Acids Bases Ch 12 in text Acids Bases

Acids & Bases Ch 12 in text

Acids Bases Please Write 1. Tastes sour. 1. Taste bitter 2. Turns litmus red 2. Feel slippery 3. React with metals to produce H 2 gas 3. Turns litmus blue 4. Conduct electricity 5. p. H < 7 5. p. H > 7
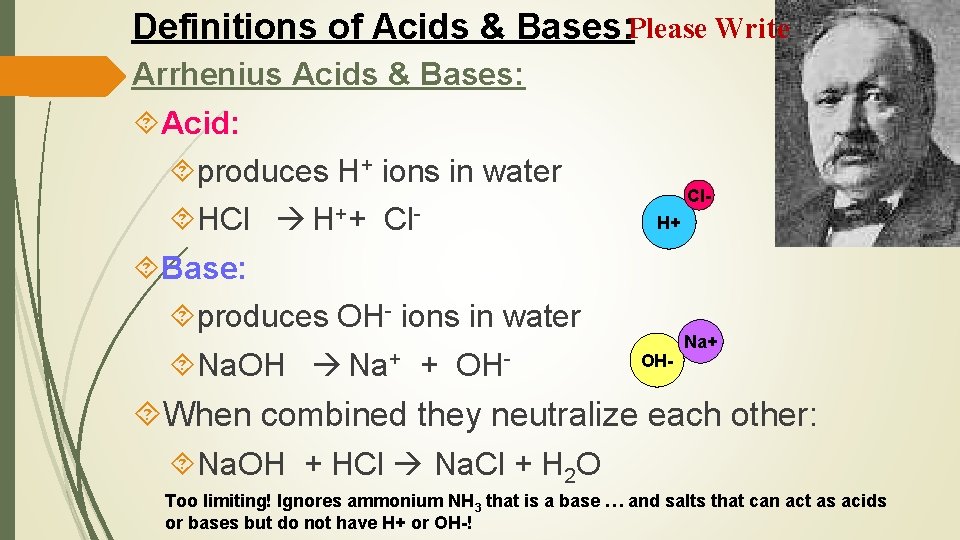
Definitions of Acids & Bases: Please Write Arrhenius Acids & Bases: Acid: produces H+ ions in water HCl H++ Cl Base: produces OH- ions in water Na. OH Na+ + OH- Cl. H+ OH- Na+ When combined they neutralize each other: Na. OH + HCl Na. Cl + H 2 O Too limiting! Ignores ammonium NH 3 that is a base … and salts that can act as acids or bases but do not have H+ or OH-!
