Acids Bases and Salts Chapter 19 1 1

Acids, Bases, and Salts Chapter 19 1
![1. Properties of Acids (w/ an example of an acid) [green, no dot] 2. 1. Properties of Acids (w/ an example of an acid) [green, no dot] 2.](http://slidetodoc.com/presentation_image/a1fb5578f64bec94fcb6435da01fb699/image-2.jpg)
1. Properties of Acids (w/ an example of an acid) [green, no dot] 2. Properties of Bases ((w/ an example of a base) [green, w/dot] 3. Similarities between Acid & Bases [yellow] 4. Types of Acids (Monoprotic, diprotic, Triprotic) w/ example of each [red] 5. Arrhenius Acids and Bases [black] 6. Brønsted-Lowry Acids and Bases [white] 7. Lewis Acids and Bases [blue] 2

19. 1 Acid- Base Theories • Properties of Acids – Taste sour – React with metals to form H 2 gas – Will change the color of and acid-base indicator • Turns blue litmus paper RED • Properties of Bases – Taste bitter – feel slippery – Will change the color of and acid-base indicator • Turns red litmus paper BLUE • BOTH – Can be strong or weak electrolytes in aqueous solutions – React with the other to produce salt (an ionic compound) and water 3
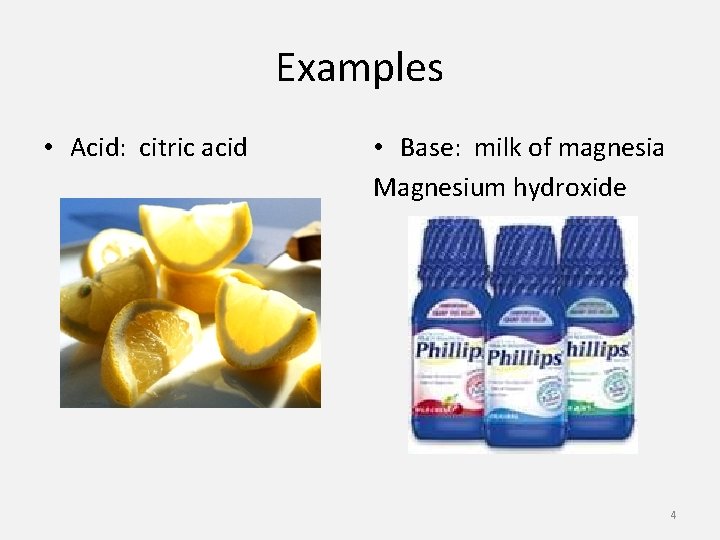
Examples • Acid: citric acid • Base: milk of magnesia Magnesium hydroxide 4
Types of acids • Monoprotic acids are acids that contain one ionizable hydrogen. Nitric acid: HNO 3 • Diprotice acids are acids that contain two ionizable hydrogens. Sulfuric acid: H 2 SO 4 • Triprotic acids are acids that contain three ionizable hydrogens. Phosphoric acid: H 3 PO 4 5
Arrhenius Acids and Bases • Svante Arrhenius in 1887 posed a way of defining acids and bases • Arrhenius Acids – Are hydrogen-containing compounds that ionize to yield hydrogen ions (H+) [H+ producer] HCl + H 2 O H 3 O+ + Cl− NH 4+ + H 2 O H 3 O+ + NH 3 • Arrhenius Bases – Are compounds that ionize to yield hydroxide ions (OH-) in aqueous solutions. [OH- producer] NH 3 + H 2 O NH 4+ + OH− CH 3 COO− + H 2 O CH 3 COOH + OH− 6 Arrhenius definiton incoporates the FEWEST number of compunds
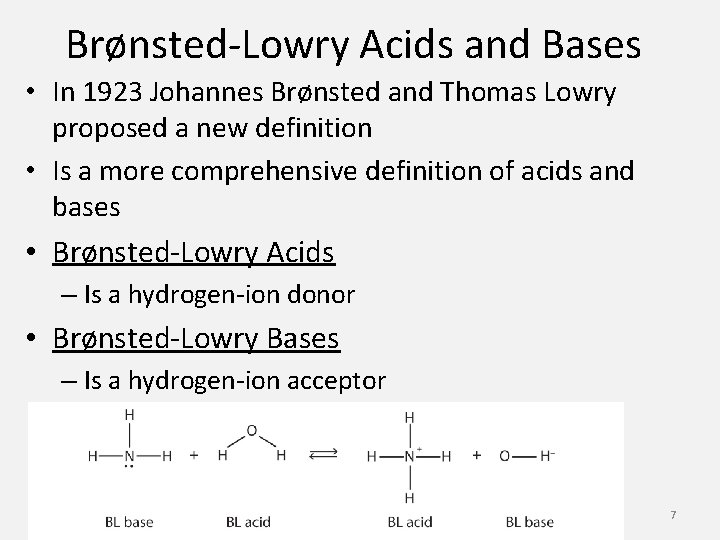
Brønsted-Lowry Acids and Bases • In 1923 Johannes Brønsted and Thomas Lowry proposed a new definition • Is a more comprehensive definition of acids and bases • Brønsted-Lowry Acids – Is a hydrogen-ion donor • Brønsted-Lowry Bases – Is a hydrogen-ion acceptor 7
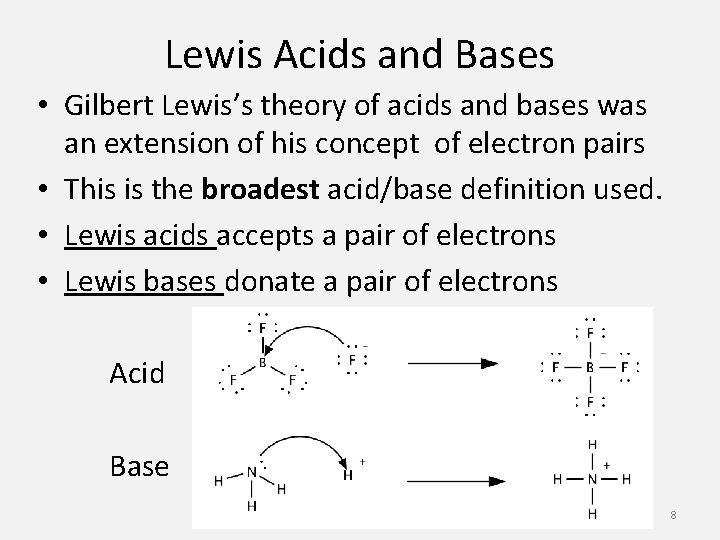
Lewis Acids and Bases • Gilbert Lewis’s theory of acids and bases was an extension of his concept of electron pairs • This is the broadest acid/base definition used. • Lewis acids accepts a pair of electrons • Lewis bases donate a pair of electrons Acid Base 8
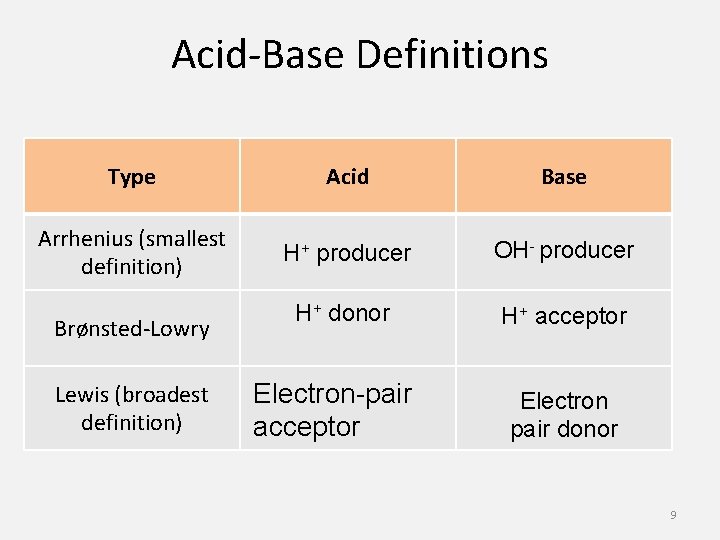
Acid-Base Definitions Type Acid Base Arrhenius (smallest definition) H+ producer OH- producer Brønsted-Lowry H+ donor H+ acceptor Lewis (broadest definition) Electron-pair acceptor Electron pair donor 9

