Acids Bases Honors Chemistry Mrs Partridge Definition of
Acids & Bases Honors Chemistry Mrs. Partridge

Definition of p. H • p. H comes from the French word “pouvior hydrogene” which means hydrogen POWER! • So therefore, p. H is a measure of the hydrogen concentration in solution

Properties of Acids q Acids taste sour q Acids effect indicators q Blue litmus turns red q Methyl orange turns red q Acids have a p. H lower than 7 q Acids are proton (hydrogen ion, H+) donors q Acids react with certain active metals, produce H 2 (g) and an ionic compound q Aqueous solutions are electrolytes – can conduct electricity q Acids react with carbonates q Acids neutralize bases
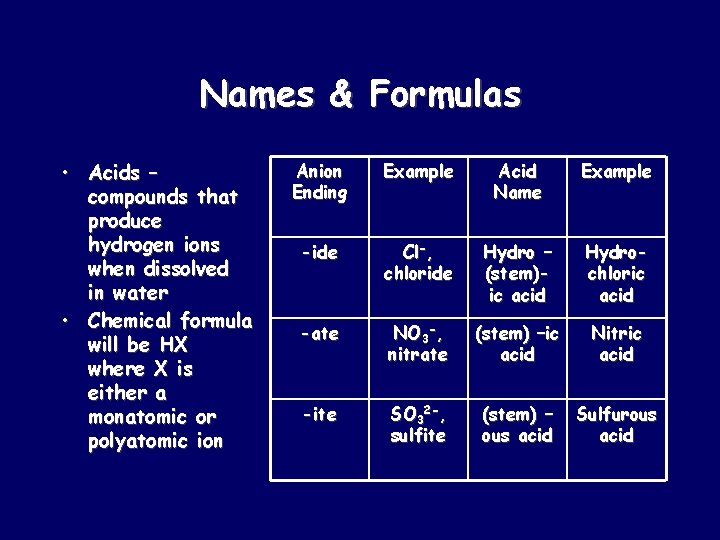
Names & Formulas • Acids – compounds that produce hydrogen ions when dissolved in water • Chemical formula will be HX where X is either a monatomic or polyatomic ion Anion Ending Example Acid Name Example -ide Cl-, chloride Hydro – (stem)ic acid Hydrochloric acid -ate NO 3 -, nitrate (stem) –ic acid Nitric acid -ite SO 32 -, sulfite (stem) – ous acid Sulfurous acid

Acids you must know: Strong Acids Weak Acids Sulfuric acid, H 2 SO 4 Phosphoric acid, H 3 PO 4 Hydrochloric acid, HCl Acetic acid, HC 2 H 3 O 2 Nitric acid, HNO 3

Sulfuric Acid q Highest volume production of any chemical q Used in in the U. S. the production of paper production of fertilizers petroleum refining

Nitric Acid • Used in the production of fertilizers • Used in the production of explosives • Nitric acid is a volatile acid – its reactive components evaporate easily • Stains proteins (including skin!)

Hydrochloric Acid • Used in the pickling of steel • Used to purify magnesium from sea water • Part of gastric juice, it aids in the digestion of protein • Sold commercially as “Muriatic acid”

Phosphoric Acid o A flavoring agent in sodas o Used in the manufacture of detergents o Used in the manufacture of fertilizers o Not a common laboratory reagent

