Acids Bases and Salts AcidBase Theories What is
Acids, Bases and Salts Acid-Base Theories

What is an Acid? The term acid comes from the Latin term acere, which means "sour". ex. Lemon juice, vinegar, and many other foods taste sour.

Acids • Taste sour • Corrosive • React with some metals to produce H 2 • Change Litmus to red acid= red • React with bases to form water and salt. Bases • • Bases taste bitter Feel slippery Corrosive Change Litmus to blue base = Blue • Become less basic when mixed with acids.

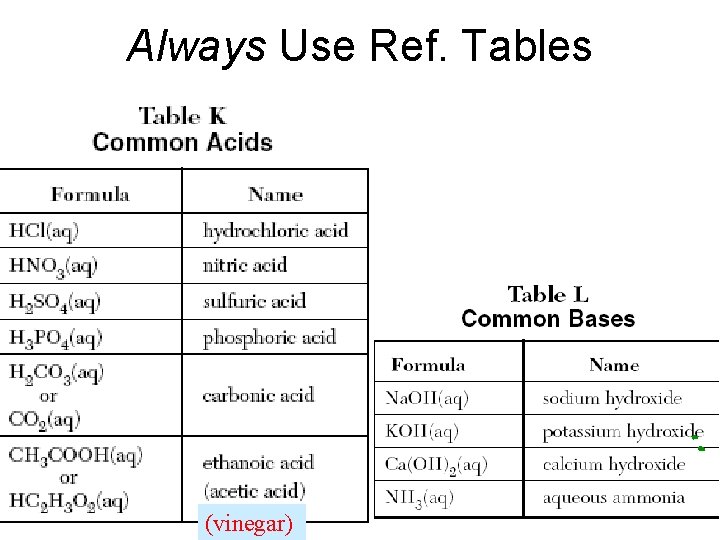
Always Use Ref. Tables (vinegar)
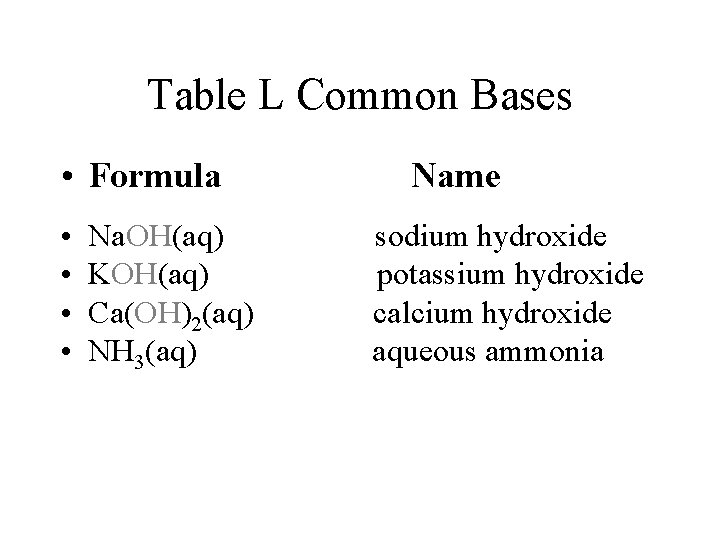
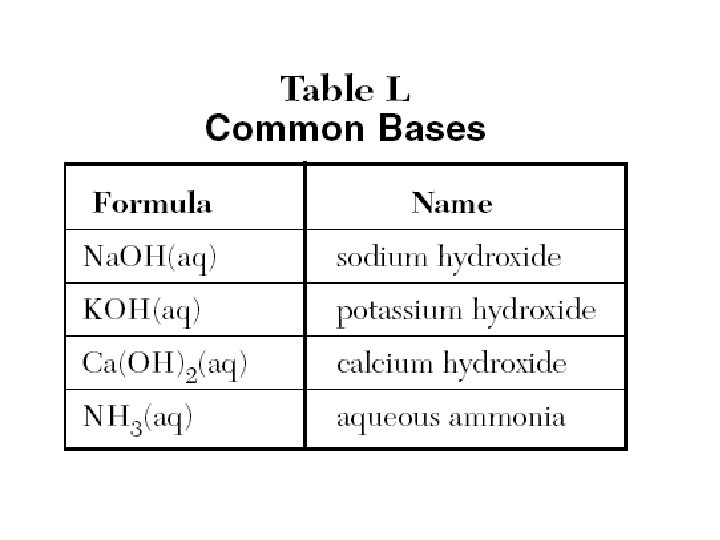
Table L Common Bases • Formula • • Na. OH(aq) KOH(aq) Ca(OH)2(aq) NH 3(aq) Name sodium hydroxide potassium hydroxide calcium hydroxide aqueous ammonia

