Acids Bases Indicators An indicator is an organic

Acids & Bases
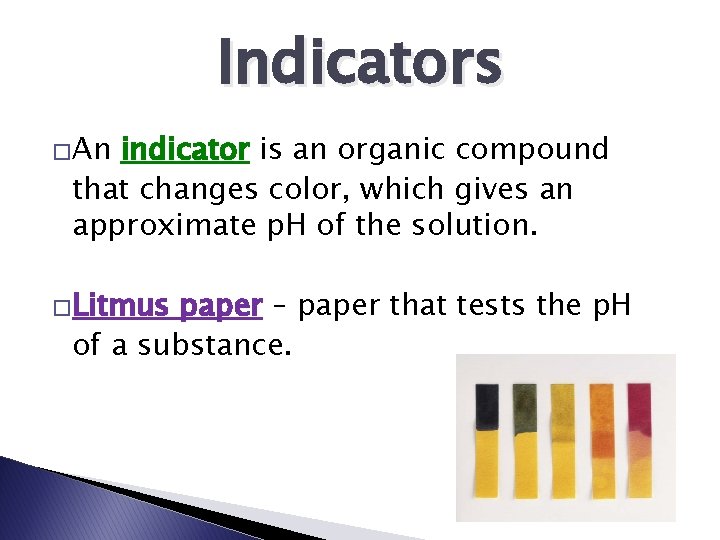
Indicators �An indicator is an organic compound that changes color, which gives an approximate p. H of the solution. �Litmus paper – paper that tests the p. H of a substance.
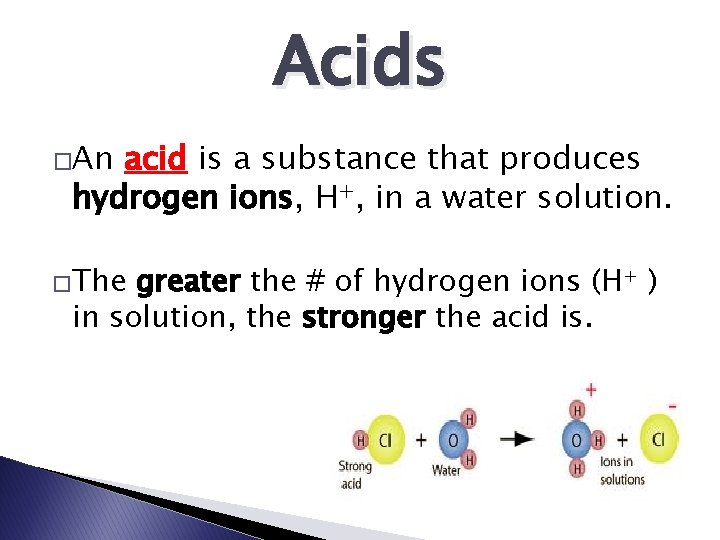
Acids �An acid is a substance that produces hydrogen ions, H+, in a water solution. �The greater the # of hydrogen ions (H+ ) in solution, the stronger the acid is.

Acids �Acids have a p. H value less than p. H 7. �Acids turn blue litmus paper red.

Properties of an Acid �Acids taste sour. �Acids are corrosive to metals. �They become less acidic when mixed with bases. The green areas are where you would taste sour foods such as a lemon.

Common Acids � Sulfuric Acid- H 2 SO 4 (most widely produced industrial chemical) � Carbonic � Boric Acid - H 2 CO 3 (found in soft drinks) Acid - H 3 BO 3 (roach & ant killer) � Hydrochloric � Nitric Acid- HCl (found in the stomach) Acid- HNO 3 (used in fertilizers)

