Acids Bases and Solutions Table of Contents Understanding
Acids, Bases, and Solutions Table of Contents Understanding Solutions Concentration and Solubility Describing Acids and Bases in Solution Digestion and p. H
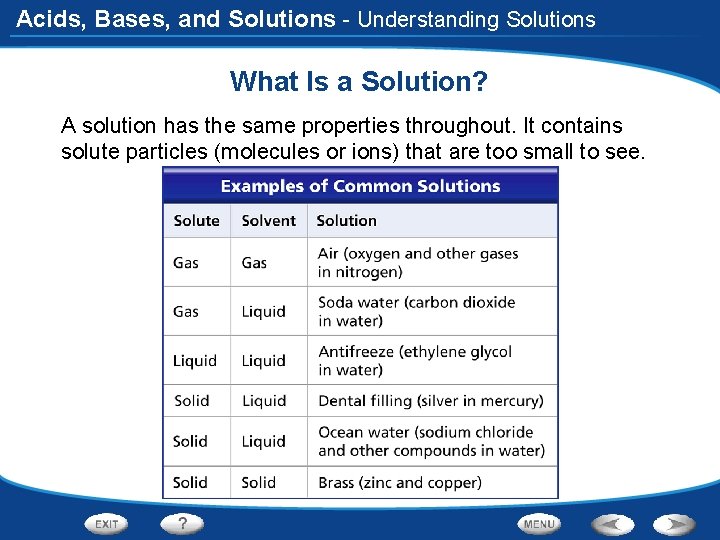
Acids, Bases, and Solutions - Understanding Solutions What Is a Solution? A solution has the same properties throughout. It contains solute particles (molecules or ions) that are too small to see.

Acids, Bases, and Solutions - Understanding Solutions What Is a Solution? Solutions can be made from any combinations of solids, liquids, and gases.

Acids, Bases, and Solutions - Understanding Solutions Colloids and Suspensions Colloids and suspensions are mixtures that have properties different from those of solutions.
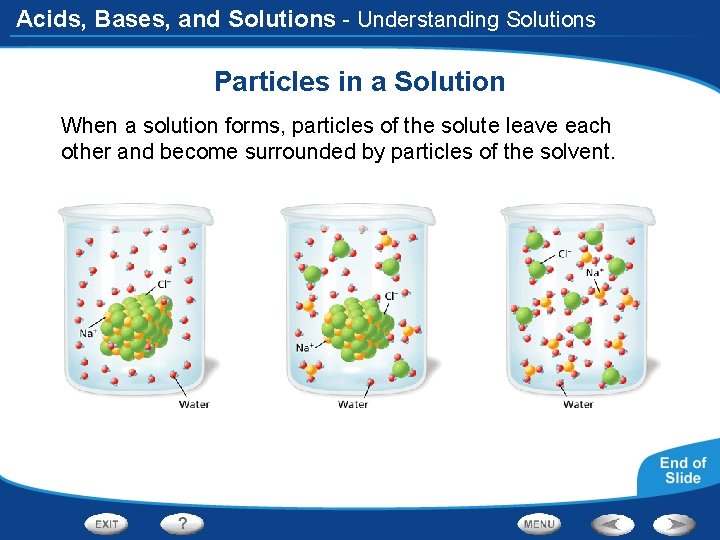
Acids, Bases, and Solutions - Understanding Solutions Particles in a Solution When a solution forms, particles of the solute leave each other and become surrounded by particles of the solvent.

Acids, Bases, and Solutions - Understanding Solutions Salt Dissolving in Water Activity Click the Active Art button to open a browser window and access Active Art about salt dissolving in water.
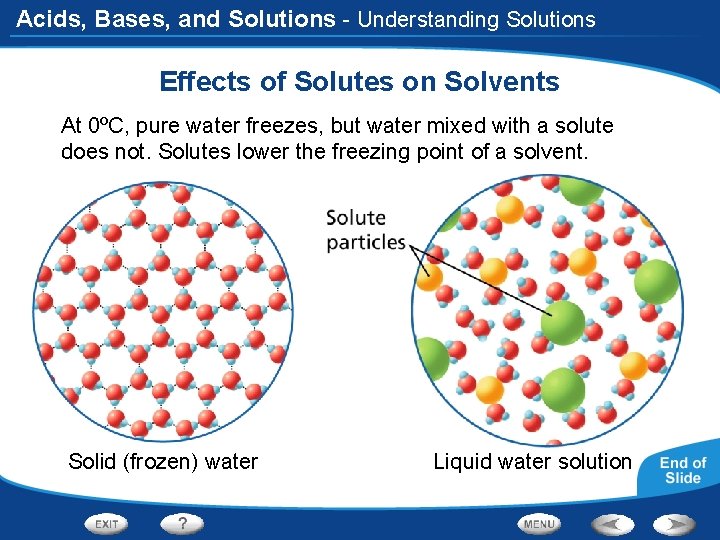
Acids, Bases, and Solutions - Understanding Solutions Effects of Solutes on Solvents At 0ºC, pure water freezes, but water mixed with a solute does not. Solutes lower the freezing point of a solvent. Solid (frozen) water Liquid water solution