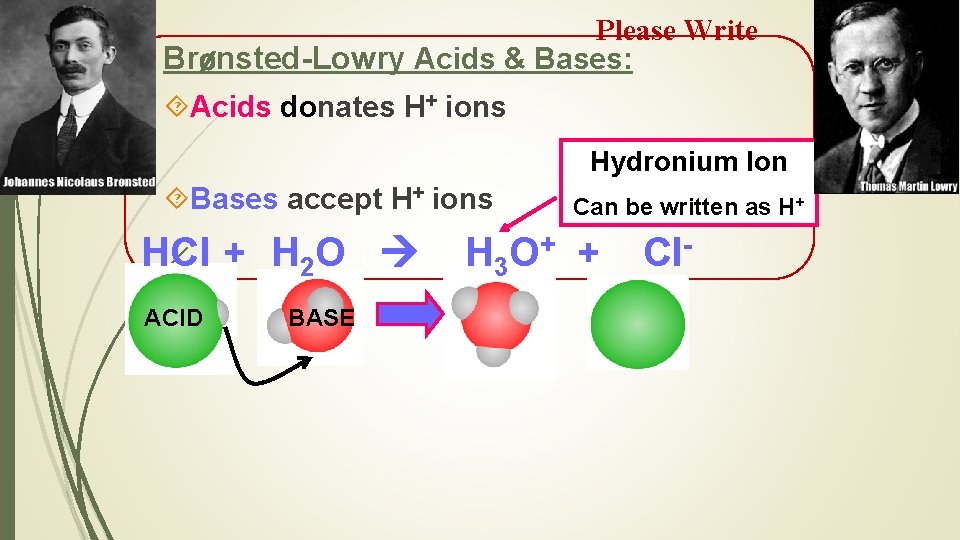
Please Write Brønsted-Lowry Acids & Bases: Acids donates H+ ions Hydronium Ion Bases accept H+ ions HCl + H 2 O ACID BASE Can be written as H+ H 3 O + + Cl-
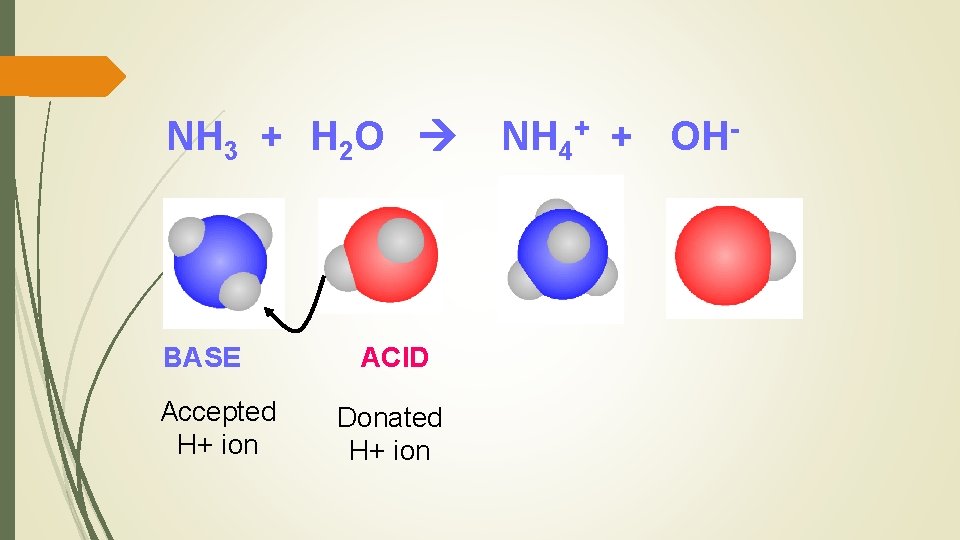
NH 3 + H 2 O BASE Accepted H+ ion ACID Donated H+ ion NH 4+ + OH-
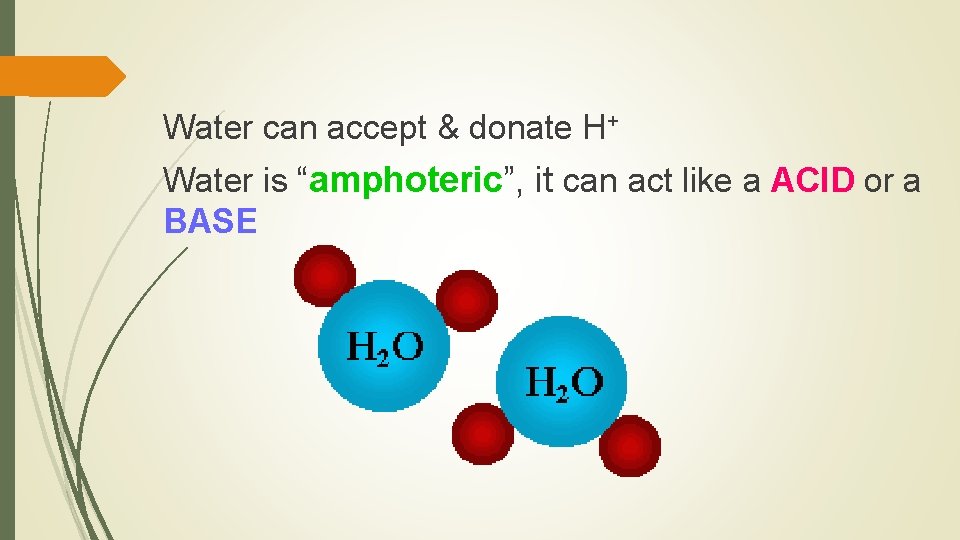
Water can accept & donate H+ Water is “amphoteric”, it can act like a ACID or a BASE

Please Write Each acid has a corresponding base conjugate. It is the particle that remains after the H+ is lost. …… base ……………acid conjugate. It is the particle that is formed after the H+ is gained.
