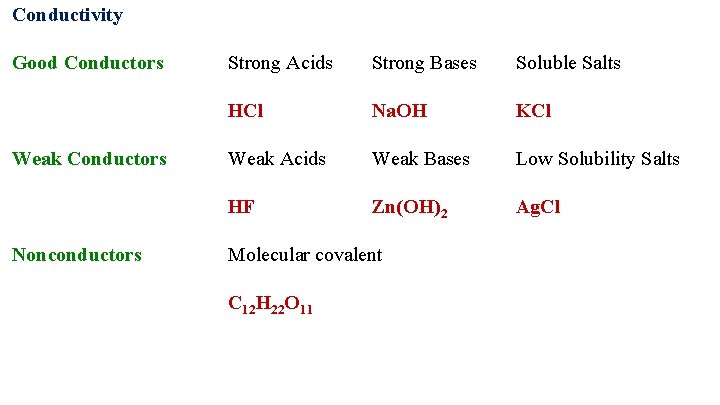
Conductivity Good Conductors Weak Conductors Nonconductors Strong Acids Strong Bases Soluble Salts HCl Na. OH KCl Weak Acids Weak Bases Low Solubility Salts HF Zn(OH)2 Ag. Cl Molecular covalent C 12 H 22 O 11
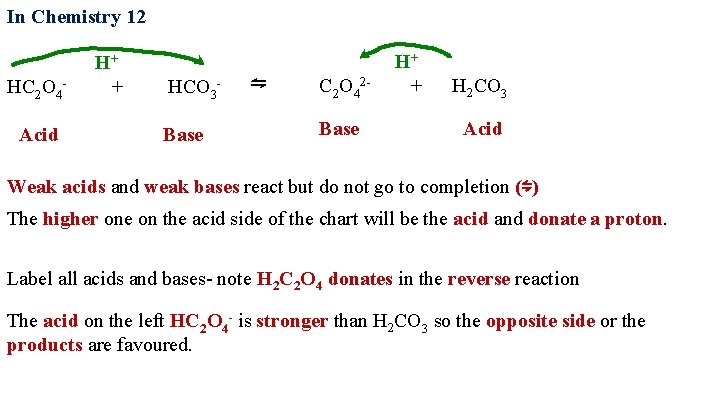
In Chemistry 12 H+ ⇋ HC 2 O 4 - + HCO 3 - Acid Base H+ C 2 O 42 - + H 2 CO 3 Base Acid Weak acids and weak bases react but do not go to completion (⇋) The higher one on the acid side of the chart will be the acid and donate a proton. Label all acids and bases- note H 2 C 2 O 4 donates in the reverse reaction The acid on the left HC 2 O 4 - is stronger than H 2 CO 3 so the opposite side or the products are favoured.

Opposite side Favoured Stronger acid Reactants ⇋ Products There are more products at equilibrium
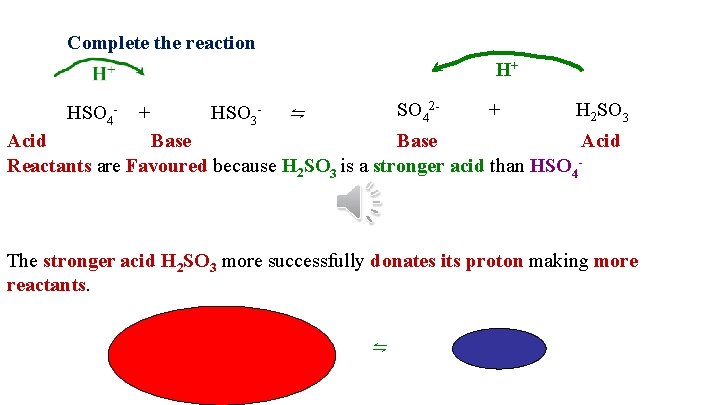
Complete the reaction H+ SO 42 - + H 2 SO 3 HSO 4 - + HSO 3 - ⇋ Acid Base Acid Reactants are Favoured because H 2 SO 3 is a stronger acid than HSO 4 - The stronger acid H 2 SO 3 more successfully donates its proton making more reactants. ⇋
- Slides: 27