Acids, Bases, and Solutions - Understanding Solutions Identifying Main Ideas As you read the section “What is a Solution? ”, write the main idea in a graphic. Then write three supporting details. Main Idea A solution is a well mixed mixture that contains a solvent and at least one solute. Detail The solvent is the substance present in the largest amount. A solute is a substance present in a smaller amount than the solvent. A solution has the same properties throughout. Detail A solution contains particles that are too small to see.
Acids, Bases, and Solutions - Understanding Solutions Universal Solvent Click the Video button to watch a movie about universal solvent.

Acids, Bases, and Solutions End of Section: Understanding Solutions
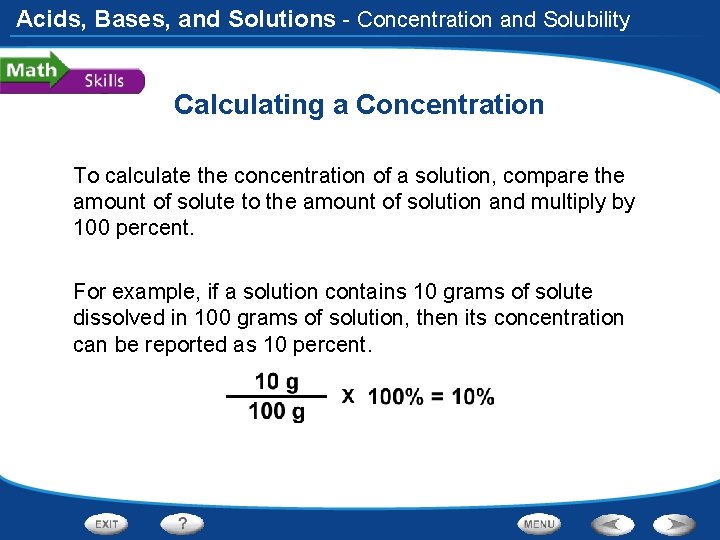
Acids, Bases, and Solutions - Concentration and Solubility Calculating a Concentration To calculate the concentration of a solution, compare the amount of solute to the amount of solution and multiply by 100 percent. For example, if a solution contains 10 grams of solute dissolved in 100 grams of solution, then its concentration can be reported as 10 percent.