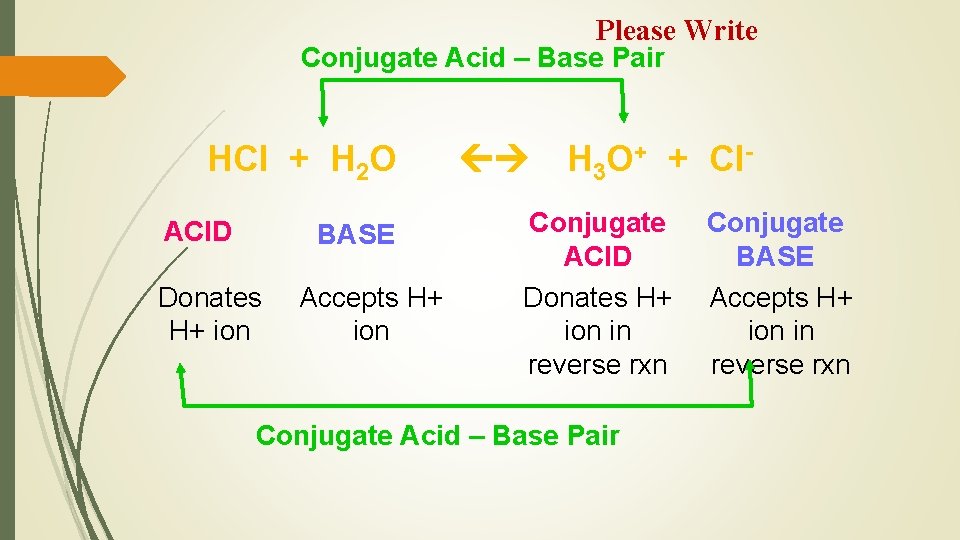
Please Write Conjugate Acid – Base Pair HCl + H 2 O ACID BASE Donates H+ ion Accepts H+ ion H 3 O+ + Cl- Conjugate ACID Donates H+ ion in reverse rxn Conjugate Acid – Base Pair Conjugate BASE Accepts H+ ion in reverse rxn
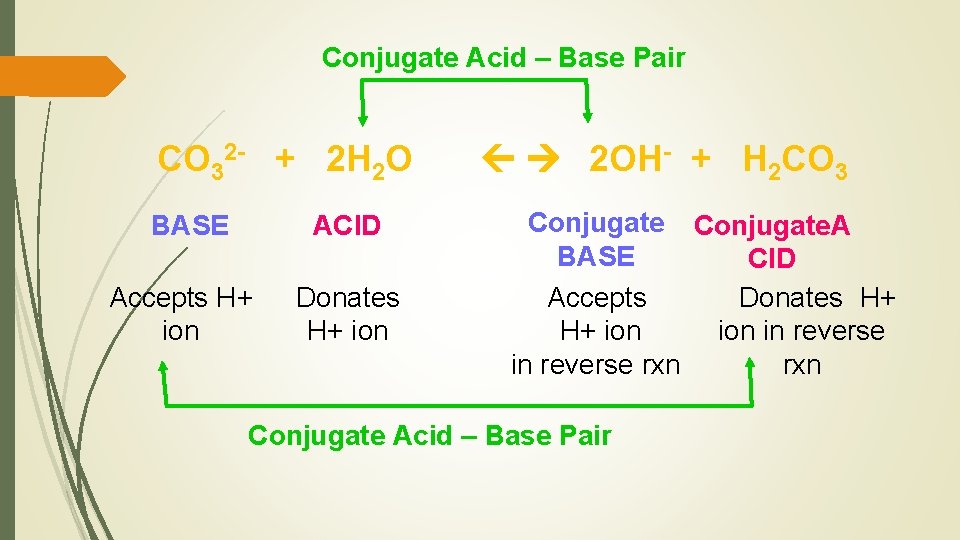
Conjugate Acid – Base Pair CO 32 - + 2 H 2 O BASE ACID Accepts H+ ion Donates H+ ion 2 OH- + H 2 CO 3 Conjugate. A BASE CID Accepts Donates H+ H+ ion in reverse rxn Conjugate Acid – Base Pair
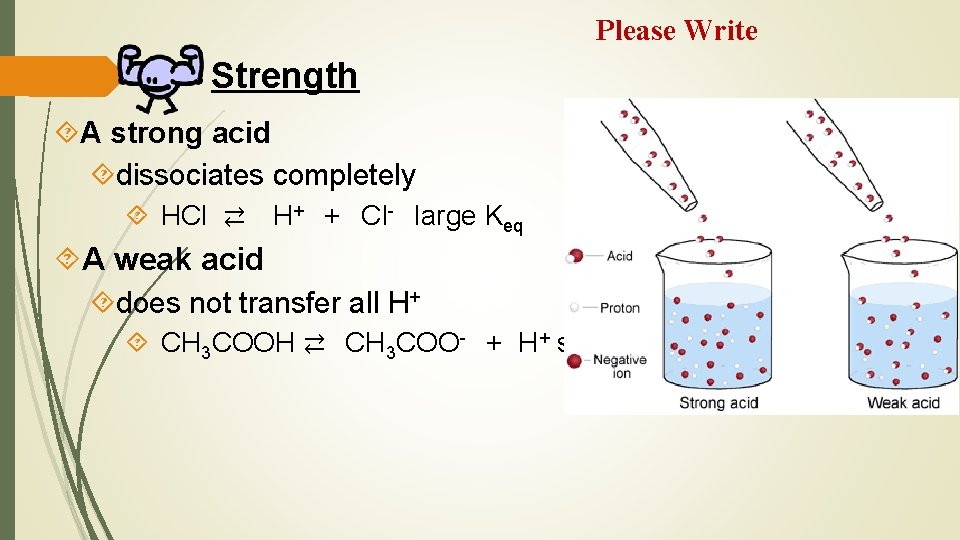
Please Write Strength A strong acid dissociates completely HCl ⇄ H+ + Cl- large Keq A weak acid does not transfer all H+ CH 3 COOH ⇄ CH 3 COO- + H+ small Keq

Please Write A strong base has a high affinity for H+ ions ex. Na. OH ⇄ Na+ + OH- large Keq (many OH- to accept H+) A weak base has a low affinity for H+ ions ex. NH 3 + H 2 O ⇄ NH 4+ + OH- small Keq

Kw: Please Write Water is also in a equilibrium rxn: H 2 O ⇄ H + + OH- = 1 x 10 -14 Few molecules are ionized. [H+]=[H 3 O+] when water is present 2 H 2 O + energy Can be simplified to H 3 O + + OH-

![At 25℃ Kw = [H+][OH-] 1. 0 x 10 -14 = [x][x] 1. At 25℃ Kw = [H+][OH-] 1. 0 x 10 -14 = [x][x] 1.](http://slidetodoc.com/presentation_image_h2/a7b5448cd0d601f1dfa1e261fe5ce774/image-14.jpg)
At 25℃ Kw = [H+][OH-] 1. 0 x 10 -14 = [x][x] 1. 0 x 10 -14 = [x]2 1. 0 x 10 -7 = [x] Please Write p. H = 7 [H+] = 1. 0 x 10 -7 M [OH-] = 1. 0 x 10 -7 M At other temperatures? H 2 O + E ⇄ H + + OH- At lower temperatures Kw will be lower. (shift left, less products) Higher temperatures Kw will be higher.
![If you add Na. OH to water. Practice [OH-]goes up. [H+]goes down. What If you add Na. OH to water. Practice [OH-]goes up. [H+]goes down. What](http://slidetodoc.com/presentation_image_h2/a7b5448cd0d601f1dfa1e261fe5ce774/image-15.jpg)
