Acids Bases Arrhenius definition of Acids Bases Acid

Acids & Bases

Arrhenius definition of Acids & Bases Acid: substance that contains hydrogen and ionizes to produce hydrogen ions in aqueous solutions Base: substance that contains a hydroxide group and dissociates (breaks down) to produce a hydroxide ion in aqueous solution
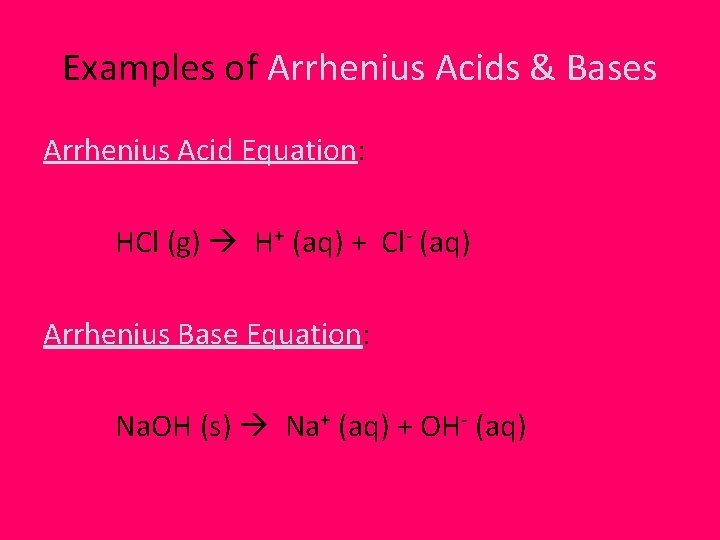
Examples of Arrhenius Acids & Bases Arrhenius Acid Equation: HCl (g) H+ (aq) + Cl- (aq) Arrhenius Base Equation: Na. OH (s) Na+ (aq) + OH- (aq)
Other Models of Acids & Bases Bronsted-Lowry Model An acid is a hydrogen-ion donor; a base is a hydrogen-ion acceptor Lewis Model A Lewis acid is an electron-pair acceptor and a Lewis base is an electron-pair donor

Common Properties of Acids • Produce H+ ions in water (the hydronium ion is a hydrogen ion attached to a water molecule) • Taste is sour • Corrodes metal • Electrolytic • Reacts with bases to form salt and water • p. H is less than 7 • Turns blue litmus paper to red

Common Properties of Bases • • Produce OH- ions in water Bitter, chalky taste Electrolytes Feel soapy, slippery React with acids to form salts and water p. H greater than 7 Turns red litmus paper blue
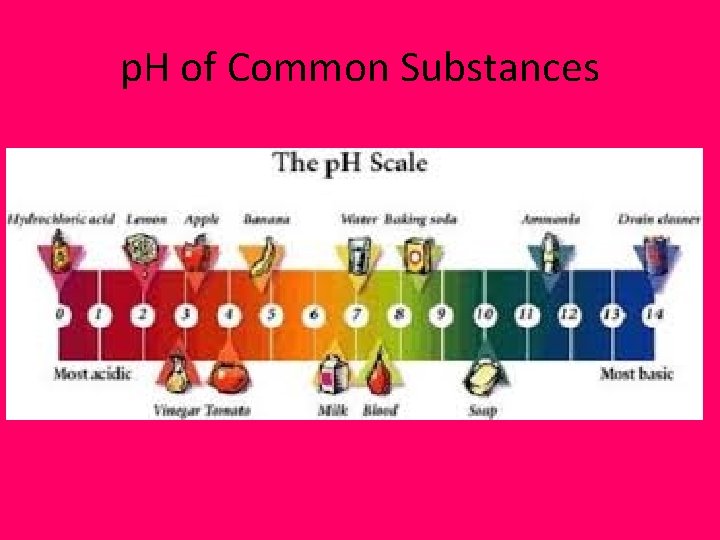
p. H of Common Substances
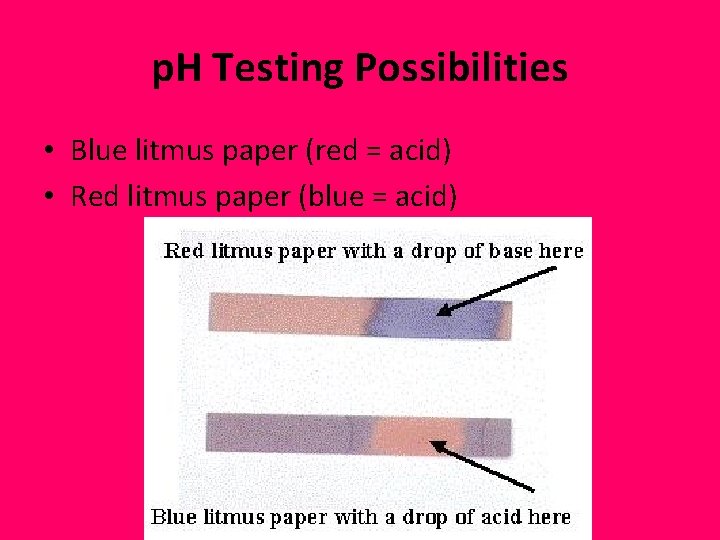
p. H Testing Possibilities • Blue litmus paper (red = acid) • Red litmus paper (blue = acid)

• p. H hydrion paper (multi-colored) • p. H meter (7 neutral, <7 acid, >7 base) • Universal indicator (multi-colored)

• Indicators like phenolphthalein (purple-pink in presence of a base) • Natural indicators like red cabbage, radishes

Acid/Base Titration • A titration is a carefully controlled neutralization reaction. • You have a solution of an unknown concentration of an acid or base & a second solution of known concentration called your standard solution. • The goal is to find the end point (the total moles of H+ donated by acid is equal to the total moles of H+ accepted by the base)
- Slides: 11