Svante Arrhenius 1859 - 1927 Swedish chemist, proposed theories of electrolytic disassociation (acids/bases) and greenhouse effect.
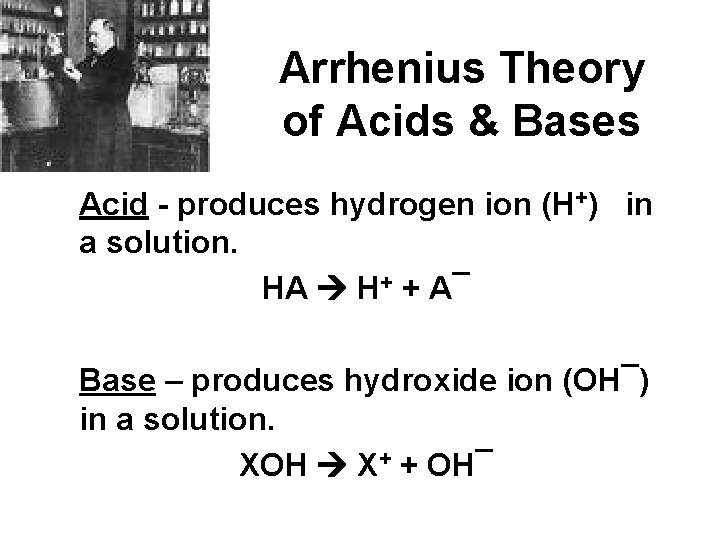
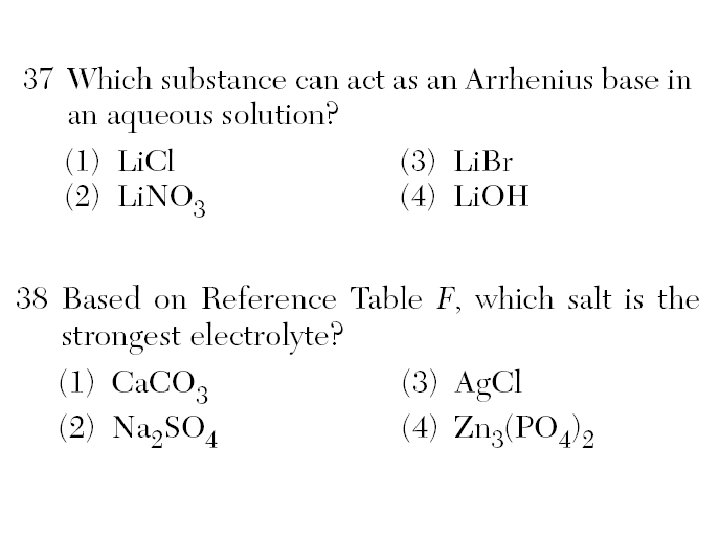
Arrhenius Theory of Acids & Bases Acid - produces hydrogen ion (H+) in a solution. HA H+ + A¯ Base – produces hydroxide ion (OH¯) in a solution. XOH X+ + OH¯

Nature of the Hydrogen Ion or Proton • Proton is so small that does not exist isolated in water. • H + + H 2 O H 3 O + • Hydronium Ion

Nature of Hydroxide Ion • The presence of OH- makes the substance an electrolyte and a base. • Alcohols contain the OH group but are NOT IONIC COMPOUNDS therefore alcohols are NOT bases. • C H O are molecular compounds NOT ionic compounds!!!!

HOW TO NAME ACIDS • NO OXYGEN IN FORMULA • HYDRO ----- ACID HCl Hydrochloric acid HBr Hydrobromic acid HF Hydrofluoric acid H 2 S Hydrosulfuric acid • OXOA CIDS • CONTAIN OXYGEN IN FORMULA • ENDING • OUS if ion ends ITE • IC if ion ends in ATE • Use table E
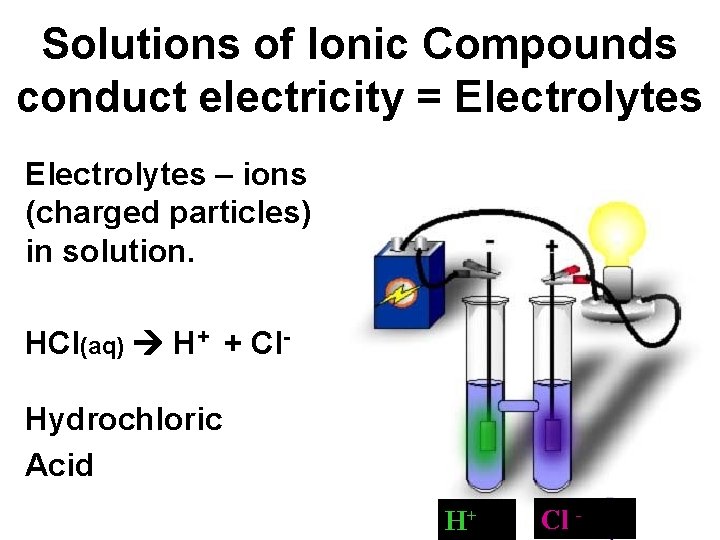
Solutions of Ionic Compounds conduct electricity = Electrolytes – ions (charged particles) in solution. HCl(aq) H+ + Cl. Hydrochloric Acid H+ Cl -
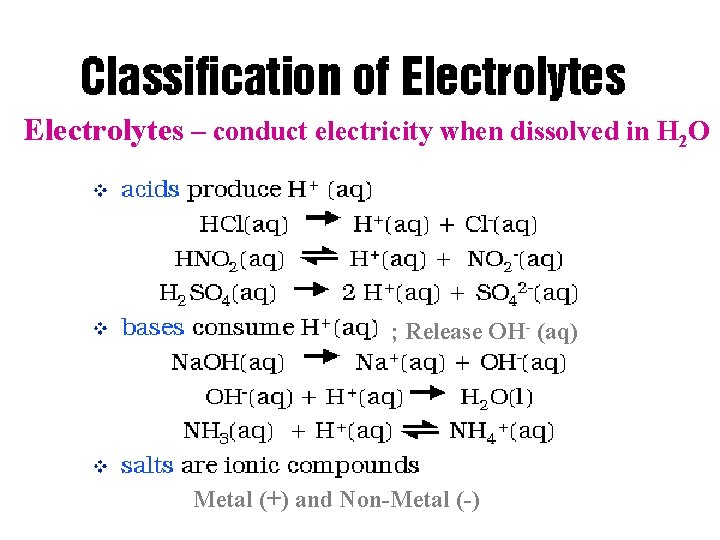
Solutions Electrolytes – conduct electricity when dissolved in H 2 O ; Release OH- (aq) Metal (+) and Non-Metal (-)