Acids, Bases, and Solutions - Concentration and Solubility Calculating a Concentration Practice Problem A solution contains 12 grams of solute dissolved in 36 grams of solution. What is the concentration of the solution? 33%
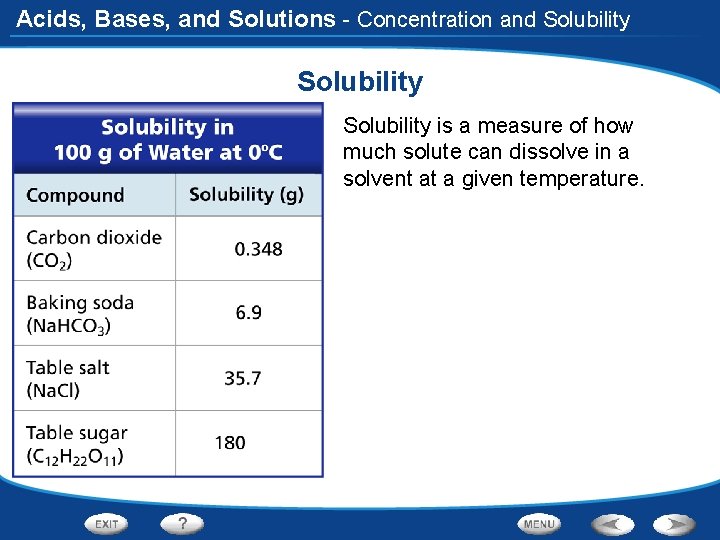
Acids, Bases, and Solutions - Concentration and Solubility is a measure of how much solute can dissolve in a solvent at a given temperature.
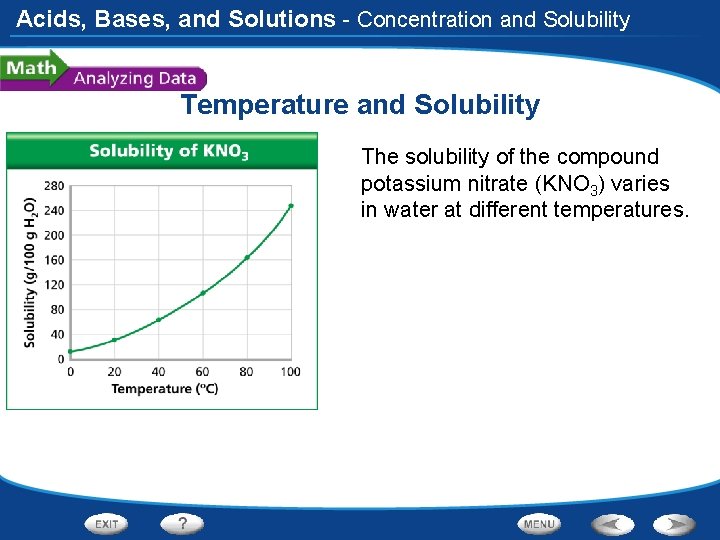
Acids, Bases, and Solutions - Concentration and Solubility Temperature and Solubility The solubility of the compound potassium nitrate (KNO 3) varies in water at different temperatures.
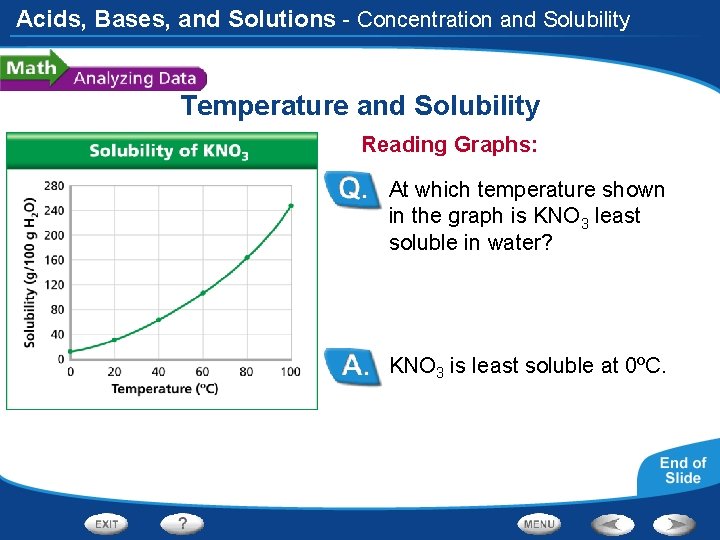
Acids, Bases, and Solutions - Concentration and Solubility Temperature and Solubility Reading Graphs: At which temperature shown in the graph is KNO 3 least soluble in water? KNO 3 is least soluble at 0ºC.