Bases �Any substance that forms hydroxide ions, OH , in a water solution is a base. �The greater the # of hydroxide ions (OH-) in solution, the stronger the base is.
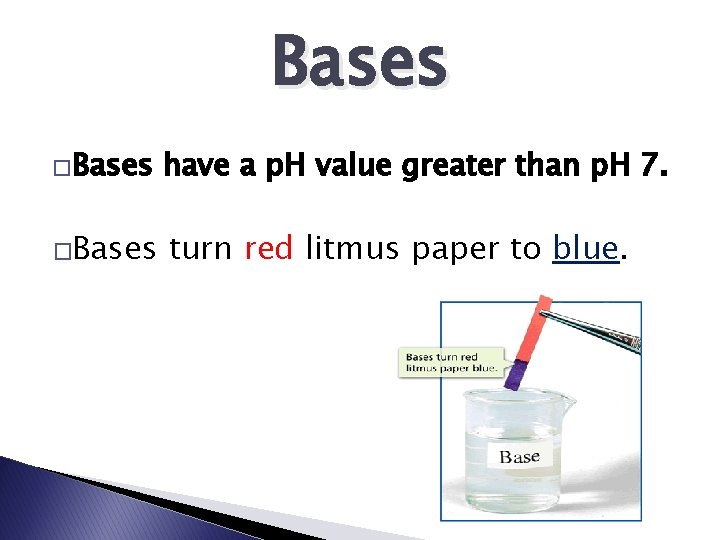
Bases �Bases have a p. H value greater than p. H 7. �Bases turn red litmus paper to blue.

Properties of a Base �Bases feel slippery, like soapy water. �Bases taste bitter. �They become less basic when mixed with acids. The green area is where you would taste bitter foods such as baking soda.

Common Bases � Sodium Hydroxide – Na. OH (called lye, used in drain cleaning products) � Potassium batteries) � Calcium Hydroxide – KOH (used in alkaline Hydroxide - Ca(OH)2 (used in cement) � Ammonia- NH 3 (common household cleaner) *Ammonia is a base that does not contain –OH.
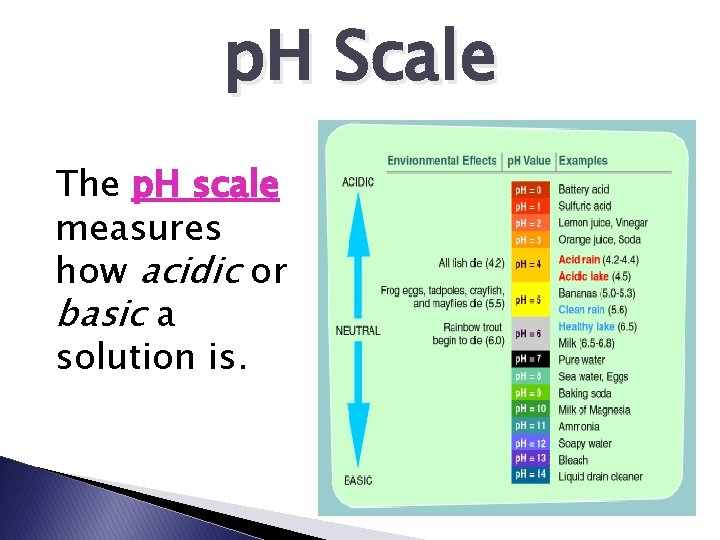
p. H Scale The p. H scale measures how acidic or basic a solution is.
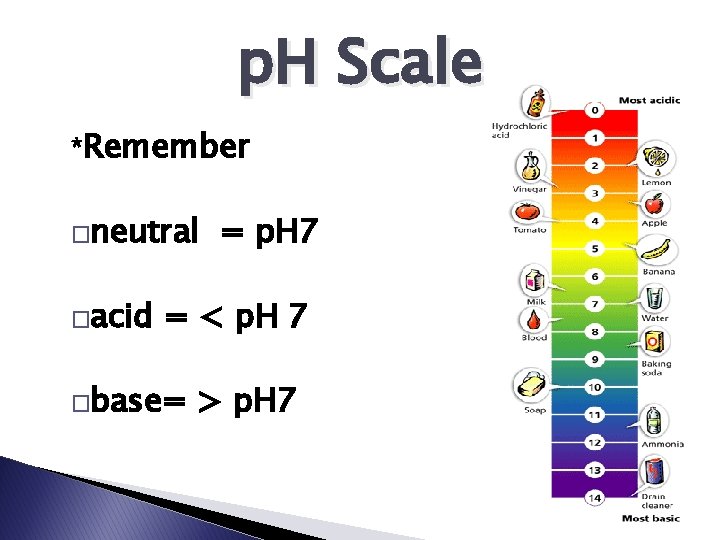
p. H Scale *Remember �neutral �acid = p. H 7 = < p. H 7 �base= > p. H 7

� Mystery Pitcher � http: //www. hometrainingtools. com/chemistry-ph- newsletter/a/1767/
- Slides: 13