Solutions = Salts (ionic), acids, & bases bright Salts = ionic (+) (-) dim Weak Acid or Strong Acid & Base Weak Base dark Covalent = no ions
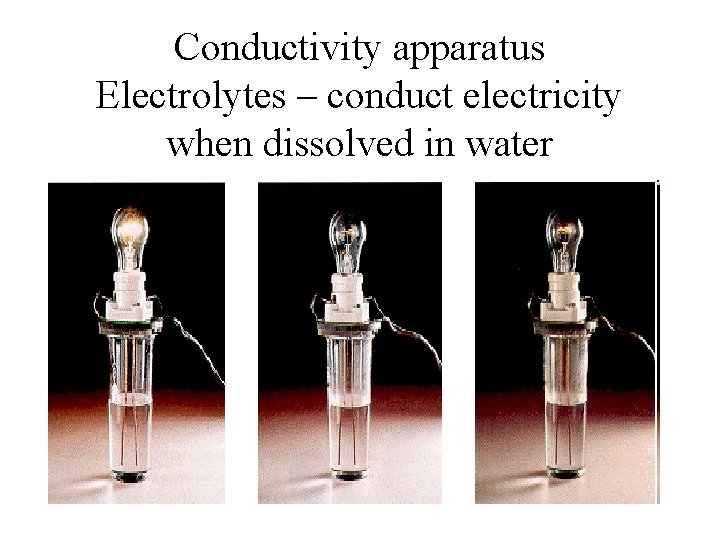
Conductivity apparatus Electrolytes – conduct electricity when dissolved in water

STRONG ACIDS • COMPLETELY DISSOCIATED • If the solution is 2 M in HCl it produces 2 mol of H + and 2 mol of Cl-

WEAK ACIDS • Partially dissociated. Molecules stay together and only some of them dissociate.
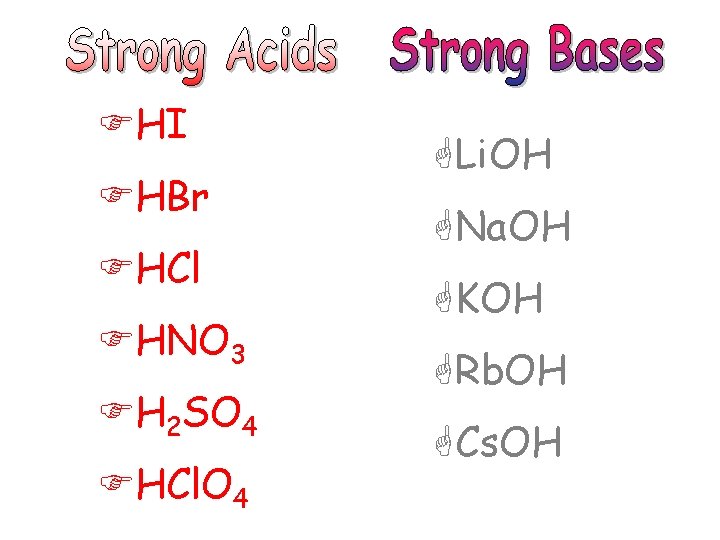
FHI FHBr FHCl FHNO 3 FH 2 SO 4 FHCl. O 4 GLi. OH GNa. OH GKOH GRb. OH GCs. OH

Naming Acids

Binary Acids - composed of hydrogen + 1 other element Ex. HCl Hydrochloric Acid Binary Acids begin with “hydro -” followed by name of other element; modified with an ending of “-ic”
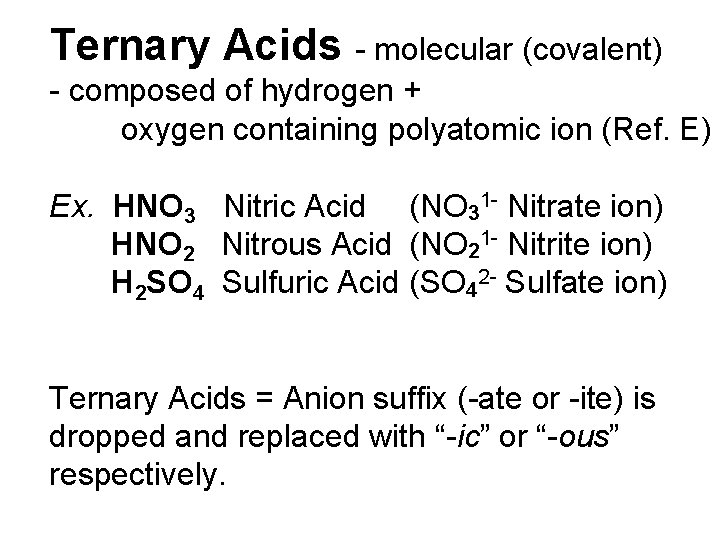
Ternary Acids - molecular (covalent) - composed of hydrogen + oxygen containing polyatomic ion (Ref. E) Ex. HNO 3 Nitric Acid (NO 31 - Nitrate ion) HNO 2 Nitrous Acid (NO 21 - Nitrite ion) H 2 SO 4 Sulfuric Acid (SO 42 - Sulfate ion) Ternary Acids = Anion suffix (-ate or -ite) is dropped and replaced with “-ic” or “-ous” respectively.
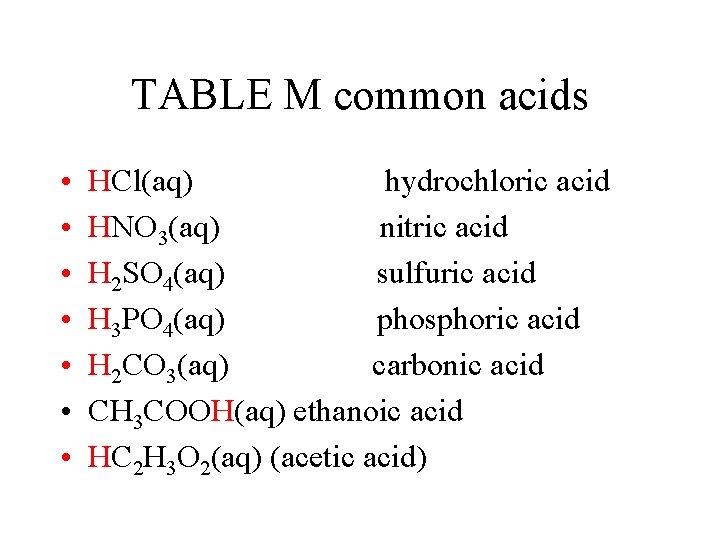
TABLE M common acids • • HCl(aq) hydrochloric acid HNO 3(aq) nitric acid H 2 SO 4(aq) sulfuric acid H 3 PO 4(aq) phosphoric acid H 2 CO 3(aq) carbonic acid CH 3 COOH(aq) ethanoic acid HC 2 H 3 O 2(aq) (acetic acid)