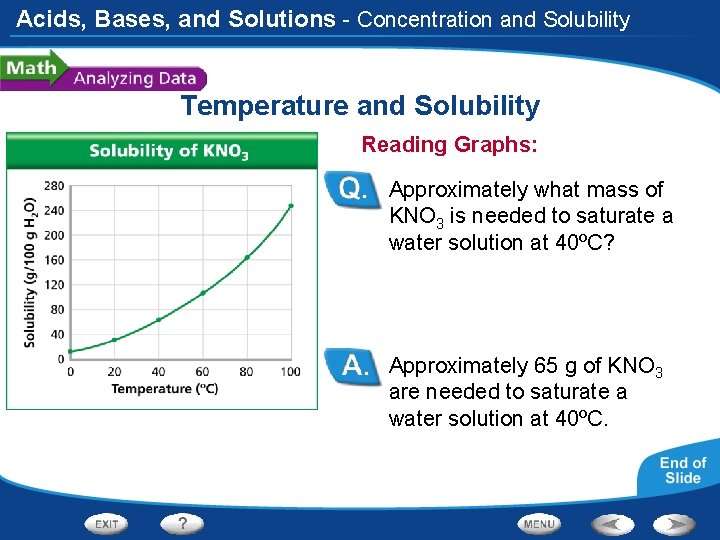
Acids, Bases, and Solutions - Concentration and Solubility Temperature and Solubility Reading Graphs: Approximately what mass of KNO 3 is needed to saturate a water solution at 40ºC? Approximately 65 g of KNO 3 are needed to saturate a water solution at 40ºC.
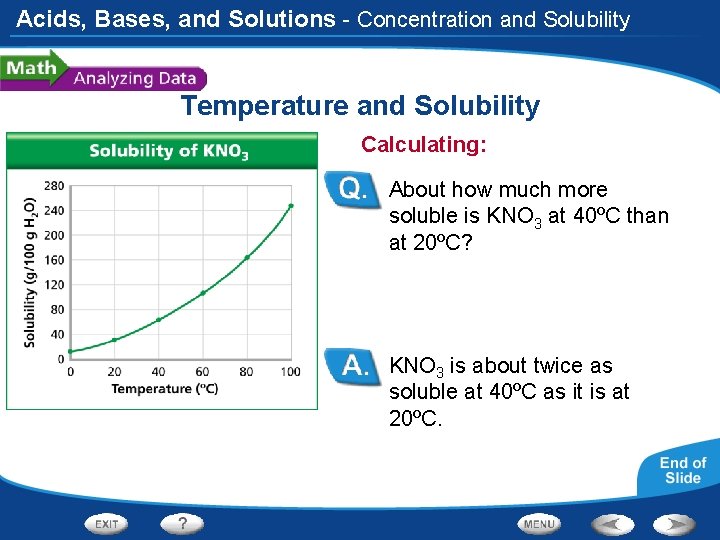
Acids, Bases, and Solutions - Concentration and Solubility Temperature and Solubility Calculating: About how much more soluble is KNO 3 at 40ºC than at 20ºC? KNO 3 is about twice as soluble at 40ºC as it is at 20ºC.
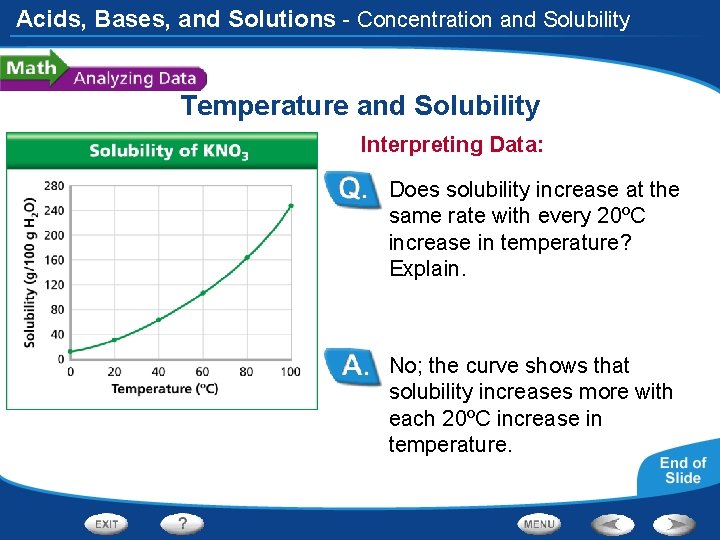
Acids, Bases, and Solutions - Concentration and Solubility Temperature and Solubility Interpreting Data: Does solubility increase at the same rate with every 20ºC increase in temperature? Explain. No; the curve shows that solubility increases more with each 20ºC increase in temperature.