Acid, Bases & Salt Video • Pre Test 1. 2. 3. 4. 5. True False (about equal) True* (when in water can form acidic/basic solutions) 6. False 7. True 8. True 9. False (Base has p. H above 7) 10. False (stomach acids) Video Quiz 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. True False [acids react w/ metals] Neutralization False [acids produce H+ ion] True Hydrogen ions True False {only 1 hydrogen ion True Acid rain
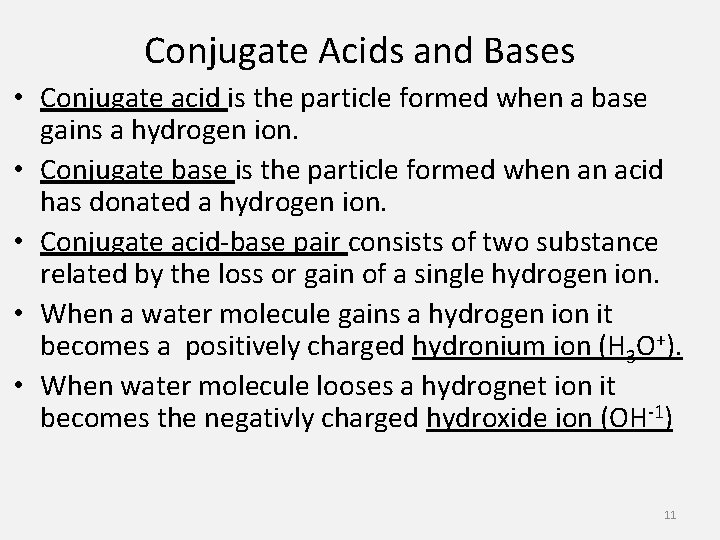
Conjugate Acids and Bases • Conjugate acid is the particle formed when a base gains a hydrogen ion. • Conjugate base is the particle formed when an acid has donated a hydrogen ion. • Conjugate acid-base pair consists of two substance related by the loss or gain of a single hydrogen ion. • When a water molecule gains a hydrogen ion it becomes a positively charged hydronium ion (H 3 O+). • When water molecule looses a hydrognet ion it becomes the negativly charged hydroxide ion (OH-1) 11
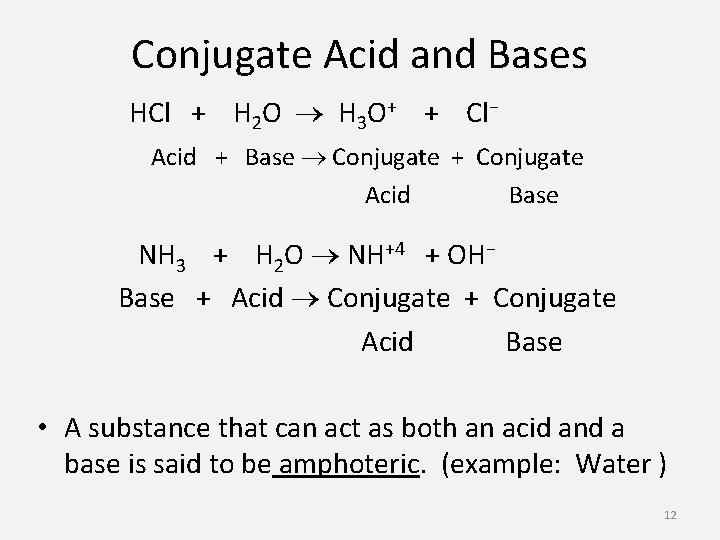
Conjugate Acid and Bases HCl + H 2 O H 3 O+ + Cl− Acid + Base Conjugate + Conjugate Acid Base NH 3 + H 2 O NH+4 + OH− Base + Acid Conjugate + Conjugate Acid Base • A substance that can act as both an acid and a base is said to be amphoteric. (example: Water ) 12
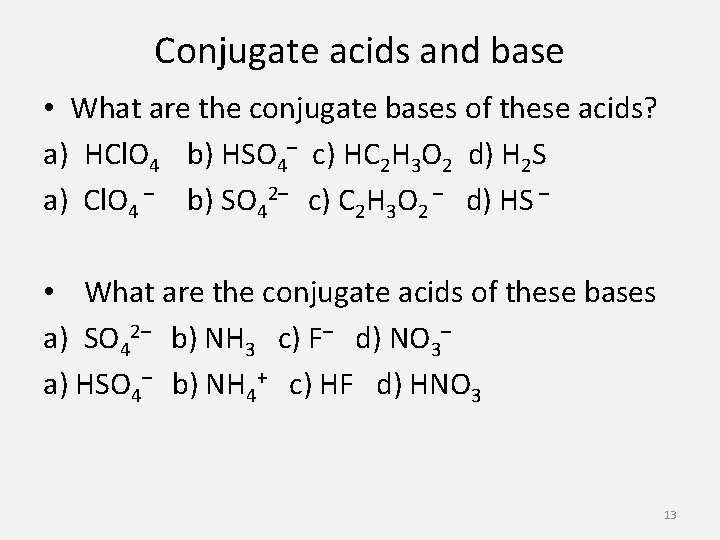
Conjugate acids and base • What are the conjugate bases of these acids? a) HCl. O 4 b) HSO 4– c) HC 2 H 3 O 2 d) H 2 S a) Cl. O 4 – b) SO 42– c) C 2 H 3 O 2 – d) HS – • What are the conjugate acids of these bases a) SO 42– b) NH 3 c) F– d) NO 3– a) HSO 4– b) NH 4+ c) HF d) HNO 3 13
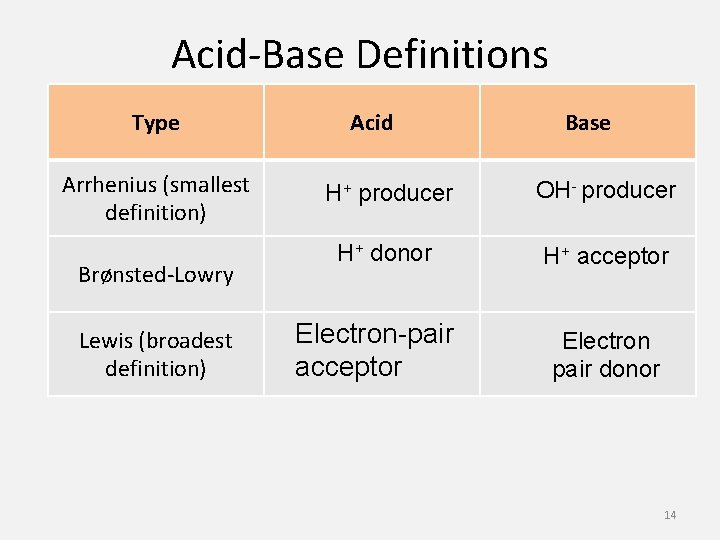
Acid-Base Definitions Type Acid Base Arrhenius (smallest definition) H+ producer OH- producer Brønsted-Lowry H+ donor H+ acceptor Lewis (broadest definition) Electron-pair acceptor Electron pair donor 14