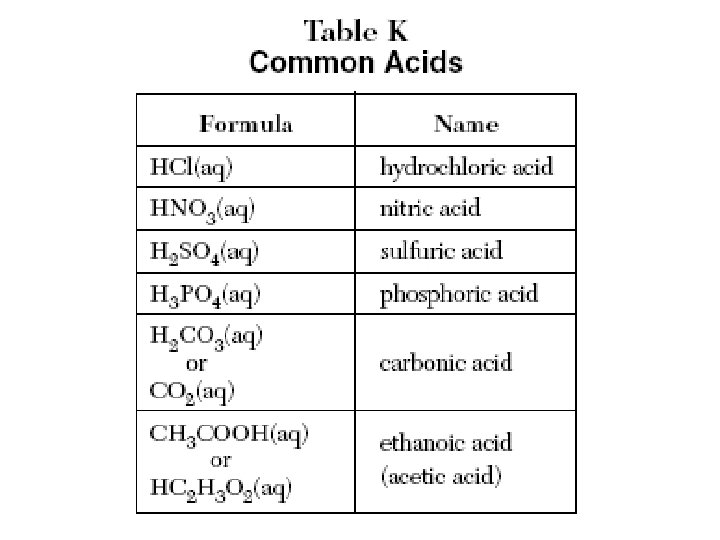
Common Acids – Ref. K Nitric acid (HNO 3) 1) Most is used to make fertilizer 2) Well known for making explosives Hydrochloric acid (HCl) 1) Most is used to clean metals (pickling) 2) Also called muriatic acid Sulfuric acid (H 2 SO 4) 1) The most widely used chemical in the world 2) Most of it is used to make fertilizer 3) It is a good dehydrating agent 4) It is used in car batteries

Properties of Bases 1) Bases feel slippery 2) Bases are electrolytes 3) Bases are corrosive, poisonous, and can cause severe burns (Lye = conc. Na. OH) 4) Bases turn litmus blue; Blue = Base 5) Bases = p. H greater than 7 Big in Base 6) Bases neutralize acids

Common Bases – Ref. L Ammonia NH 3 1) The most widely used base 2) Used in household cleaning materials 3) Used as fertilizer; adds nitrogen to soil Calcium hydroxide (caustic lime) Ca(OH)2 1) Used to make mortar and plaster 2) Used to help neutralize acid soil Sodium hydroxide (Lye) Na. OH 1) One of the strongest bases 2) Used in oven cleaners and drain cleaners
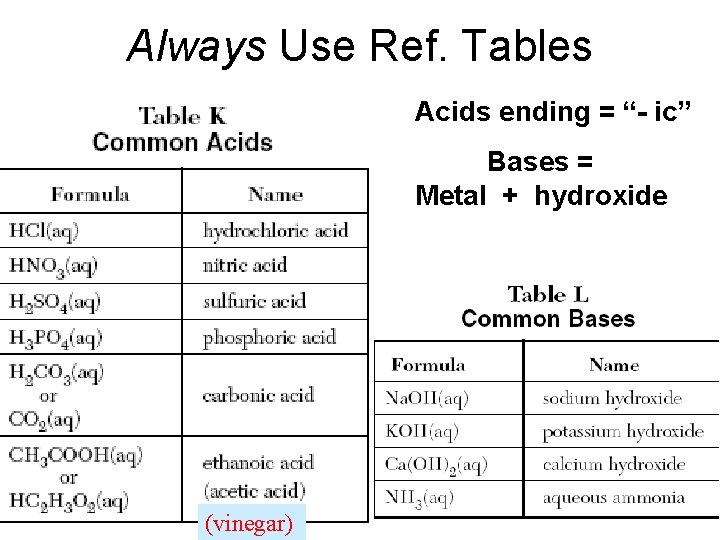
Always Use Ref. Tables Acids ending = “- ic” Bases = Metal + hydroxide (vinegar)

Naming acids worksheet 1. 2. 3. 4. 5. 6. 7. 8. 9. Sulfuric acid Hydrobromic acid Acetic acid Phosphoric acid Hydrosulfuric acid Hydrochloric acid Hypochorous acid Perchloric acid Sulfurous acid 10. Hydroiodic acid 11. Sulfuric acid 12. Chromic acid 13. Permanganic acid 14. Carbonic acid 15. Hydrofluoric acid 16. Oxalic acid 17. Nitric acid 18. Chlorous acid
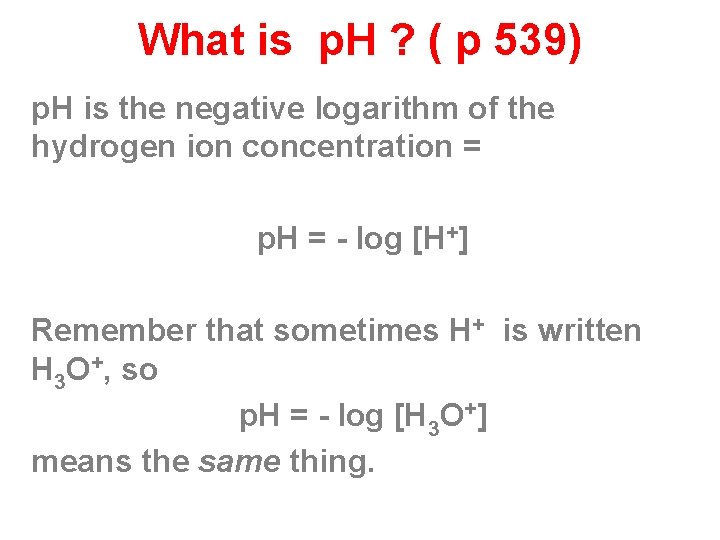
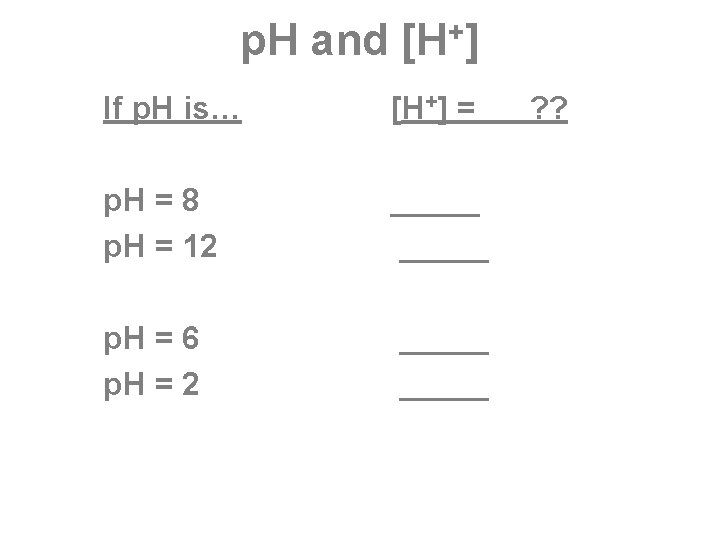
What is p. H ? ( p 539) p. H is the negative logarithm of the hydrogen ion concentration = p. H = - log [H+] Remember that sometimes H+ is written H 3 O+, so p. H = - log [H 3 O+] means the same thing.