Acetic Acid v Used in the manufacture of plastics v Used in making pharmaceuticals v Acetic acid is the acid present in vinegar
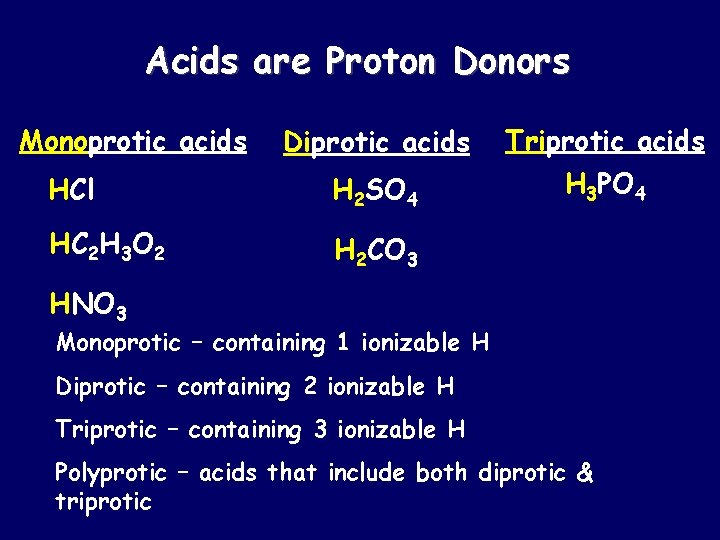
Acids are Proton Donors Monoprotic acids Diprotic acids Triprotic acids HCl H 2 SO 4 H 3 PO 4 HC 2 H 3 O 2 H 2 CO 3 HNO 3 Monoprotic – containing 1 ionizable H Diprotic – containing 2 ionizable H Triprotic – containing 3 ionizable H Polyprotic – acids that include both diprotic & triprotic
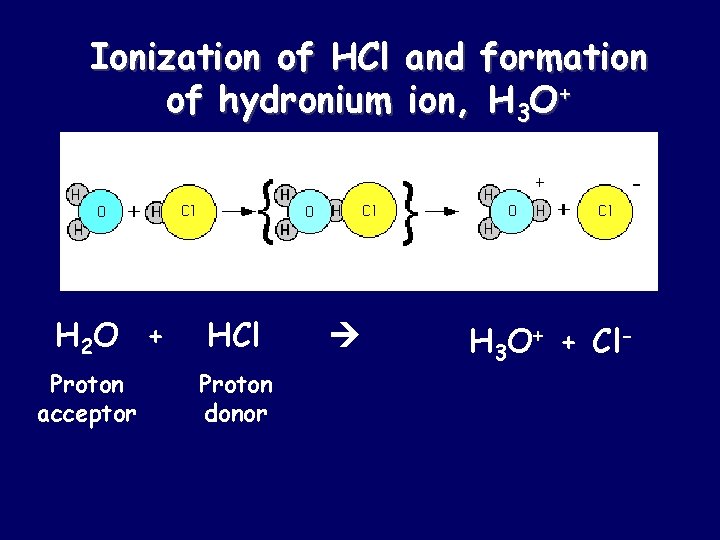
Ionization of HCl and formation of hydronium ion, H 3 O+ H 2 O + Proton acceptor HCl Proton donor H 3 O+ + Cl-