Acids, Bases, and Solutions - Concentration and Solubility Building Vocabulary After you read the section, carefully note the definition of each Key Term. Also note other details in the paragraph that contains the definition. Use all this information to write a meaningful sentence using the Key Terms: Examples: unsaturated solution dilute solution An unsaturated can that continue to dissolve A dilute solution is a mixture has only a little more solute. dissolved in a certain amount of solvent. supersaturated concentrated solution solubility A supersaturated concentrated solution is has one more that has dissolved a lot of soluteisdissolved than predicted in bythe its same solubility amount at theofgiven solvent. temperature. Solubility is a measure of how much solute can dissolve in a solvent at a given temperature. A saturated solution contains so much solute that no more dissolves. saturated solution

Acids, Bases, and Solutions - Concentration and Solubility Links on Solubility Click the Sci. Links button for links on solubility.

Acids, Bases, and Solutions End of Section: Concentration and Solubility
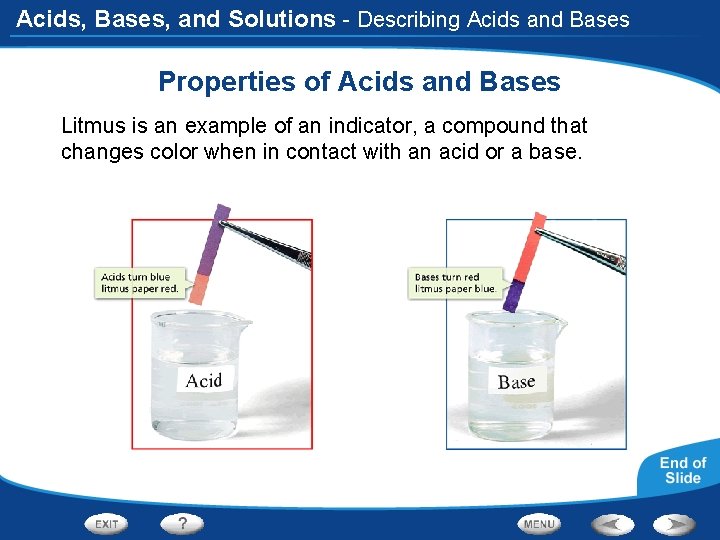
Acids, Bases, and Solutions - Describing Acids and Bases Properties of Acids and Bases Litmus is an example of an indicator, a compound that changes color when in contact with an acid or a base.

Acids, Bases, and Solutions - Describing Acids and Bases Uses of Acids and Bases Acids and bases have many uses around the home and in industry.

Acids, Bases, and Solutions - Describing Acids and Bases Asking Questions Before you read, preview the red headings. In a graphic organizer like the one below, ask a what question for each heading. As you read, write answers to your questions. Question Answer What is an acid? An acid is a substance that tastes sour, reacts with metals and carbonates, and turns blue litmus paper red. What is a base? A base is a substance that tastes bitter, feels slippery, and turns red litmus paper blue. What are uses of acids and bases? Uses of acids include cleaning products, fertilizers, and car batteries; uses of bases include cleaning products, baking ingredients, and cement manufacturing.

Acids, Bases, and Solutions - Describing Acids and Bases Links on Acids and Bases Click the Sci. Links button for links on acids and bases.