May 20 DO NOW! • Find the p. H for • A) 0. 0001 M HCl • B) 0. 01 M HBr • C) 0. 001 M Na. OH
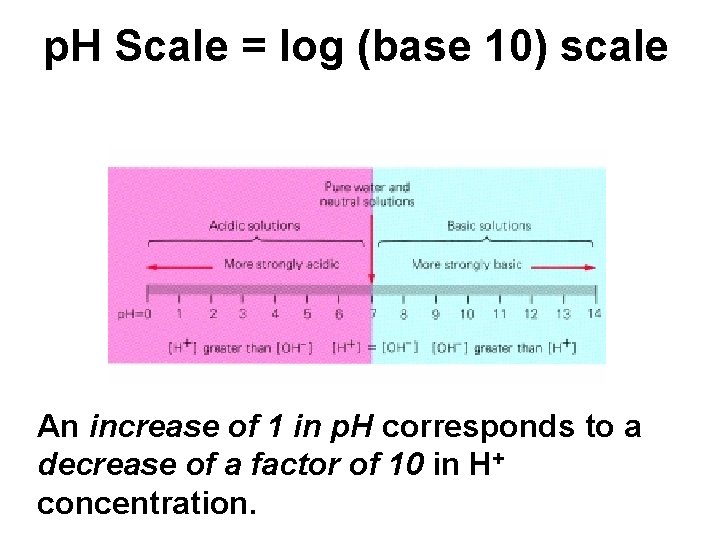
p. H Scale = log (base 10) scale p. H is. . Big in Base & Blue An increase of 1 in p. H corresponds to a decrease of a factor of 10 in H+ concentration.
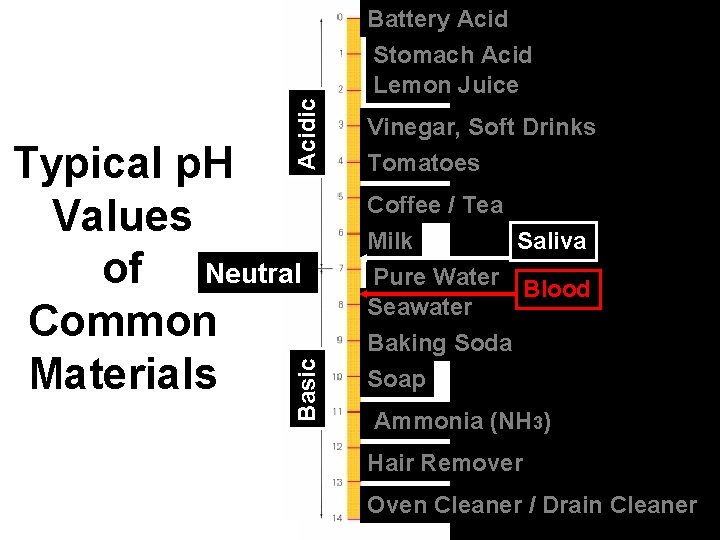
Acidic Basic Typical p. H Values of Neutral Common Materials Battery Acid Stomach Acid Lemon Juice Vinegar, Soft Drinks Tomatoes Coffee / Tea Milk Saliva Pure Water Blood Seawater Baking Soda Soap Ammonia (NH 3) Hair Remover Oven Cleaner / Drain Cleaner
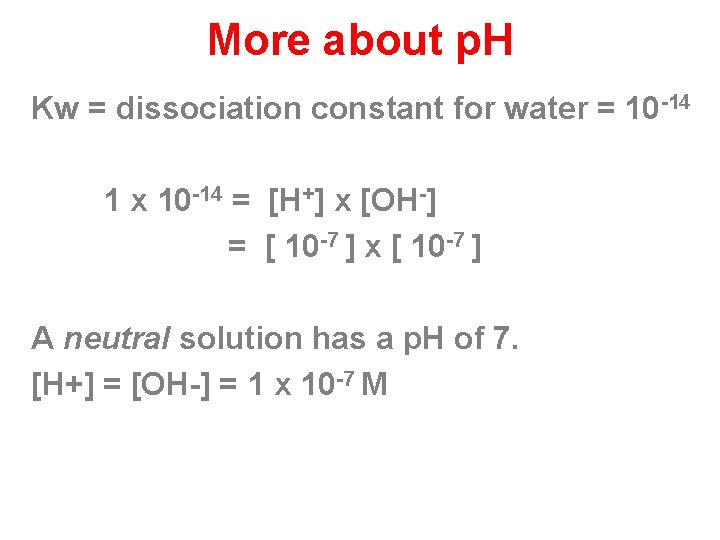
More about p. H Kw = dissociation constant for water = 10 -14 1 x 10 -14 = [H+] x [OH-] = [ 10 -7 ] x [ 10 -7 ] A neutral solution has a p. H of 7. [H+] = [OH-] = 1 x 10 -7 M

Finding p. H for a solution of a strong acid or strong base • For strong acid the concentration of the acid is the same as H+ • For strong bases the concentration of the base is the same as OH-.