Strong Acids vs. Weak Acids Strong acids are assumed to be 100% ionized in solution (good proton donors). HCl H 2 SO 4 HNO 3 Weak acids are usually less than 5% ionized in solution (poor proton donors). H 3 PO 4 HC 2 H 3 O 2 Organic acids
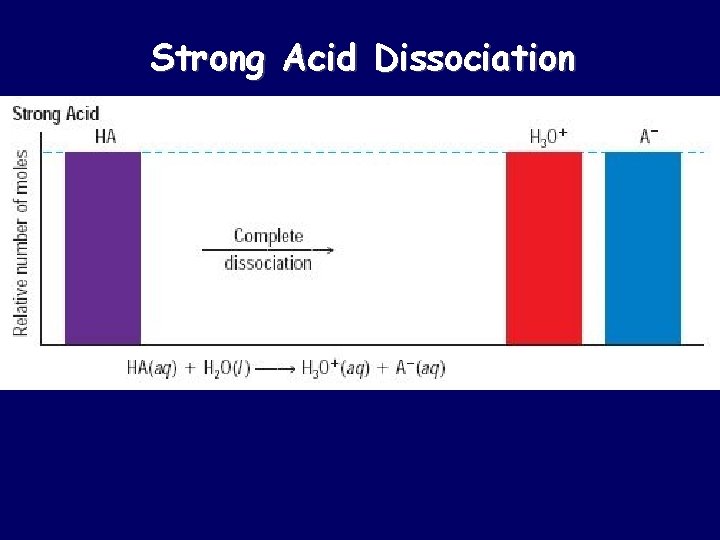
Strong Acid Dissociation
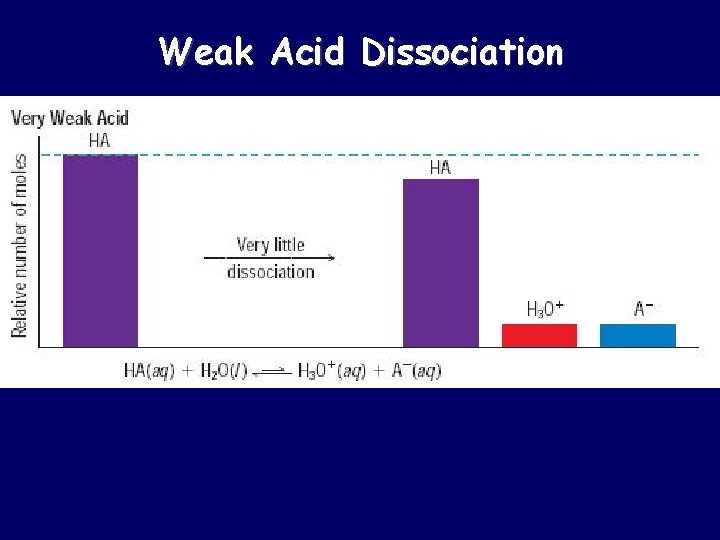
Weak Acid Dissociation
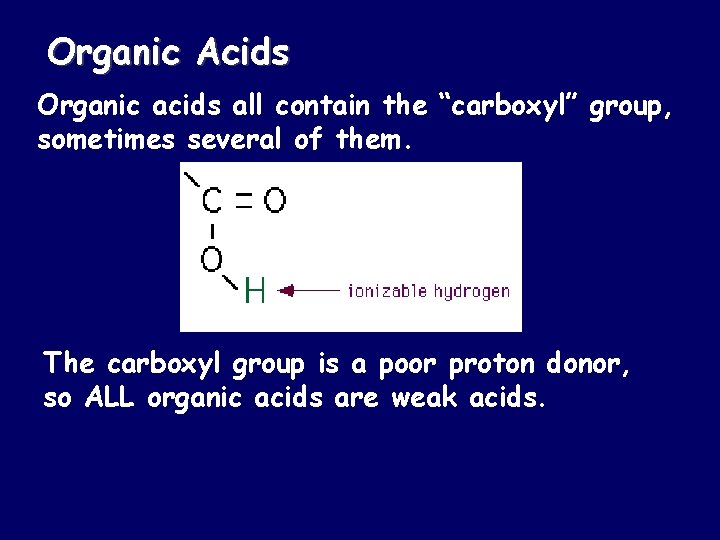
Organic Acids Organic acids all contain the “carboxyl” group, sometimes several of them. The carboxyl group is a poor proton donor, so ALL organic acids are weak acids.

Examples of Organic Acids Citric acid in citrus fruit Malic acid in sour apples Deoxyribonucleic acid, DNA Amino acids, the building blocks of protein q Lactic acid in sour milk and sore muscles q Butyric acid in rancid butter q q

Acids Effect Indicators Blue litmus paper turns red in contact with an acid.
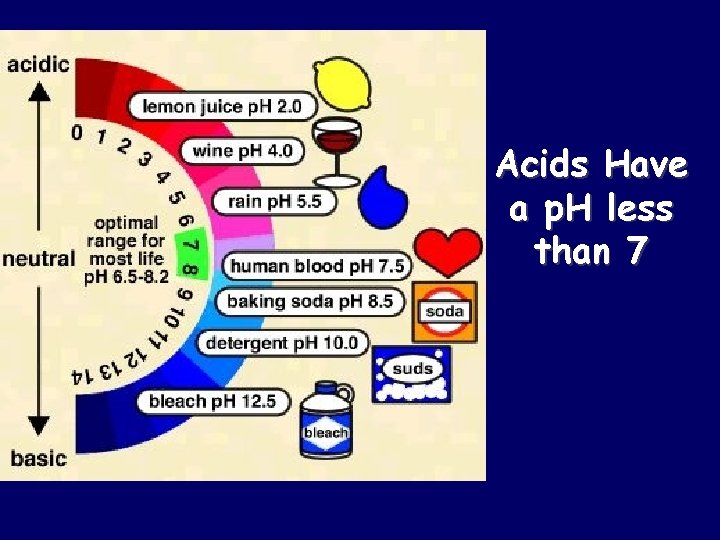
Acids Have a p. H less than 7

Acids React with Active Metals Acids react with active metals to form salts and hydrogen gas. Mg + 2 HCl Mg. Cl 2 + H 2(g)
Acids React with Carbonates 2 HC 2 H 3 O 2 + Na 2 CO 3 2 Na. C 2 H 3 O 2 + H 2 O + CO 2
Products of Neutralization HCl + Na. OH Na. Cl + H 2 O H 2 SO 4 + Ca(OH)2 Ca. SO 4 + 2 H 2 O HNO 3 + KOH KNO 3 + H 2 O The products of neutralization are always salt and _______. water a ______
Acids Neutralize Bases HCl + Na. OH Na. Cl + H 2 O Neutralization reactions ALWAYS produce a salt and water.

