Acids Bases Chapter 21 Properties of Acids Bases

Acids & Bases Chapter 21

Properties of Acids & Bases l Acids l Taste sour l React with carbonates & bicarbonates to make CO 2 l React with metals to make H 2 gas (acids corrode metals) l Turn litmus paper red

Properties of Acids & Bases l Taste bitter l Feel slippery (emulsify fats & proteins) l Turn litmus paper blue l React with acids l Both Acids & Bases: l Are electrolytes & conduct electricity l Neutralize each other, forming salt & water
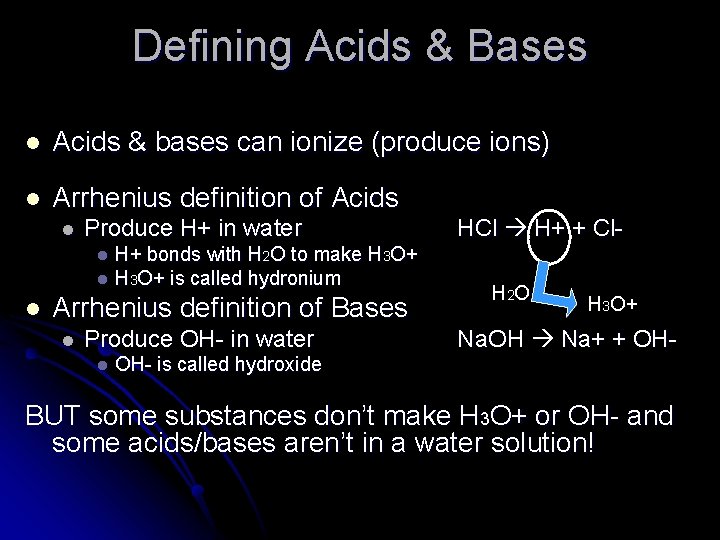
Defining Acids & Bases l Acids & bases can ionize (produce ions) l Arrhenius definition of Acids l Produce H+ in water H+ bonds with H 2 O to make H 3 O+ l H 3 O+ is called hydronium HCl H+ + Cl- l l Arrhenius definition of Bases l Produce OH- in water l H 2 O H 3 O+ Na. OH Na+ + OH- is called hydroxide BUT some substances don’t make H 3 O+ or OH- and some acids/bases aren’t in a water solution!
Defining Acids & Bases l Bronsted-Lowry definition of Acids l Acids donate protons (H+) l May l not form hydronium Bronsted-Lowry definition of Bases l Bases accept protons l Don’t have to be dissolved in water l Some compounds can behave as either Whether some substances act as acids or bases depends on other reactants

l NH 3 accepts a proton to become NH 4+ l It is a Bronsted-Lowry base l This reaction doesn’t have to happen in water l It is not an Arrhenius base because it doesn’t form OH-
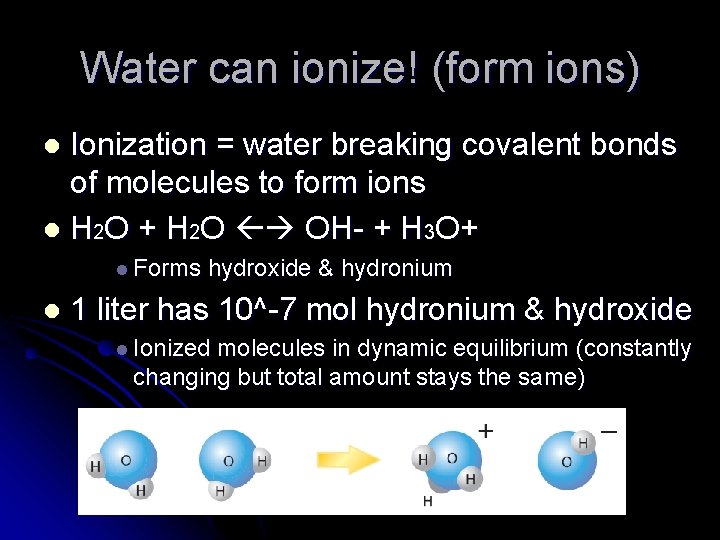
Water can ionize! (form ions) Ionization = water breaking covalent bonds of molecules to form ions l H 2 O + H 2 O OH- + H 3 O+ l l Forms l hydroxide & hydronium 1 liter has 10^-7 mol hydronium & hydroxide l Ionized molecules in dynamic equilibrium (constantly changing but total amount stays the same)