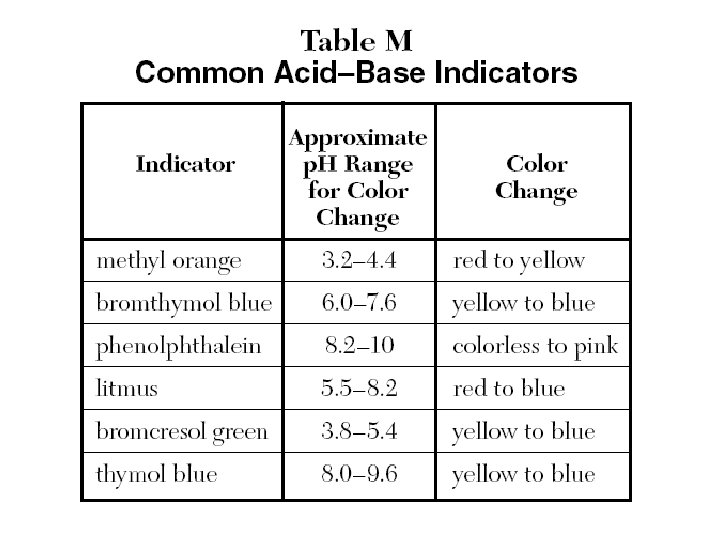
Acid-Base Indicators • A substance that have different colors at different p. H. • They are used to find out the p. H of solutions.
Always Use Ref. Tables (vinegar)

May 22 • Acid – Base Reactions • A) Acids with metals - More detail next unit • B) Neutralization reactions and titration • Section 3 in textbook STUDY!!!! (P 548)
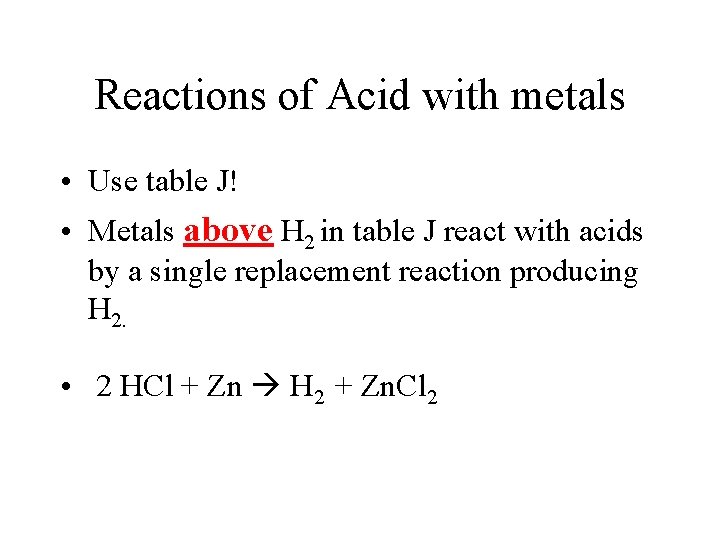
Reactions of Acid with metals • Use table J! • Metals above H 2 in table J react with acids by a single replacement reaction producing H 2. • 2 HCl + Zn H 2 + Zn. Cl 2
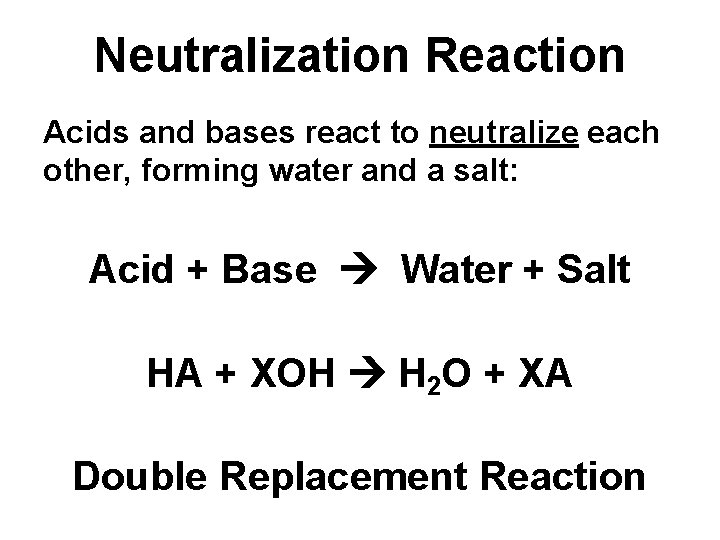
Neutralization Reaction Acids and bases react to neutralize each other, forming water and a salt: Acid + Base Water + Salt HA + XOH H 2 O + XA Double Replacement Reaction

May 23 • Titration – Practice problems • Homework – take home test • MAKE SURE YOU TAKE SCANTRON SHEET AND TEST QUESTIONS. • DUE TUESDAY MAY 27 at the beginning of the period!
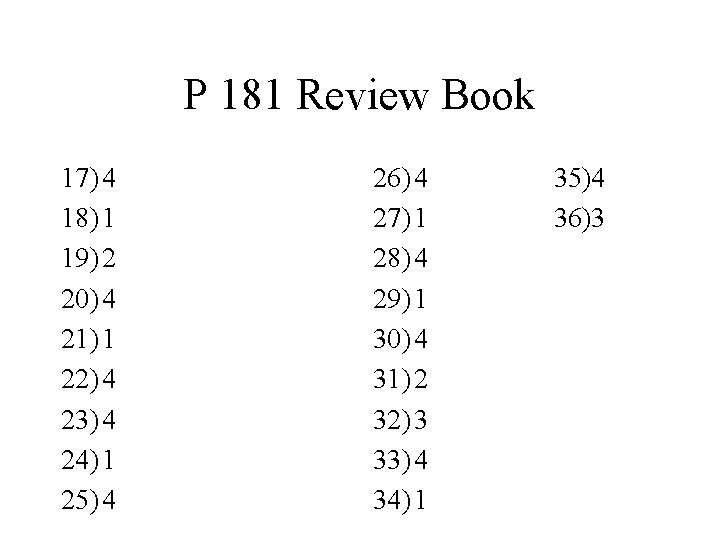
P 181 Review Book 17) 4 18) 1 19) 2 20) 4 21) 1 22) 4 23) 4 24) 1 25) 4 26) 4 27) 1 28) 4 29) 1 30) 4 31) 2 32) 3 33) 4 34) 1 35)4 36)3