Effects of Acid Rain on Marble (calcium carbonate) George Washington: BEFORE George Washington: AFTER

Properties of Bases q Bases taste bitter q Bases effect indicators q Red litmus turns blue q Phenolphthalein turns purple q Bases have a p. H greater than 7 q Bases are proton (hydrogen ion, H+) acceptors q Solutions of bases feel slippery q Bases neutralize acids
Examples of Bases Ø Ø Sodium hydroxide (lye), Na. OH Potassium hydroxide, KOH Magnesium hydroxide, Mg(OH)2 Calcium hydroxide (lime), Ca(OH)2
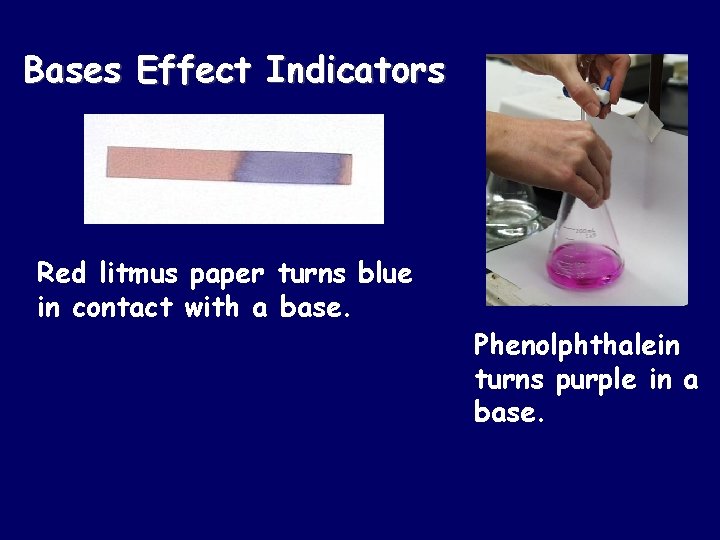
Bases Effect Indicators Red litmus paper turns blue in contact with a base. Phenolphthalein turns purple in a base.
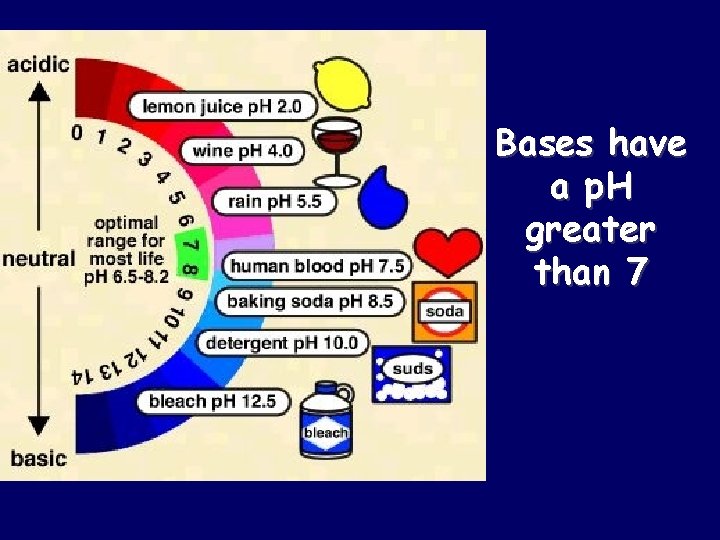
Bases have a p. H greater than 7
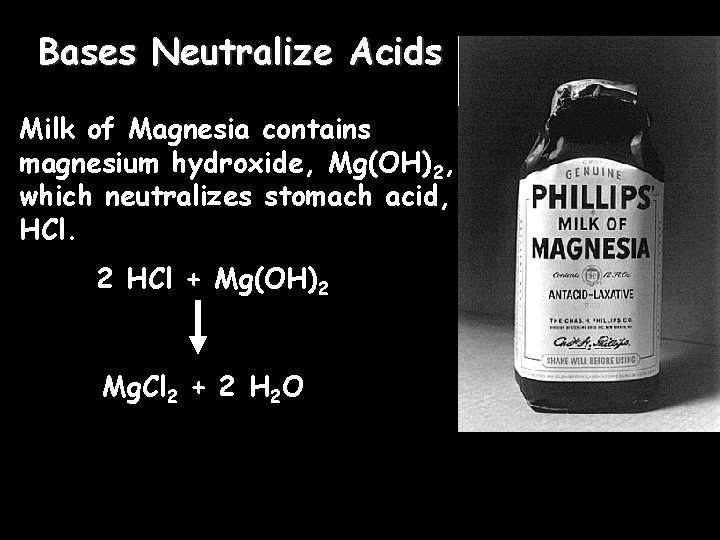
Bases Neutralize Acids Milk of Magnesia contains magnesium hydroxide, Mg(OH)2, which neutralizes stomach acid, HCl. 2 HCl + Mg(OH)2 Mg. Cl 2 + 2 H 2 O
![Inequalities • An acid solution is one in which [H+] is GREATER THAN the Inequalities • An acid solution is one in which [H+] is GREATER THAN the](http://slidetodoc.com/presentation_image_h2/2f8bf4d88de77887b19feb52d084745f/image-30.jpg)
Inequalities • An acid solution is one in which [H+] is GREATER THAN the [OH-] • A basic solution is one in which the [OH-] is greater than [H+] • Basic solutions are also known as alkaline solutions
![Neutral Solutions • • • The [OH-] is equal to the [H+] p. H Neutral Solutions • • • The [OH-] is equal to the [H+] p. H](http://slidetodoc.com/presentation_image_h2/2f8bf4d88de77887b19feb52d084745f/image-31.jpg)
Neutral Solutions • • • The [OH-] is equal to the [H+] p. H = 7 Neutralization: – An acid and base react in an aqueous solutions to produce a salt and water – Double Replacement Reaction – HCl + Na. OH Na. Cl + HOH (H 2 O) – One mole of H+ reacts with one mole of OH-. This stoichiometric ratio means that one mole of acid will neutralize one mole of base

