Acids Bases And the Indicators that tell the

Acids & Bases And the Indicators that tell the story….

Acids & Bases are Based on WATER! • Depending on the amount of H+ or OHions, the “water” becomes either acidic or basic (aka alkaline) • The p. H scale determines the strength of the acid/base • The p. H scale runs from 0 -14 – Low numbers are acidic – High numbers are alkaline – “ 7” is neutral Water

Acids • Acids are a chemical substance which contains more H+ ions than OH- ions. • An acid is able to donate electrons • H+ is called Hydronium • The more H+ ions in solution, the stronger the acid.

Bases (Alkali) • Bases are a chemical substance which contains more OHions than H+ ions. • A base is able to accept electrons • OH- is called hydroxide • The more OH- ions in solution, the stronger the base.
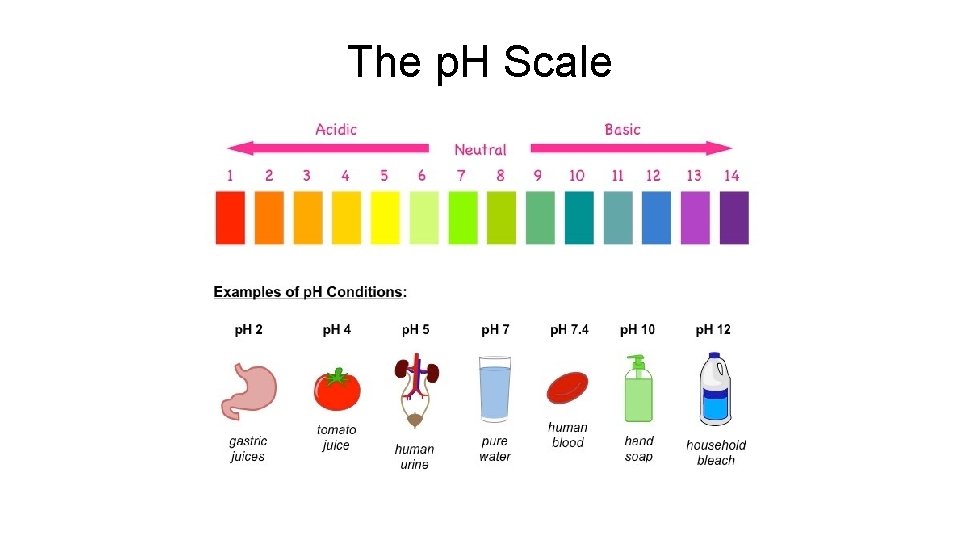
The p. H Scale

Characteristics of Acids • Taste Sour • Conduct Electricity • Corrosive, which means they break down certain substances. Many acids can corrode fabric, skin and wood • Some acids react strongly with metals • Turns blue litmus paper red • Turn p. H Paper Yellow Red • Depending on strength of acid

Acids • Remember… Acids are solutions with a higher amount of Hydrogen ions (H+) • 99% of the time, their formula begins with “H” (which stands for the Hydrogen ion) • HCl = Hydrochloric Acid • HNO 3 = Nitric Acid • H 2 SO 4 = Sulfuric Acid

Characteristics of Bases aka - Alkalis • Feel Slippery (Think SOAP!) • Taste Bitter • Corrosive (Just as corrosive as Acids!) • Can conduct electricity. (Think alkaline batteries. ) • Do not react with metals • Turns red litmus paper blue • Turn p. H paper Green Blue • Turn Phenolphthalein Pink/Magenta

Bases • Remember… Basic (Alkali) solutions have a higher amount of Hydroxide ions (OH-) • 99% of the time, their formula ends with “OH” (which stands for the Hydroxide ion) • Na. OH = Sodium Hydroxide • KOH = Potassium Hydroxide • Ba(OH)2 = Barium Hydroxide

To Neutralize… • Neutralization is a chemical reaction in which an acid and a base react with each other • In a neutralization reaction, the point is to bring the solution back to equilibrium • No excess of hydrogen or hydroxide ions present in solution • One “H+” for each “OH-”… • H+ OH = Water! (Which is neutral)

Neutralization • To neutralize (counter-act) an acid, you add a base • To neutralize (counter-act) a base, you add an acid A Neutralization Reaction always produces a salt and water! Acid + Base = Salt + Water Neutralization is a type of Hydrolysis (breaking something apart and producing water (hydro) as one of the products)

Salts • A salt is formed between the reaction of an acid and a base • Usually, a neutral salt is formed when a strong acid and a strong base are neutralized in the reaction • The H+ is removed from the acid and the OH- is removed from the base • H + OH = H 2 O • The other elements combine to form a salt • A salt is an ionic compound which is made up of two groups of oppositely charged ions • A Metal + A Non-metal

Salts CAN BE acidic or basic (or neutral)! • Adding a strong acid to a strong base should bring the p. H of the solution back to neutral • A salt formed between a strong acid and a weak base is an acid salt • A salt formed between a strong base and a weak acid is a basic salt

What happens when… • What happens when a solid salt solution is dissolved in water? • It is hydrolyzed (broken down) into an acid and a base • Example: Na. Cl (Sodium Chloride) – Table Salt • In water, it is broken into Na+ and Cl- ions • The Sodium (Na) metal and a Hydroxide ion (OH) combine to form a base (Na. OH) • The Chlorine (Cl) non-metal and a Hydrogen ion (H) combine to form an acid (HCl)

Indicators • Acid - base indicators (aka p. H indicators) are substances which change color in different p. H levels • There are many different types of indicators: Litmus Paper (Red & Blue) p. H Paper Universal Bromothymol Blue Thymol Blue Methyl Orange Phenolyphthalein Bromocresol Green

Indicator Acid Neutral Base Red Litmus Blue Red Blue Litmus Red Blue Universal Indicator Red/Brownish Green Blue/Violet Yellow Green Blue Methyl Orange Red Orange Yellow Phenolphthalein Clear/Colorless Bromothymol Blue Thymol Blue p. H Paper Pink/Magenta Red Yellow Blue Reddish/Brown Green Navy
- Slides: 16