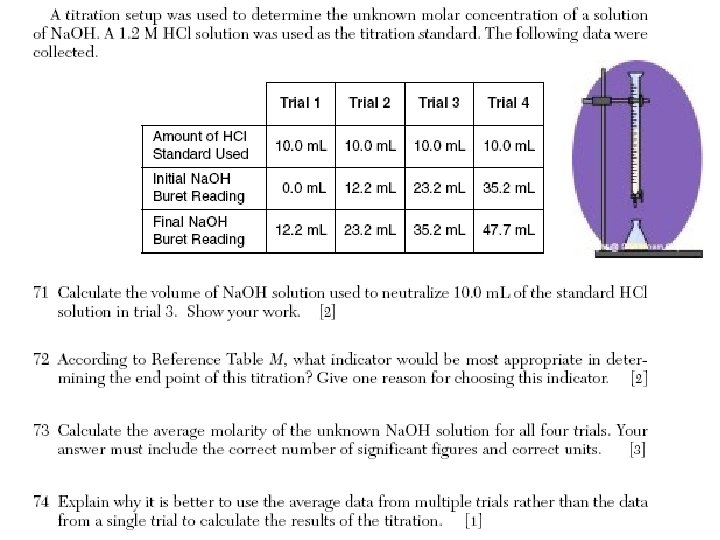
Titration • Is a process that uses a neutralization to determine the concentration of an acid or a base. Concentration in molarity is the amount of moles of solute per liter of solution. When the reaction of neutralization is 1: 1 we use the following formula in a titration • Ma x Va = Mb x V b
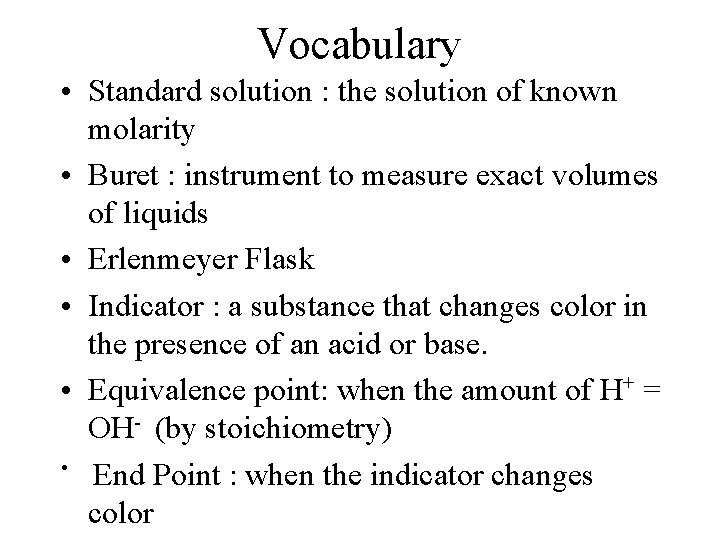
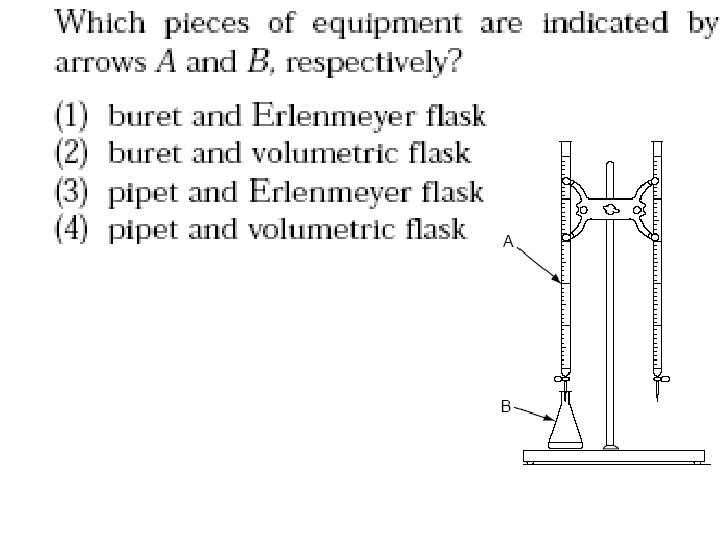
Vocabulary • Standard solution : the solution of known molarity • Buret : instrument to measure exact volumes of liquids • Erlenmeyer Flask • Indicator : a substance that changes color in the presence of an acid or base. • Equivalence point: when the amount of H+ = OH- (by stoichiometry) • End Point : when the indicator changes color
Buret • Instrument to measure exact volumes of liquids
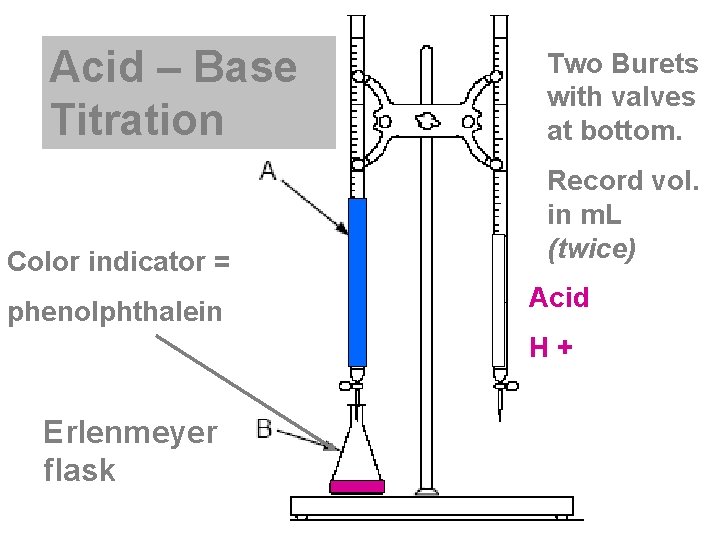
Acid – Base Titration Color indicator = phenolphthalein Two Burets with valves at bottom. Record vol. in m. L (twice) Acid H + Base Erlenmeyer flask OH -
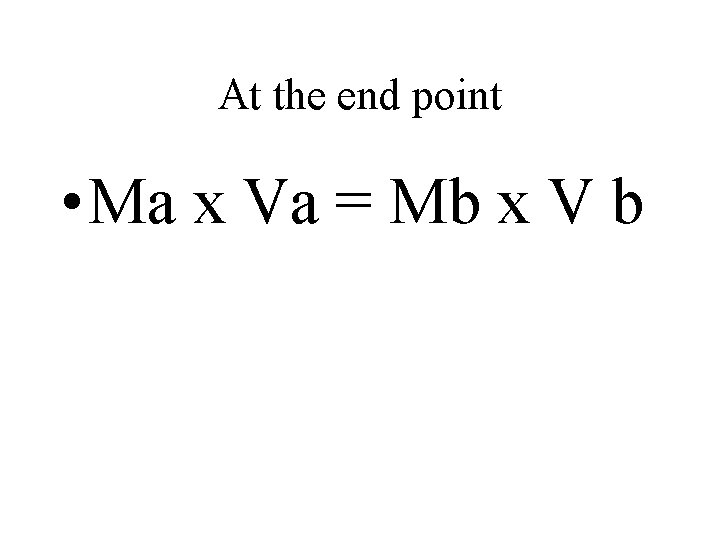
At the end point • Ma x Va = Mb x V b
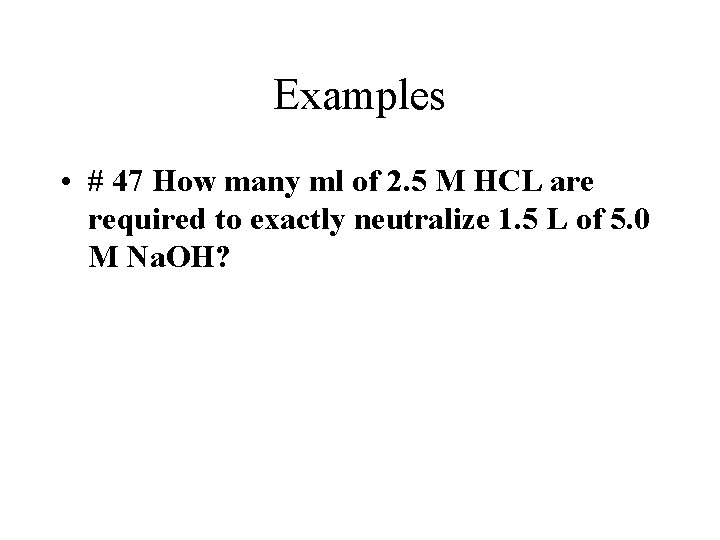
Examples • # 47 How many ml of 2. 5 M HCL are required to exactly neutralize 1. 5 L of 5. 0 M Na. OH?

