Acids Bases Part 2 Neutralization Titrations Neutralization Reactions

Acids & Bases Part 2: Neutralization & Titrations
Neutralization Reactions Neutralization reactions occur when acids and bases react to form water and salt. The resulting products are neutral because there is no excess of H+ or OH- ions. Acid + Base → Water + Salt HCl(aq) + Na. OH(aq) → H 2 O(l) + Na. Cl(aq) Net ionic equation: H+ (aq) + OH- (aq) → H 2 O (l)
Neutralization Reactions Continued Another type of neutralization reaction is when an acid reacts with a carbonate compound. Acid + Carbonate → Salt + H 2 O + CO 2 HCl (aq) + Mg. CO 3 (aq) → Mg. Cl 2 (aq) + H 2 O(l) + CO 2 (g)
Neutralization Reaction Practice Write the complete chemical equations for the following acid base reactions: 1. Hydrobromic acid reacts with potassium hydroxide 2. Sulfuric acid reacts with lithium hydroxide 3. Chloric acid (HCl. O 3) reacts with potassium carbonate 4. Phosphoric acid reacts with sodium hydroxide

Neutralization Reaction Practice Answers 1. HBr (aq) + KOH (aq) → H 2 O (l) + KBr (aq) 2. H 2 SO 4 (aq) + 2 Li. OH → 2 H 2 O (l) + Li 2 SO 4 (aq) 3. HCl. O 3 (aq) + K 2 CO 3 (aq) → 2 KCl. O 3 (aq) + H 2 O (l) + CO 2 (g) 4. H 3 PO 4 (aq) + 3 Na. OH (aq) → 3 H 2 O (l) + Na 3 PO 4 (aq)
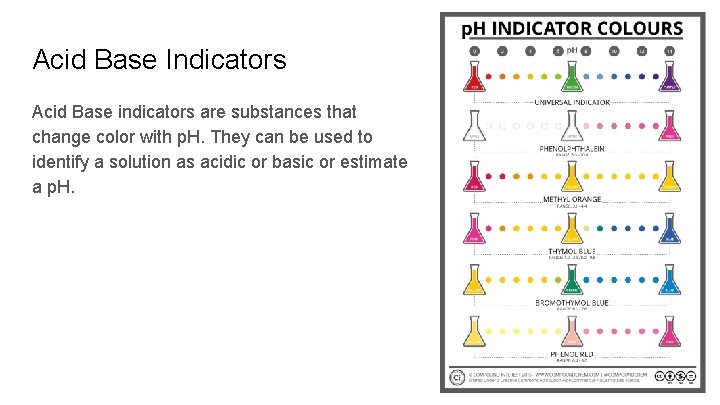
Acid Base Indicators Acid Base indicators are substances that change color with p. H. They can be used to identify a solution as acidic or basic or estimate a p. H.
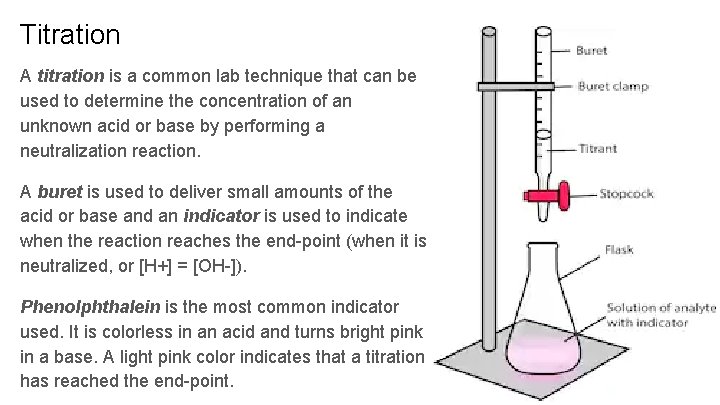
Titration A titration is a common lab technique that can be used to determine the concentration of an unknown acid or base by performing a neutralization reaction. A buret is used to deliver small amounts of the acid or base and an indicator is used to indicate when the reaction reaches the end-point (when it is neutralized, or [H+] = [OH-]). Phenolphthalein is the most common indicator used. It is colorless in an acid and turns bright pink in a base. A light pink color indicates that a titration has reached the end-point.