Amphoteric Substances that can act as either an acid or a base Example: water

Acidic and Basic Anhydrides • The term anhydrous means without water • An acidic anhydride is an acid which has had the water removed – Example: HNO 3 N 2 O 5 • A basic anhydride is a base which has had the water removed – Example: Li. OH Li 2 O
Concentrated vs Dilute • Molarity is often used to express the concentration of an acid or base • Molarity (M) = moles (mol) / volume in liters (v) • Note: strong acids and weaks acids have the capability of being concentrated or dilute!
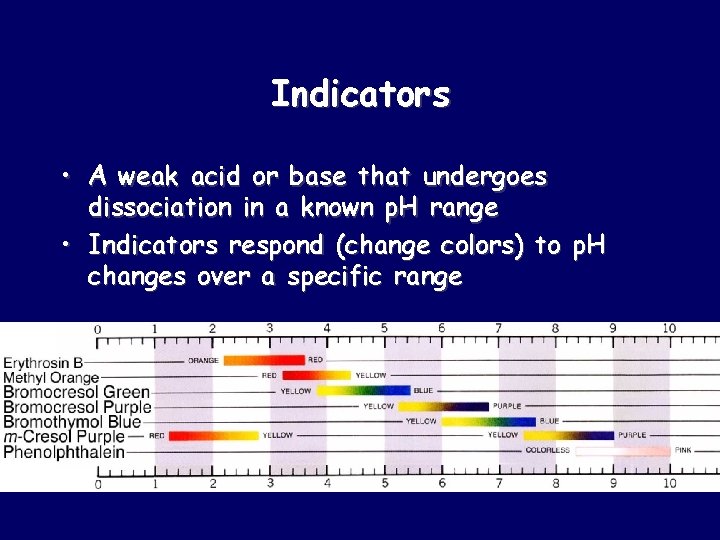
Indicators • A weak acid or base that undergoes dissociation in a known p. H range • Indicators respond (change colors) to p. H changes over a specific range
Net Ionic Equations for Acids and Bases When writing the net ionic equation for a neutralization reaction, strong acids and bases are broken up into their ions, while weak acids and bases are kept as a complete molecule.
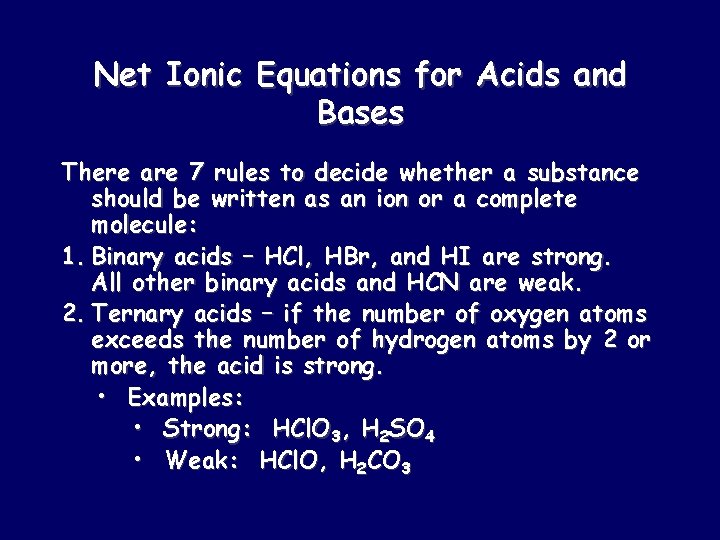