19. 1 Review 1. What are the properties of acids and bases? 2. How did Arrhenius define and acid and base? 3. How are acids and bases defined by the Brønsted-Lowry theory? 4. What is the Lewis-theory of acids and bases? 5. Identify the following as monoprotic, diprotic or triprotic: a) H 2 CO 3 b) H 3 PO 4 c) HCl d) H 2 SO 4 15
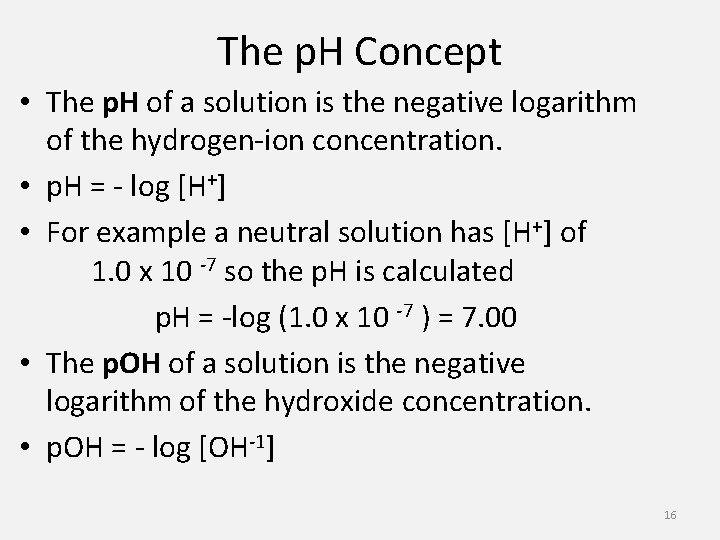
The p. H Concept • The p. H of a solution is the negative logarithm of the hydrogen-ion concentration. • p. H = - log [H+] • For example a neutral solution has [H+] of 1. 0 x 10 -7 so the p. H is calculated p. H = -log (1. 0 x 10 -7 ) = 7. 00 • The p. OH of a solution is the negative logarithm of the hydroxide concentration. • p. OH = - log [OH-1] 16
![p. H and Significant Figures • A [H+] of 6. 0 x 10 -5 p. H and Significant Figures • A [H+] of 6. 0 x 10 -5](http://slidetodoc.com/presentation_image/a1fb5578f64bec94fcb6435da01fb699/image-17.jpg)
p. H and Significant Figures • A [H+] of 6. 0 x 10 -5 has two significant figures • The p. H is recorded with two decimal places 4. 22 • A [H+] of 6. x 10 -5 has one significant figures • The p. H is recorded with one decimal places 4. 2 17
![Calculating p. H practice 1. [H+] of 1. 0 x 10 -11 p. H Calculating p. H practice 1. [H+] of 1. 0 x 10 -11 p. H](http://slidetodoc.com/presentation_image/a1fb5578f64bec94fcb6435da01fb699/image-18.jpg)
Calculating p. H practice 1. [H+] of 1. 0 x 10 -11 p. H = -log (1. 0 x 10 -11 ) = 11. 00 2. [H+] of 6. 0 x 10 -5 p. H = -log (6. 0 x 10 -5 ) = 4. 22 3. [H+] of 4. 0 x 10 -3 p. H = -log (4. 0 x 10 -3 ) = 2. 40 4. [H+] of 9. 0 x 10 -9 p. H = -log (9. 0 x 10 -9 ) = 8. 05 18
![Calculating p. OH • p. OH = - log [OH-] 1. [OH-] of 1. Calculating p. OH • p. OH = - log [OH-] 1. [OH-] of 1.](http://slidetodoc.com/presentation_image/a1fb5578f64bec94fcb6435da01fb699/image-19.jpg)
Calculating p. OH • p. OH = - log [OH-] 1. [OH-] of 1. 0 x 10 -3 p. OH = -log (1. 0 x 10 -3 ) = 3. 00 2. [OH-] of 6. 0 x 10 -7 p. OH = -log (6. 0 x 10 -7 ) = 6. 22 3. [OH-] of 2. 0 x 10 -4 p. OH = -log (2. 0 x 10 -4 ) = 3. 70 4. [OH-] of 7. 2 x 10 -9 p. OH = -log (7. 2 x 10 -9 ) =. 14 19
![• A solution in which [H+] is greater than 1. 0 x 10 • A solution in which [H+] is greater than 1. 0 x 10](http://slidetodoc.com/presentation_image/a1fb5578f64bec94fcb6435da01fb699/image-20.jpg)
• A solution in which [H+] is greater than 1. 0 x 10 -7 has a p. H less than 7. 0 and is acidic. • The p. H of a neutral solution is 7. 0 • A solution in which [H+] is less than 1. 0 x 10 -7 has a p. H greater than 7. 0 and is basic. • A simple relationship between p. H and p. OH allows you to calculate the other if one is known. p. H + p. OH = 14 20
![Calculating concentration from p. H/p. OH • Using p. H to find [H+] since Calculating concentration from p. H/p. OH • Using p. H to find [H+] since](http://slidetodoc.com/presentation_image/a1fb5578f64bec94fcb6435da01fb699/image-21.jpg)
Calculating concentration from p. H/p. OH • Using p. H to find [H+] since p. H= -log [H+] Then [H+] = 10(-p. H) • Calculate the [H+] for the solution p. H= 3. 00 [H+]= 10(-p. H) [H+] = 10(-3. 00) = 1. 0 x 10 -3 M H+ 0. 00100 M H+ • Using p. H to find [OH-1] since p. OH= -log [OH-1] Then [OH-1] = 10(-p. OH) • Calculate the [OH-1] for the solution p. OH= 7. 67 [OH-1]= 10(-p. OH) [OH-1] = 10(-7. 67) = 2. 14 x 10 -8 21
![p. H to p. OH • If [H+1] is 2. 5 x 10 -4 p. H to p. OH • If [H+1] is 2. 5 x 10 -4](http://slidetodoc.com/presentation_image/a1fb5578f64bec94fcb6435da01fb699/image-22.jpg)
p. H to p. OH • If [H+1] is 2. 5 x 10 -4 what is the p. OH p. H = -log [2. 5 x 10 -4 ] = 3. 60 p. OH = 14 – 3. 60 = 10. 40 • If the [OH-1] is 6. 8 x 10 -12 what is the p. H p. OH = -log [6. 8 x 10 -12 ] = 11. 17 p. H = 14 – 11. 17 = 2. 83 22
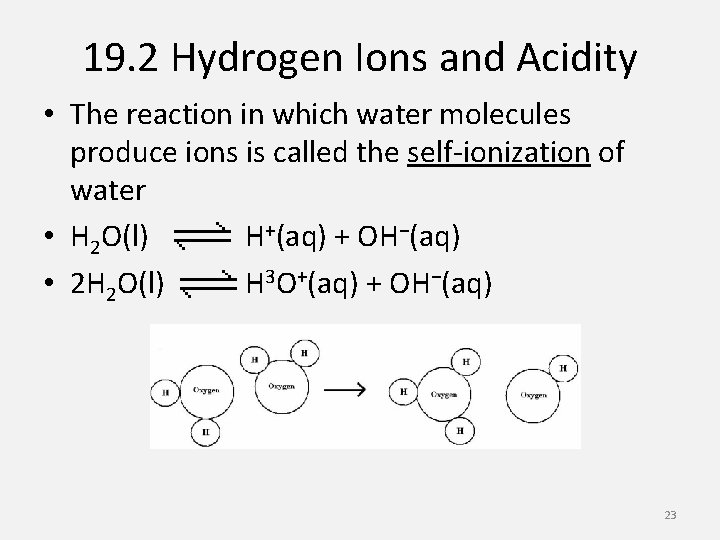
19. 2 Hydrogen Ions and Acidity • The reaction in which water molecules produce ions is called the self-ionization of water • H 2 O(l) H+(aq) + OH−(aq) • 2 H 2 O(l) H 3 O+(aq) + OH−(aq) 23
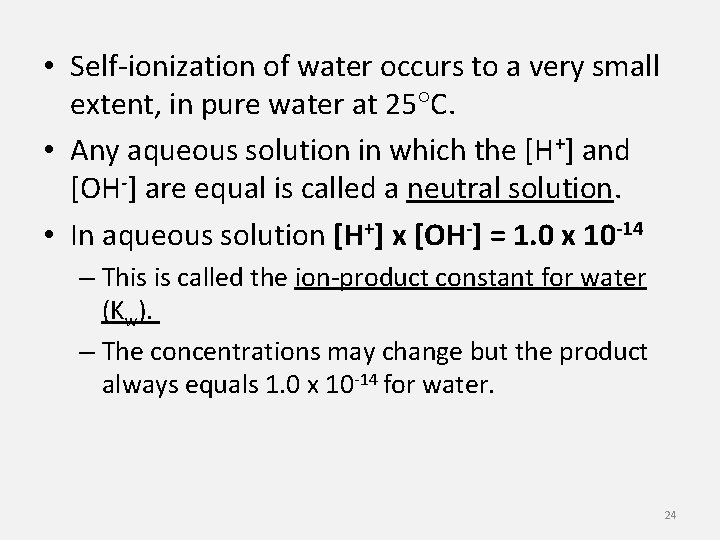
• Self-ionization of water occurs to a very small extent, in pure water at 25 C. • Any aqueous solution in which the [H+] and [OH-] are equal is called a neutral solution. • In aqueous solution [H+] x [OH-] = 1. 0 x 10 -14 – This is called the ion-product constant for water (Kw). – The concentrations may change but the product always equals 1. 0 x 10 -14 for water. 24
![Acidic Solutions • An acidic solution is one in which the [H+] is greater Acidic Solutions • An acidic solution is one in which the [H+] is greater](http://slidetodoc.com/presentation_image/a1fb5578f64bec94fcb6435da01fb699/image-25.jpg)
Acidic Solutions • An acidic solution is one in which the [H+] is greater than the [OH-]. • The [H+] of an acidic solution is greater than 1. 0 x 10 -7 M HCl(g) H+ (aq) + Cl− (aq) 25
![Basic Solution • An basic solution is one in which the [H+] is less Basic Solution • An basic solution is one in which the [H+] is less](http://slidetodoc.com/presentation_image/a1fb5578f64bec94fcb6435da01fb699/image-26.jpg)
Basic Solution • An basic solution is one in which the [H+] is less than the [OH-]. • The [H+] of an acidic solution is less than 1. 0 x 10 -7 M Na. OH Na+ + OH− • Basic solutions are also known as alkaline solutions. 26
![• What is the [OH-] if the [H+] is 1. 0 x 10 • What is the [OH-] if the [H+] is 1. 0 x 10](http://slidetodoc.com/presentation_image/a1fb5578f64bec94fcb6435da01fb699/image-27.jpg)
• What is the [OH-] if the [H+] is 1. 0 x 10 -3? remember: [OH-] x [H+] = 1. 0 x 10 -14 [OH-] = 1. 0 x 10 -14/ 1. 0 x 10 -3= 1. 0 x 10 -11 • What is the [H+] if the [OH-] is 1. 0 x 10 -8 ? [H+] = 1. 0 x 10 -14/ 1. 0 x 10 -8= 1. 0 x 10 -6 • Classify each solution as acidic, basic, or neutral: a) [H+] = 6. 0 x 10 -10 b) [OH-] = 3. 0 x 10 -4 c) [H+] = 6. 0 x 10 -7 d) [H+] = 1. 0 x 10 -7 27
![• What is the [H+] of a solution with a p. OH of • What is the [H+] of a solution with a p. OH of](http://slidetodoc.com/presentation_image/a1fb5578f64bec94fcb6435da01fb699/image-28.jpg)
• What is the [H+] of a solution with a p. OH of 3. 12? Is it acidic, basic, or neutral? p. H= 14 - p. OH p. H= 14 – 3. 12 = 10. 88 [H+]= 10(-p. H) [H+] = 10(-10. 88) = 1. 3 x 10 -11 it is a Basic solution • What is the [H+] of a solution with a p. OH of 9. 18? Is it acidic, basic, or neutral? p. H= 14 - p. OH p. H= 14 – 9. 18 = 4. 82 [H+]= 10(-p. H) [H+] = 10(-4. 82) = 1. 5 x 10 -5 it is an acidic solution 28
![Concentration to p. H and back • Possible equations p. H = -log [H+1] Concentration to p. H and back • Possible equations p. H = -log [H+1]](http://slidetodoc.com/presentation_image/a1fb5578f64bec94fcb6435da01fb699/image-29.jpg)
Concentration to p. H and back • Possible equations p. H = -log [H+1] = 10 -p. H p. OH = -log [OH-1] p. H + p. OH =14 [OH-1] = 10 -p. OH [H+1]x[OH-1]=10 -14 • If the [OH-1] = 4. 68 x 10 -3 determine the p. OH, p. H, [H+1] and if acidic/basic/neutral. -3 ] = 2. 33 = - log [4. 68 x 10 o p. OH o p. H = 14 -2. 33 = 11. 67 o [H+1] =10 -11. 67 = 2. 14 x 10 -12 o Substance is Basic because p. H is 11. 67 29