If you add Na. OH to water. Practice [OH-]goes up. [H+]goes down. What is the p. H of the solution if you add enough Na. OH such that [OH-]= 1 x 10 -5 M what is the p. H? Kw = [H+][OH-] 1. 0 x 10 -14 = [H+](1 x 10 -5 ) [H+]=1 x 10 -9 p. H = 9
![Please Write [H+] [OH-] p. H p. OH SA WA WATER WB 1 x Please Write [H+] [OH-] p. H p. OH SA WA WATER WB 1 x](http://slidetodoc.com/presentation_image_h2/a7b5448cd0d601f1dfa1e261fe5ce774/image-16.jpg)
Please Write [H+] [OH-] p. H p. OH SA WA WATER WB 1 x 10 -1 = 0. 1 M 1 x 10 -5 1 x 10 -7 1 x 10 -13 1 x 10 -9 5 1 x 10 -7 7 9 7 1 13 p. H 2 vs p. H 1 = 10 x stronger p. H + p. OH = 14 SB 1 x 10 -8 1 x 10 -6 1 x 10 -12 8 12 2 6 1 x 10 -2 [H+]=1 x 10 -p. H = - log[H+] p. OH = -log[OH-]
![1. Find the [H+] and [OH-] of the following p. H solutions without using 1. Find the [H+] and [OH-] of the following p. H solutions without using](http://slidetodoc.com/presentation_image_h2/a7b5448cd0d601f1dfa1e261fe5ce774/image-17.jpg)
1. Find the [H+] and [OH-] of the following p. H solutions without using a calculator: A) p. H=4 B) p. H=12 C) p. H=9 D) p. H=7 E) p. H=8 2. Find the p. H of the following solutions without a calculator: A) [H+]=1. 0 x 10 -8 mol/L B) [OH-]=1. 0 x 10 -3 mol/L C) [H+]=1. 0 x 10 -5 mol/L D) [OH-]=1. 0 x 10 -9 mol/L Practice 3. Find the [H+] of these solutions: [H+]=10 -p. H A) p. H=3. 7 B) p. H=9. 8 C) p. H=6. 2 D) p. H=4. 0 E) p. H=7. 1 4. Find the p. H of the following solutions: p. H= - log [H+]. A) [H+]=4. 5 x 10 -3 mol/L B) [H+]=3. 4 x 10 -8 mol/L C) [H+]=3. 0 x 10 -7 mol/L D) [H+]=2. 5 x 10 -2 mol/L
![Solutions to Problems 1 - 4 Check answe 1. Answers A) [H+]=1 x 10 Solutions to Problems 1 - 4 Check answe 1. Answers A) [H+]=1 x 10](http://slidetodoc.com/presentation_image_h2/a7b5448cd0d601f1dfa1e261fe5ce774/image-18.jpg)