Acids, Bases, and Solutions End of Section: Describing Acids and Bases
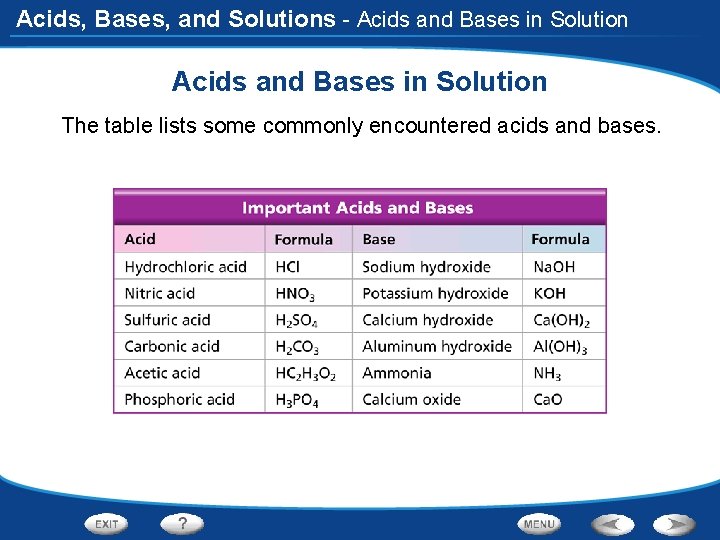
Acids, Bases, and Solutions - Acids and Bases in Solution The table lists some commonly encountered acids and bases.
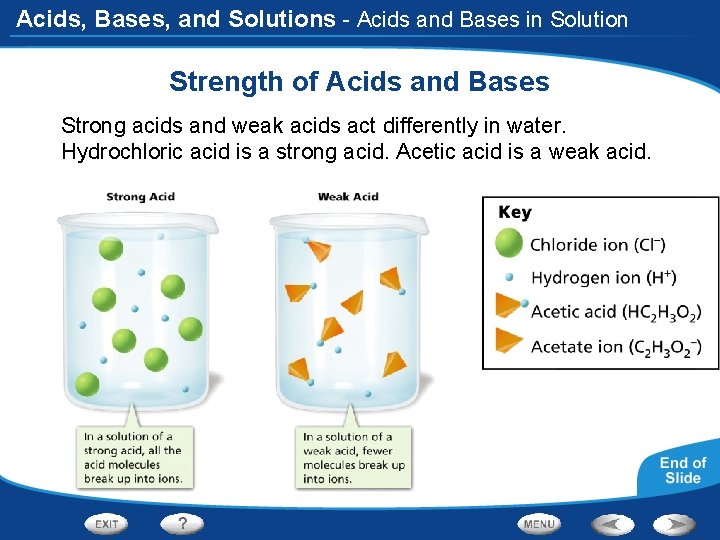
Acids, Bases, and Solutions - Acids and Bases in Solution Strength of Acids and Bases Strong acids and weak acids act differently in water. Hydrochloric acid is a strong acid. Acetic acid is a weak acid.
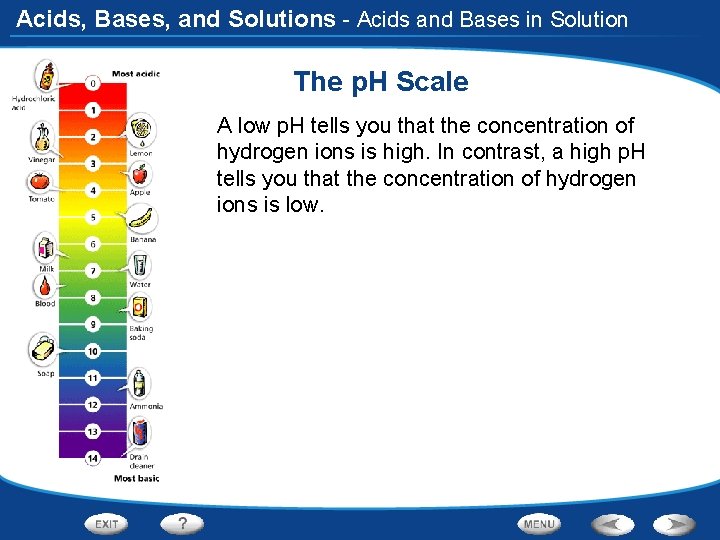
Acids, Bases, and Solutions - Acids and Bases in Solution The p. H Scale A low p. H tells you that the concentration of hydrogen ions is high. In contrast, a high p. H tells you that the concentration of hydrogen ions is low.
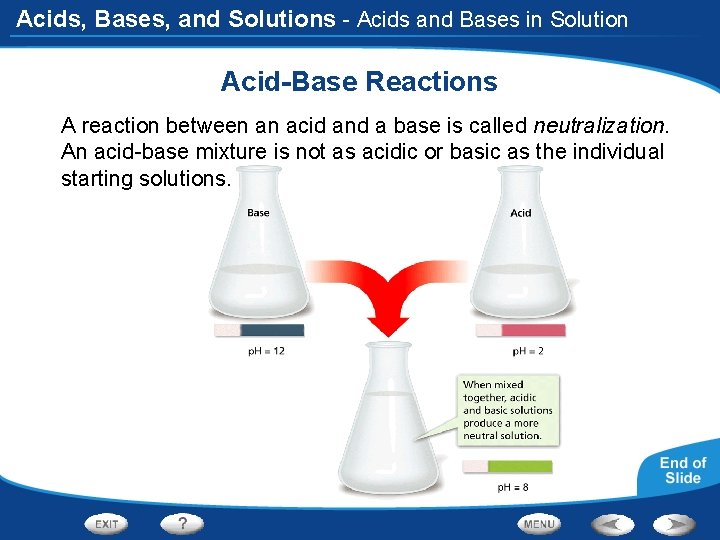