Strength of Acids & Bases • • • What does it mean to be a STRONG acid? What are the 6 strong acids? (name and formula) What does it mean to be a STRONG base? What are the 8 strong bases? (name and formula) What does it mean to be a WEAK acid or WEAK base?
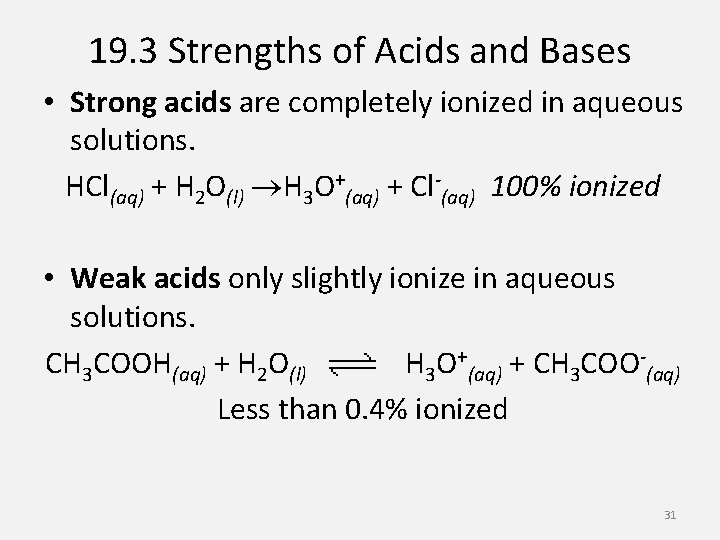
19. 3 Strengths of Acids and Bases • Strong acids are completely ionized in aqueous solutions. HCl(aq) + H 2 O(l) H 3 O+(aq) + Cl-(aq) 100% ionized • Weak acids only slightly ionize in aqueous solutions. CH 3 COOH(aq) + H 2 O(l) H 3 O+(aq) + CH 3 COO-(aq) Less than 0. 4% ionized 31