Net Ionic Equations for Acids and Bases There are 7 rules to decide whether a substance should be written as an ion or a complete molecule: 1. Binary acids – HCl, HBr, and HI are strong. All other binary acids and HCN are weak. 2. Ternary acids – if the number of oxygen atoms exceeds the number of hydrogen atoms by 2 or more, the acid is strong. • Examples: • Strong: HCl. O 3, H 2 SO 4 • Weak: HCl. O, H 2 CO 3
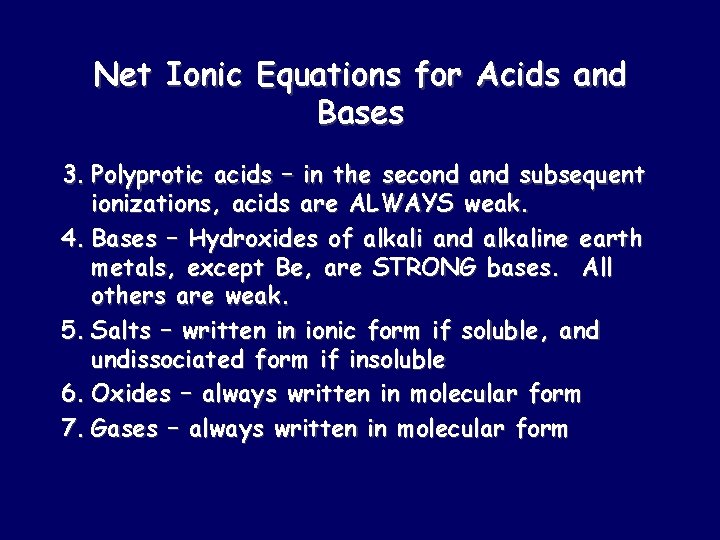
Net Ionic Equations for Acids and Bases 3. Polyprotic acids – in the second and subsequent ionizations, acids are ALWAYS weak. 4. Bases – Hydroxides of alkali and alkaline earth metals, except Be, are STRONG bases. All others are weak. 5. Salts – written in ionic form if soluble, and undissociated form if insoluble 6. Oxides – always written in molecular form 7. Gases – always written in molecular form
![p. H Concept • Expressing [H+] in molarity is cumbersome, so Sorensen proposed the p. H Concept • Expressing [H+] in molarity is cumbersome, so Sorensen proposed the](http://slidetodoc.com/presentation_image_h2/2f8bf4d88de77887b19feb52d084745f/image-40.jpg)
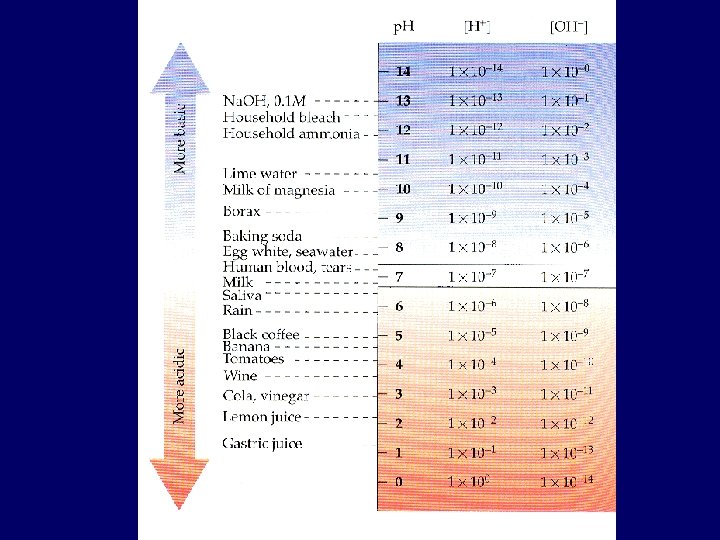
p. H Concept • Expressing [H+] in molarity is cumbersome, so Sorensen proposed the p. H scale • Range: 0 – 14