Acids, Bases, and Solutions - Acids and Bases in Solution Acid-Base Reactions A reaction between an acid and a base is called neutralization. An acid-base mixture is not as acidic or basic as the individual starting solutions.
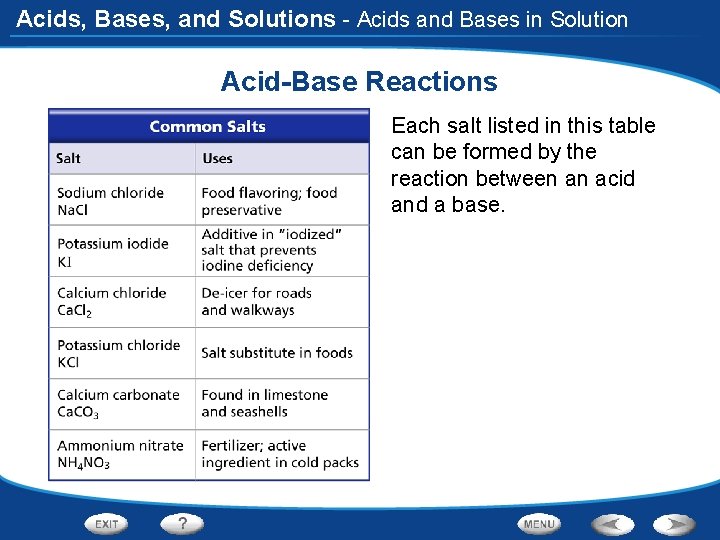
Acids, Bases, and Solutions - Acids and Bases in Solution Acid-Base Reactions Each salt listed in this table can be formed by the reaction between an acid and a base.
Acids, Bases, and Solutions - Acids and Bases in Solution Previewing Visuals When you preview, you look ahead at the material to be read. Preview Figure 21. Then write two questions that you have about the diagram in a graphic organizer like the one below. As you read, answer your questions. Neutralization Q. What is a neutral solution? A. A neutral solution is one that has a p. H close to 7. Q. What is neutralization? A. Neutralization is a reaction between an acid and a base.