Solutions to Problems 1 - 4 Check answe 1. Answers A) [H+]=1 x 10 -4, [OH-]=1 x 10 -10 mol/L B) [H+]=1 x 10 -12, [OH-]=1 x 10 -2 mol/L C) [H+]= 1 x 10 -9, [OH-]=1 x 10 -5 mol/L D) [H+]=1 x 10 -7, [OH-]=1 x 10 -7 mol/L E) [H+]=1 x 10 -8, [OH-]1 x 10 -6 mol/L 2. Answers: A) p. H=8 B) p. OH-=3 p. H= 11 C) p. H=5 D) p. OH-=9 p. H=5 3. Answers (in mol/L). remember: [H+]=10 -p. H A) 10 -3. 7=2. 0 x 10 -4 mol/L B) 10 -9. 8=1. 6 x 10 -10 mol/L C) 6. 3 x 10 -7 mol/L D) 1. 0 x 10 -4 mol/L E) 7. 9 x 10 -8 mol/L 4. Answers: p. H= - log [H+]. A) p. H= 2. 3 (-log(4. 5 x 10 -3)) B) p. H=7. 5 C) p. H=6. 5 D) p. H=1. 6
![Please Write What is the [H+] of black coffee at 25 o. C if Please Write What is the [H+] of black coffee at 25 o. C if](http://slidetodoc.com/presentation_image_h2/a7b5448cd0d601f1dfa1e261fe5ce774/image-19.jpg)
Please Write What is the [H+] of black coffee at 25 o. C if [OH -] is 1. 3 x 10 -9 M? What is the p. H? Kw = [H+][OH-] 1. 0 x 10 -14 = [H+] [1. 3 x 10 -9] 7. 69 x 10 -6 = [H+] p. H = -log [H+] p. H = - log (7. 69 x 10 -6 ) p. H = 5. 11
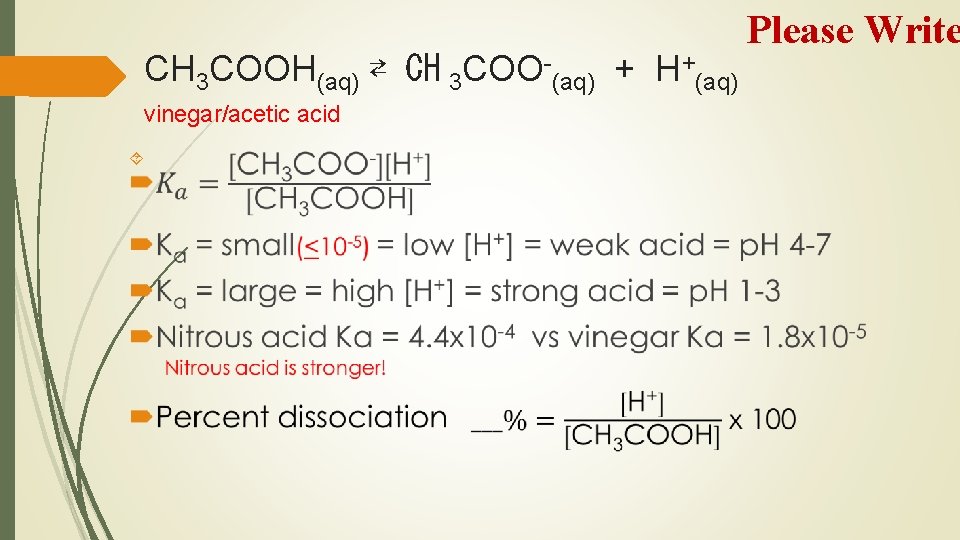
CH 3 COOH(aq) ⇄ CH 3 COO-(aq) + H+(aq) vinegar/acetic acid Please Write

Simplifying I. C. E. tables(the 5% rule) When doing an ICE table you may have to subtract a very small value from a relatively large value… for example 2. 0 mol/L – 1. 0 x 10 -4 mol/L. In this case, don’t bother doing the subtraction, since by the time you change it to show significant digits, the result will be the same: 2. 0 mol/L – 0. 0001 mol/L = 1. 9999 mol/L ≈ 2. 0 mol/L. SHORTCUT: If the number you subtract is less than 5% of the original number, you can usually skip the subtraction.