Calculating p. H • • • p. H is the negative logarithm of the [H+] p. H = -log[H+] p. OH is the negative logarithm of the [OH-]. p. OH = -log [OH-] Neutral solution has a p. OH of 7 and a p. H of 7 • For all solutions, p. OH + p. H = 14
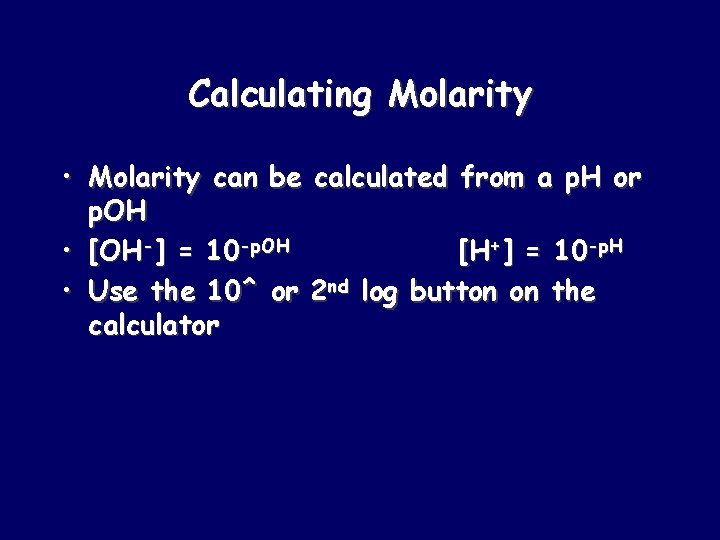
Calculating Molarity • Molarity can be calculated from a p. H or p. OH • [OH-] = 10 -p. OH [H+] = 10 -p. H • Use the 10^ or 2 nd log button on the calculator
Equivalents • An equivalent is the amount of acid (or base) that will give one mole of H+ (or OH-) – 1 mole of HCl produces 1 mole of H+ – 1 mole of H 2 SO 4 produces 2 moles of H+
Normality (N) • Concentration expressed as the number of equivalents of H+ or OH- times the molarity of the solution • N = (eq)M • Example: – 0. 2 M H 2 SO 4 0. 4 N H 2 SO 4 – 0. 15 M H 3 PO 4 0. 45 N H 3 PO 4
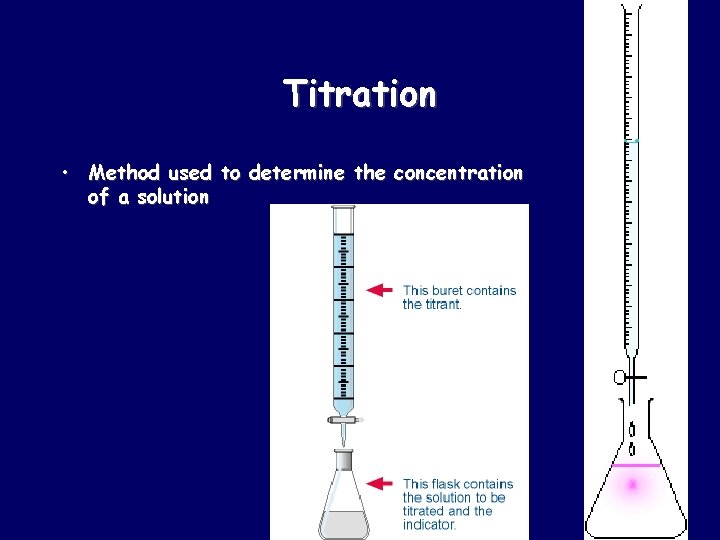
Titration • Method used to determine the concentration of a solution
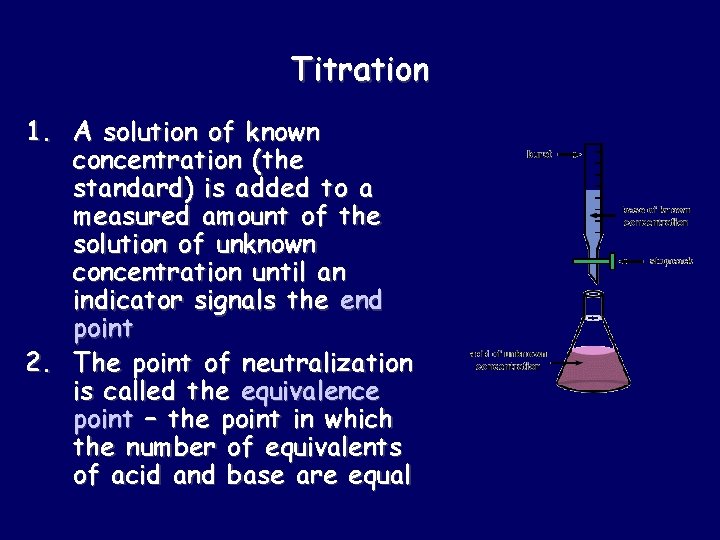