Acids, Bases, and Solutions - Acids and Bases in Solution More on the p. H Scale Click the PHSchool. com button for an activity about the p. H scale.

Acids, Bases, and Solutions - Acids and Bases in Solution p. H Click the Video button to watch a movie about p. H.

Acids, Bases, and Solutions End of Section: Acids and Bases in Solution
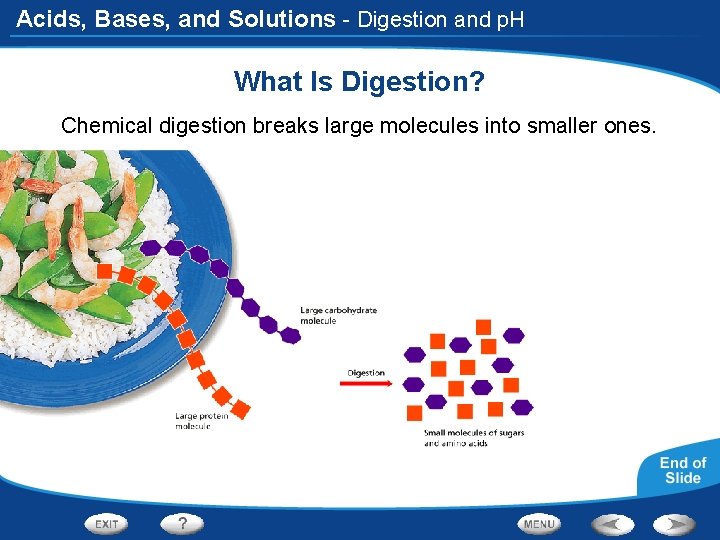
Acids, Bases, and Solutions - Digestion and p. H What Is Digestion? Chemical digestion breaks large molecules into smaller ones.
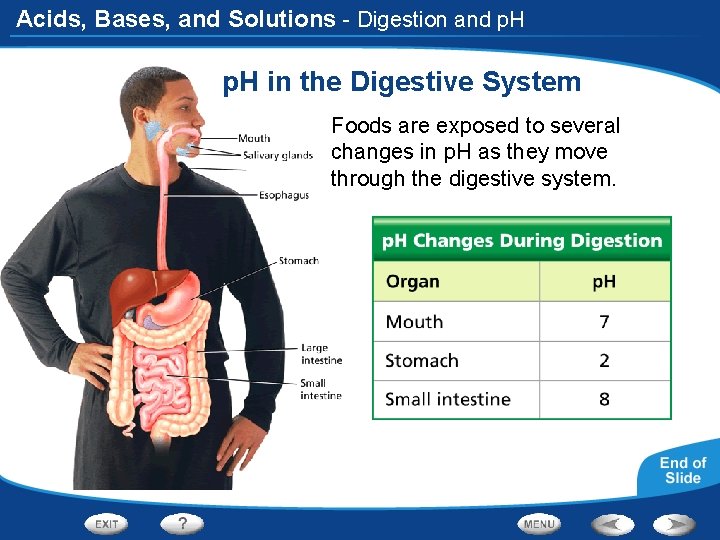
Acids, Bases, and Solutions - Digestion and p. H in the Digestive System Foods are exposed to several changes in p. H as they move through the digestive system.
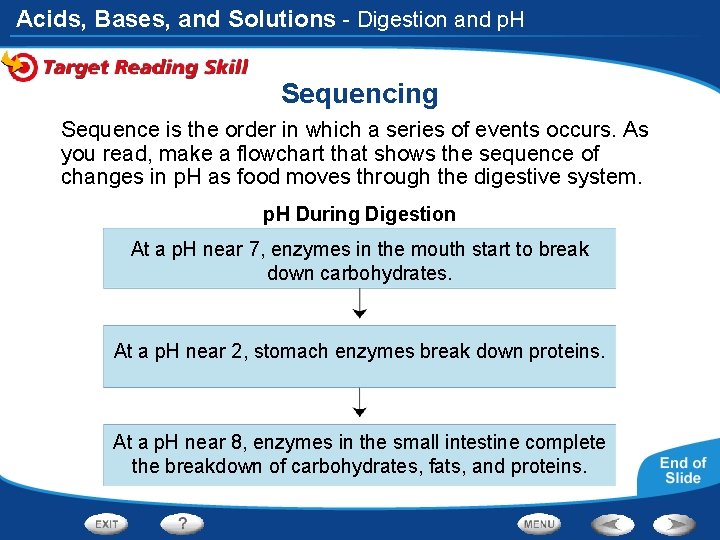
Acids, Bases, and Solutions - Digestion and p. H Sequencing Sequence is the order in which a series of events occurs. As you read, make a flowchart that shows the sequence of changes in p. H as food moves through the digestive system. p. H During Digestion At a p. H near 7, enzymes in the mouth start to break down carbohydrates. At a p. H near 2, stomach enzymes break down proteins. At a p. H near 8, enzymes in the small intestine complete the breakdown of carbohydrates, fats, and proteins.

Acids, Bases, and Solutions - Digestion and p. H Links on Digestion and p. H Click the Sci. Links button for links on digestion and p. H.

Acids, Bases, and Solutions End of Section: Digestion and p. H
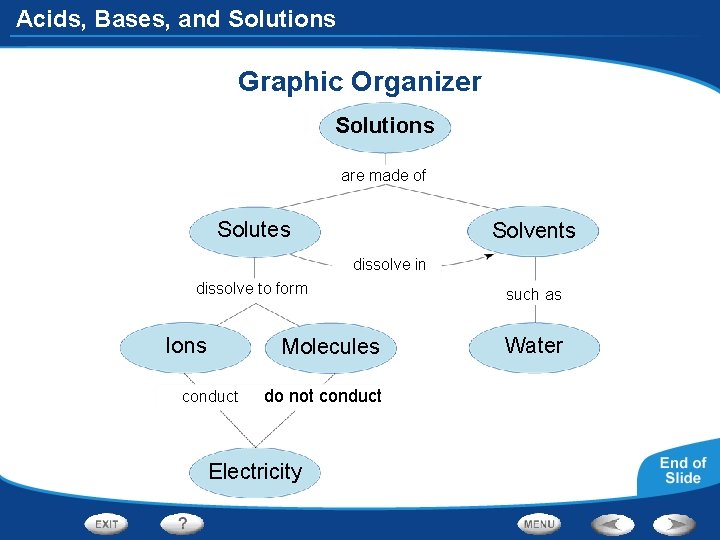
Acids, Bases, and Solutions Graphic Organizer Solutions are made of Solutes Solvents dissolve in dissolve to form Ions Molecules conduct do not conduct Electricity such as Water

Acids, Bases, and Solutions End of Section: Graphic Organizer
- Slides: 42