Homework: SRQ 21 A 1 -5

Acids can be described by how many H they have to give… l Monoprotic Acids l Only l Diprotic Acids l Can l donate one hydrogen (Ex: HCl) give two hydrogen Polyprotic Acids H H l Can donate more than one H l Ex: H 2 SO 4, H 2 CO 3, H 3 C 6 H 5 O 7 l. H that can be donated are called ionizable l Ionizable H written 1 st
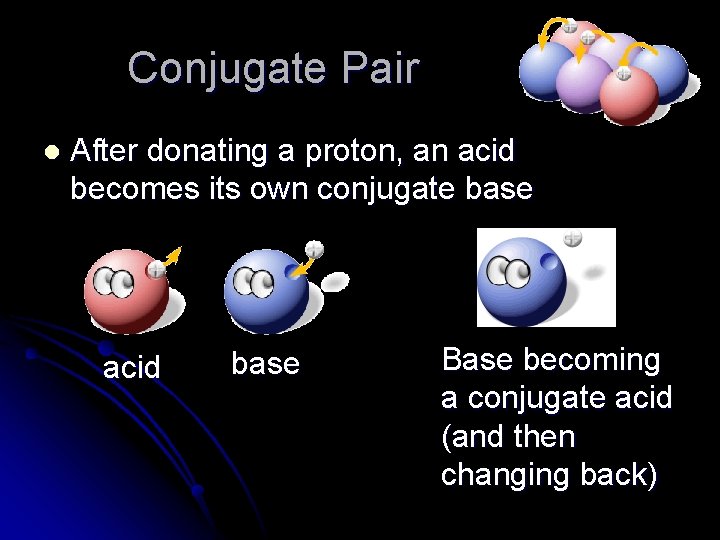
Conjugate Pair l After donating a proton, an acid becomes its own conjugate base acid base Base becoming a conjugate acid (and then changing back)
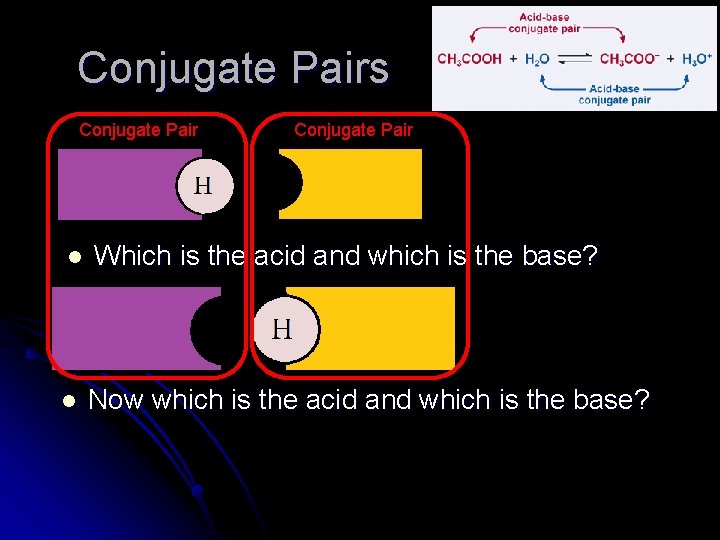
Conjugate Pairs Conjugate Pair l Which is the acid and which is the base? l Now which is the acid and which is the base?
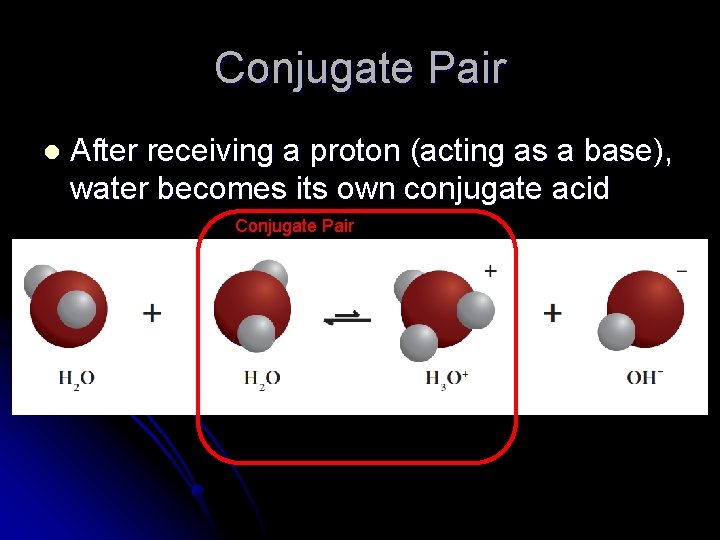
Conjugate Pair l After receiving a proton (acting as a base), water becomes its own conjugate acid Conjugate Pair

Homework: SRQ 21 A 7, 10, & 11 and CRQ 1 -3 & 5 -10
- Slides: 13