Titration 1. A solution of known concentration (the standard) is added to a measured amount of the solution of unknown concentration until an indicator signals the end point 2. The point of neutralization is called the equivalence point – the point in which the number of equivalents of acid and base are equal
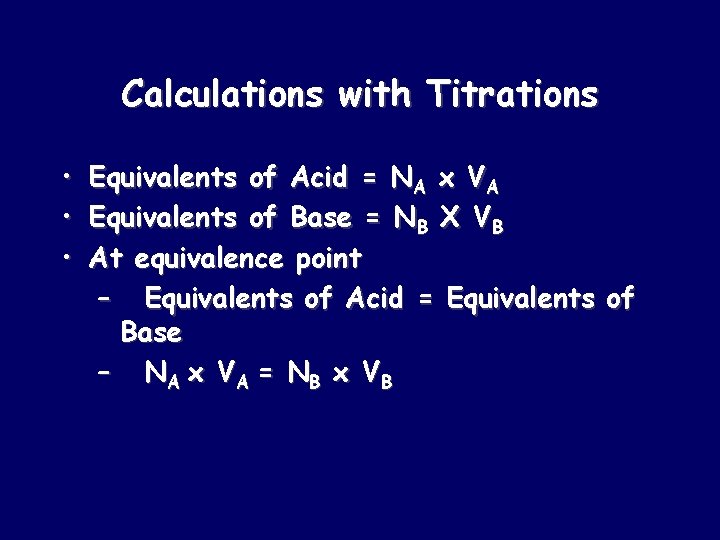
Calculations with Titrations • • • Equivalents of Acid = NA x VA Equivalents of Base = NB X VB At equivalence point – Equivalents of Acid = Equivalents of Base – NA x VA = NB x V B
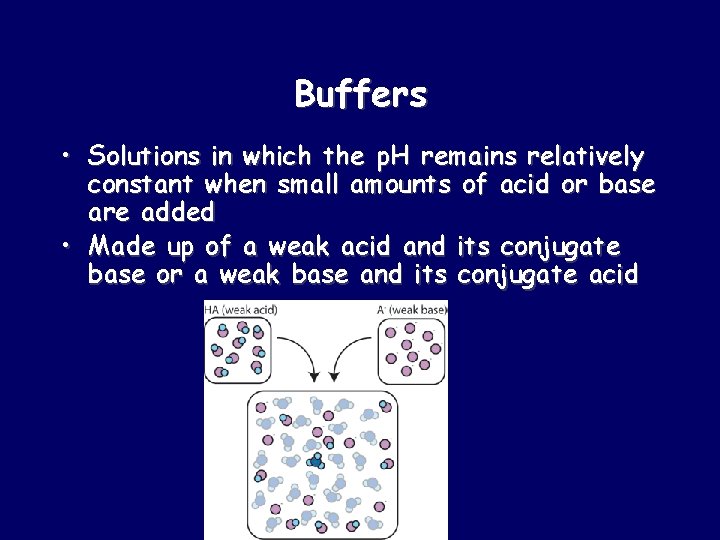
Buffers • Solutions in which the p. H remains relatively constant when small amounts of acid or base are added • Made up of a weak acid and its conjugate base or a weak base and its conjugate acid

Buffers • Buffer capacity is the amount of acid or base that can be added to a buffer solution before a significant change in p. H occurs • 2 buffer systems are found in blood and are crucial in maintaining the p. H of the body

- Slides: 52