Six strong acids!! • • • HCl HBr HI HNO 3 H 2 SO 4 HCl. O 4 hydrochloric acid hydrobromic acid hydroiodic acid nitric acid sulfuric acid perchloric acid All other acids are considered weak acids!! 32
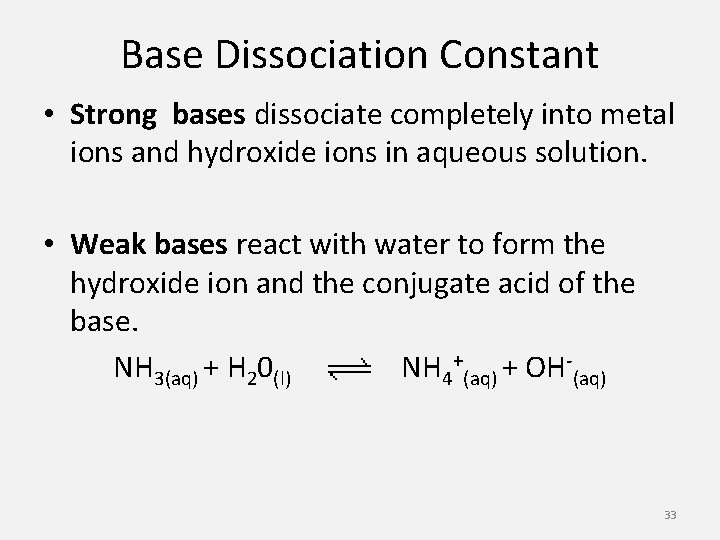
Base Dissociation Constant • Strong bases dissociate completely into metal ions and hydroxide ions in aqueous solution. • Weak bases react with water to form the hydroxide ion and the conjugate acid of the base. NH 3(aq) + H 20(l) NH 4+(aq) + OH-(aq) 33