![Please Write +] & p. H of a 0. 25 M solution of Calculate Please Write +] & p. H of a 0. 25 M solution of Calculate](http://slidetodoc.com/presentation_image_h2/a7b5448cd0d601f1dfa1e261fe5ce774/image-22.jpg)
Please Write +] & p. H of a 0. 25 M solution of Calculate the [H Ex. 2 CH 3 COOH. Ka = 1. 80 x 10 -5. CH 3 COOH H+ + CH 3 COOH H+ CH 3 COO- 0. 25 0 0 C -x +x +x E 0. 25 – x x x I Ka = [H+][CH 3 COO-] [CH 3 COOH] 1. 80 x 10 -5 = [x][x] [0. 25 – x] How do you know? x/0. 25 < 5% then the difference will be insignificant and the short cut is justified! [H+] = 2. 12 x 10 -3 M p. H = 2. 67
![Please write +] of a 0. 55 M HCN solution. Also Calculate the [H Please write +] of a 0. 55 M HCN solution. Also Calculate the [H](http://slidetodoc.com/presentation_image_h2/a7b5448cd0d601f1dfa1e261fe5ce774/image-23.jpg)
Please write +] of a 0. 55 M HCN solution. Also Calculate the [H O 3 Ex. 3 calculate the % dissociation. Ka = 4. 8 x 10 -10 HCN + H 2 O H 3 O+ + CNI C HCN H 3 O + CN- 0. 55 0 0 -x E 0. 55 – x +x +x x x Ka = [H 3 O+][CN-] [HCN] 4. 80 x 10 -10 = [x][x] [0. 55 – x] [H 3 O+] = 1. 62 x 10 -5 M Shortcut?
![Please Write % Dissociation = [H 3 O+] x 100 [HCN] % Dissociation = Please Write % Dissociation = [H 3 O+] x 100 [HCN] % Dissociation =](http://slidetodoc.com/presentation_image_h2/a7b5448cd0d601f1dfa1e261fe5ce774/image-24.jpg)
Please Write % Dissociation = [H 3 O+] x 100 [HCN] % Dissociation = [1. 62 x 10 -5] x 100 [0. 55] % Dissociation = 2. 94 x 10 -3 % Very Small % Weak Acid!