Titration General Procedure to Determine Concentration of an Acid 1) Use a volumetric pipet to add acid to an Erlenmeyer flask 2) Add a few drops of indicator to the flask 3) Fill the buret with a solution of base of known concentration and determine the starting volume 4) Add base to acid slowly while swirling the flask 5) Stop adding base when the indicator just changes color (the end-point). Determine the end volume of base. Here is a more detailed video showing the technique
Titration Calculations for Determining Concentration of an Acid The key with titration calculations is remembering that at the end-point the solution is neutral and the moles of H+ = moles of OH● Use the molarity of the base and the volume used in the buret to calculate the moles of base. (Mbase=molbase/Lbase) ● Use the dissociation equation for the base to determine the moles of OH● At the end-point, moles OH- = moles of H+ ● Using the moles of H+, determine the moles of acid using the dissociation equation for the acid. ● Lastly, use the volume of acid originally used in the flask to calculate the Molarity. (Macid=molacid/Lacid)
Titration Calculation Example You do a titration using 25. 00 m. L of an unknown concentration of H 2 SO 4. It takes 17. 85 m. L of 0. 1500 M Na. OH to reach the end-point. What is the concentration of the acid? Mbase=molbase/Lbase 17. 85 m. L * (1 L/1000 m. L) = 0. 01785 L 0. 1500 M = mol. Na. OH/0. 01785 L =0. 002678 mol Na. OH * (1 mol OH-/1 mol Na. OH) = 0. 002678 mol OHAt the endpoint, mol OH- = mol H+ =0. 002678 mol H+ * (1 mol H 2 SO 4/2 mol H+) = 0. 001339 mol H 2 SO 4 Macid = 0. 001339 mol/0. 02500 L Macid = 0. 05355 M (because H 2 SO 4 dissociates into 2 H+)

Titration Calculation Practice 1) A titration uses 28. 90 m. L of 0. 2500 M Na. OH to neutralize an acid. A. How many moles of OH- were used to neutralize the reaction? B. How many moles of H+ were in the flask? C. If the acid used was 15. 00 m. L of HCl, what is the concentration of the acid? 1) A titration uses 17. 25 m. L of 0. 100 M Na. OH to neutralize 25. 0 m. L of H 2 SO 4. What is the concentration of the acid?
Titration Calculation Practice Answers 1. A. =0. 007225 mols Na. OH = 0. 007225 mols OH- since Na. OH dissociates into 1 OH- ion B. Since at the end-point, moles OH- = mols H+, there also 0. 007225 mols H+ C. 0. 007225 mols H+ = 0. 007225 mols HCl (since HCl dissociates into 1 H+), then use M=mol/L = 0. 4817 M HCl 1. = 0. 0345 M H 2 SO 4 Remember that H 2 SO 4 dissociates into 2 H+ so once you find the moles of OH(0. 001725 mol), which is also the moles of H+, you have to divide by 2 to get the moles of H 2 SO 4: 0. 0017225 mol H+ * (1 mol H 2 SO 4/2 mol H+). Use that value to find molarity.

At Home Experiment: Cabbage Juice Indicator Red cabbage juice can be used as an acid base indicator. It’s really easy to make and super satisfying to experiment with. You only need a small amount of red cabbage too, so find a good recipe to use up the rest! Steps: -Chop some red cabbage. -Put in a pot of boiling water and cook until the water turns purple. Enjoy the smell. -Strain out the cabbage and use the juice to test some household items. -Send a picture to your teacher and impress them with your pretty colors. Mrs. Jeffery taught Coriana all about acids and bases. Here was their experiment with cabbage juice! Coriana had fun mixing everything together at the end.
- Slides: 13