Eight strong bases • • Li. OH - lithium hydroxide Na. OH - sodium hydroxide KOH - potassium hydroxide Rb. OH - rubidium hydroxide Cs. OH - cesium hydroxide Ca(OH)2 - calcium hydroxide Sr(OH)2 - strontium hydroxide Ba(OH)2 - barium hydroxide 34
![Strong Acids or Bases • [H+] for strong acids equals molarity of acid [H+]= Strong Acids or Bases • [H+] for strong acids equals molarity of acid [H+]=](http://slidetodoc.com/presentation_image/a1fb5578f64bec94fcb6435da01fb699/image-35.jpg)
Strong Acids or Bases • [H+] for strong acids equals molarity of acid [H+]= M of acid • [OH-] for strong bases equals molarity of base [OH-]= M of base • What is the [OH-] and [H+] for the following solutions a. 0. 275 M HCl b. 0. 500 M Na. OH c. 0. 200 M HNO 3 d. 0. 375 M Ba(OH)2 35
![Remember [H+] x [OH-] = 1. 0 x 10 -14 a. 0. 275 M Remember [H+] x [OH-] = 1. 0 x 10 -14 a. 0. 275 M](http://slidetodoc.com/presentation_image/a1fb5578f64bec94fcb6435da01fb699/image-36.jpg)
Remember [H+] x [OH-] = 1. 0 x 10 -14 a. 0. 275 M HCl [H+]= 0. 275 [OH-] = 3. 64 x 10 -14 b. 0. 500 M Na. OH [OH-] =0. 500 [H+]= 2. 00 x 10 -14 c. 0. 200 M HNO 3 [H+]=0. 200 [OH-] = 5. 00 x 10 -14 d. 0. 375 M Ba(OH)2 [OH-] =0. 375 [H+]= 2. 67 x 10 -14 36