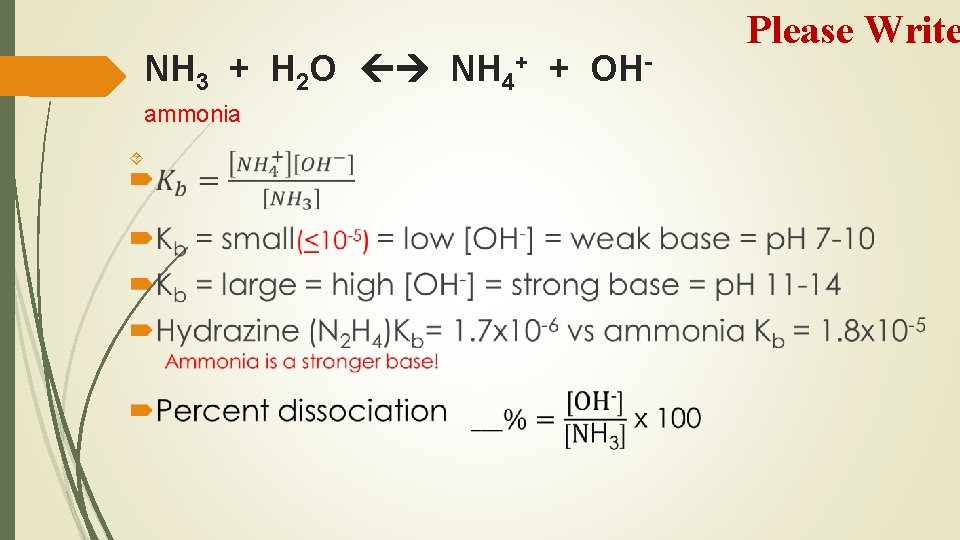
NH 3 + H 2 O NH 4+ + OHammonia Please Write

Relationship between Ka & Kb: Please Write CH 3 COOH(aq) + H 2 O ⇄ CH 3 COO-(aq) + H 3 O+(aq) CH 3 COO-(aq) + H 2 O ⇄ CH 3 COOH(aq) + OH-(aq)

Please Write Ex. 1 Calculate the Kb for CN- acting as a base in water, if the Ka of HCN = 4. 8 x 10 -10.

Please Write HCO 31 - is amphoteric. Ex. 2. If HCO 31 - was placed in water, would the solution be acting as an acid or a base? Step 1: Write out the equations acid & base rxns: HCO 31 - + H 2 O CO 32 - + H 3 O+ Ka = 4. 7 x 10 -11 HCO 31 - + H 2 O H 2 CO 3 + OH- Kb = ? 2. 13 x 10 -4 Step 2: Calculate Kb & compare to Ka Kb = 1. 0 x 10 -14 4. 7 x 10 -11 Kb = 2. 13 x 10 -4 Kb > Ka Therefore solution is BASIC
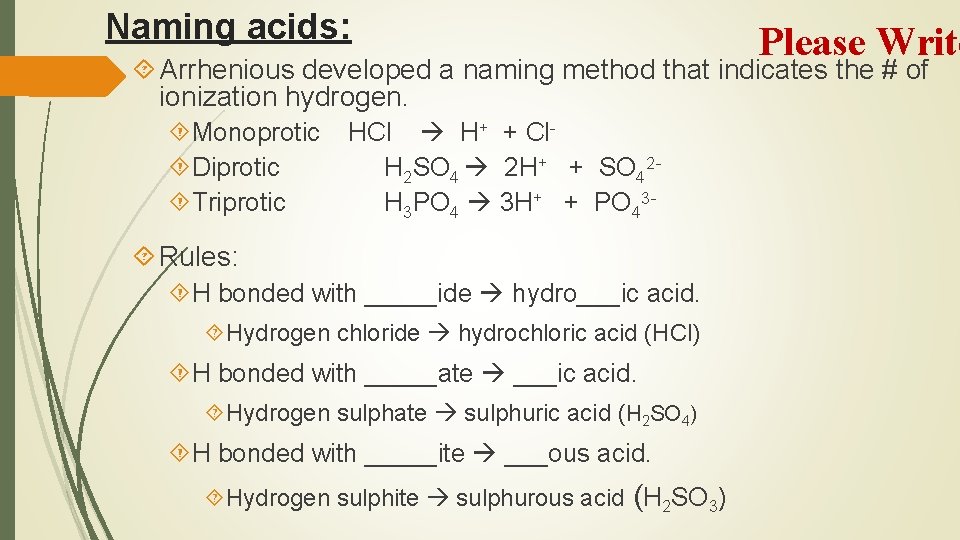
Naming acids: Please Write Arrhenious developed a naming method that indicates the # of ionization hydrogen. Monoprotic Diprotic Triprotic HCl H+ + Cl. H 2 SO 4 2 H+ + SO 42 H 3 PO 4 3 H+ + PO 43 - Rules: H bonded with _____ide hydro___ic acid. Hydrogen chloride hydrochloric acid (HCl) H bonded with _____ate ___ic acid. Hydrogen sulphate sulphuric acid (H 2 SO 4) H bonded with _____ite ___ous acid. Hydrogen sulphite sulphurous acid (H 2 SO 3)
- Slides: 29