19. 4 Neutralization Reactions • When you mix a strong acid with a strong base a neutral solution results. HCl (aq) + Na. OH (aq) Na. Cl (aq) + H 2 O (l) 2 HBr (aq) + Ba(OH)2 (aq) Ba. Br 2(aq) + 2 H 2 O (l) • A neutralization reaction is where an acid and a base react to form a salt and water 37
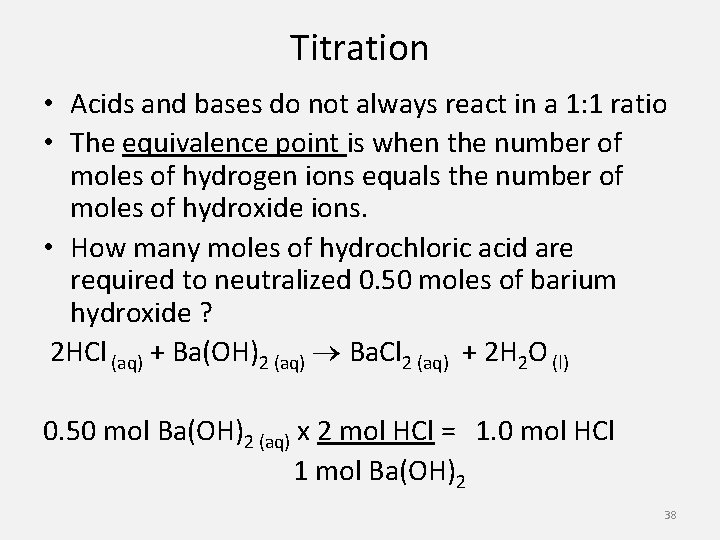
Titration • Acids and bases do not always react in a 1: 1 ratio • The equivalence point is when the number of moles of hydrogen ions equals the number of moles of hydroxide ions. • How many moles of hydrochloric acid are required to neutralized 0. 50 moles of barium hydroxide ? 2 HCl (aq) + Ba(OH)2 (aq) Ba. Cl 2 (aq) + 2 H 2 O (l) 0. 50 mol Ba(OH)2 (aq) x 2 mol HCl = 1. 0 mol HCl 1 mol Ba(OH)2 38

• The process of adding a known amount of a solution of known concentration to determine the concentration of another solution is called titration. • The solution of known concentration is called the standard solution. • We use a buret to add the standard solution. • The solution is added until the indicator changes colors. • The point at which the indicator changes colors is the end point of the titration. 39
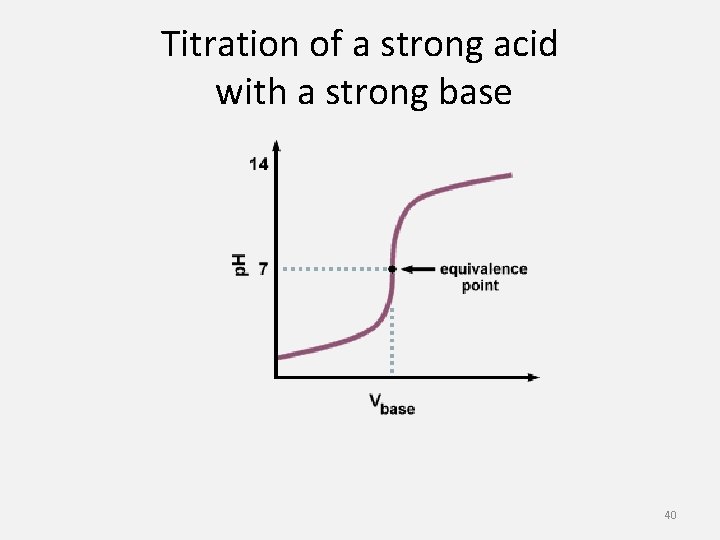
Titration of a strong acid with a strong base 40
Titration Calculations – 1 st type of problem • Determine the volume of 2. 1 M sodium hydroxide needed to titrate 435 m. L of 1. 75 M sulfuric acid. 1 st : write a balanced chemical equation 2 nd : determine the number of moles of reactant (sulfuric acid) available 3 rd: use stoichiometry to calculate the number of moles of reactant (sodium hydroxide) needed 4 th : use molarity to determine the number of m. L needed to complete the neutralization. 41

• Determine the volume of 2. 1 M sodium hydroxide needed to titrate 435 m. L of 1. 75 M sulfuric acid. 1 st : 2 Na. OH + H 2 SO 4 → Na 2 SO 4 +2 H 2 O 2 nd : 0. 435 L x [1. 75 mol/1 L]= 0. 76125 mol H 2 SO 4 3 rd: 0. 76125 mol H 2 SO 4 x 2 mol Na. OH = 1. 5225 mol Na. OH 1 mol H 2 SO 4 4 th : 1. 5225 mol Na. OH x __1 L = 0. 725 L = 725 ml 2. 1 mol 42
• Determine the volume of 3. 3 M hydrochloric acid needed to titrate 355 m. L of 2. 50 M magnesium hydroxide. 1 st : 2 HCl + Mg(OH)2 → Mg. Cl 2 +2 H 2 O 2 nd : 0. 355 L x [2. 50 mol/1 L]= 0. 8875 mol Mg(OH)2 3 rd: 0. 8875 mol Mg(OH)2 x 2 mol HCl = 1. 775 mol HCL 1 mol Mg(OH)2 4 th : 1. 775 mol HCl x __1 L = 0. 538 L = 538 ml HCl 3. 3 mol 43

Titration Calculations – 2 nd type of problem • Titration reveals that 22. 5 ml of 2. 0 M nitric acid are required to neutralize 20 ml of calcium hydroxide. What is the molarity of the calcium hydroxide? 1 st : 2 HNO 3 + Ca(OH)2 → Ca(NO 3)2 +2 H 2 O 2 nd : 0. 0225 L x [2. 0 mol/1 L]= 0. 045 mol HNO 3 3 rd: 0. 045 mol HNO 3 x 1 mol Ca(OH)2 = 0. 0225 mol Ca(OH)2 2 mol HNO 3 4 th : 0. 0225 mol Ca(OH)2 = 1. 125 M Ca(OH)2 0. 020 L 44

• Titration reveals that 11. 2 ml of 3. 5 M barium hydroxide are required to neutralize 50 ml of perchloric acid. What is the molarity of the perchloric acid? 1 st : 2 HCl. O 4 + Ba(OH)2 → Ba(Cl. O 4)2 +2 H 2 O 2 nd : 0. 0112 L x [3. 5 mol/1 L]= 0. 0392 mol Ba(OH)2 3 rd: 0. 0392 mol Ba(OH)2 x 2 mol HCl. O 4 = 0. 0784 mol HCl. O 4 1 mol Ba(OH)2 4 th : 0. 0784 mol HCl. O 4 = 1. 56 M HCl. O 4 0. 050 L 45

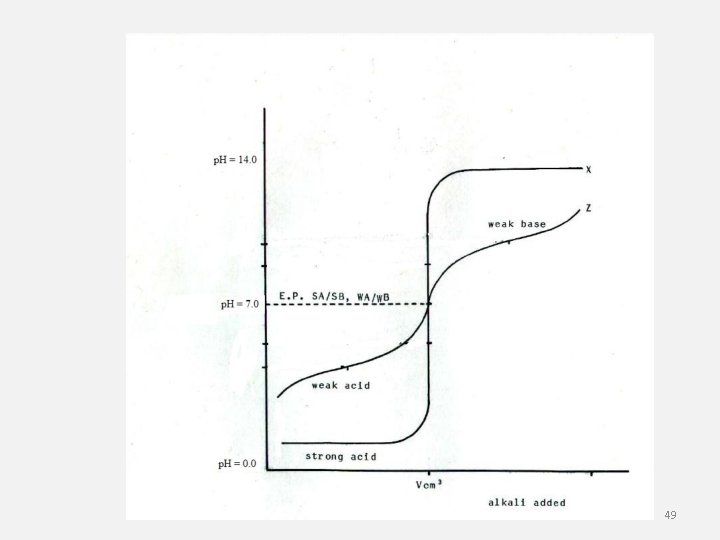
19. 5 • Salt hydrolysis the cations or anions of a dissociated salt remove hydrogen ions from or donate hydrogen ions to the water. • In general, salts that produce acidic solutions contain positive ions that release protons to water • Salts that produce basic solutions contain negative ions that attract protons from water. 46

19. 5 Salts in Solution • A salt consists of an anion from an acid and a cation from a base. It forms as the result of a neutralization reaction. • A buffer is a solution in which the p. H remains relatively constant when small amount of acid or base are added. • A buffer is a solution of a weak acid and one of its salts, or a solution of a weak base and one of its salts. • Buffers are able to resist drastic changes in p. H. 47

• The buffering capacity is the amount of acid or base that can be added to a buffer solution before a significant change in p. H occurs. • Buffer systems are crucial in maintaining human blood p. H within a narrow range. 48
49
- Slides: 49