• # 48 How many ml of. 020 M H 2 SO 4 are required to completely neutralize 40. m. L of 0. 10 M Ca(OH)2
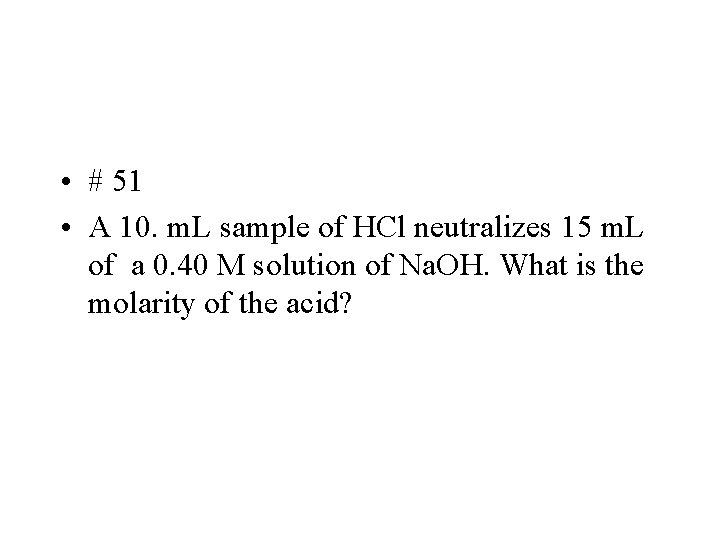
• # 51 • A 10. m. L sample of HCl neutralizes 15 m. L of a 0. 40 M solution of Na. OH. What is the molarity of the acid?





P 177 REVIEW BOOK 1) 2) 3) 4) 5) 6) 7) 8) 2 1 1 2 4 4 1 3 9) 2 10) 2 11) 4 12) 1 13) 1

P 185 answers (titration problems) 40) 4 41) 2 42) 2 43) 1 44) 4 45) 2 46) 25 ml 47) 3000 ml 48) 20 m. L 49) 50 m. L 50) 6 M

P 178 rb answers • 14. chlorate • 15 a) hydrosulfuric acid • b) hydrobromic acid • c) lithium hydroxide • d) magnesium hydroxide • 16. An electrolyte can also be a base or a salt. An indicator can be added to test if is an acid or a base.

Page 187 answers (p. H and indicators) 61) 4 62) 1 63) 3 64) 3 65) 3 66) 2 67) 1 68) 1 69) 4 70) 2 71) Bromocresol green 72) Bet 5. 4 and 6 73) A blue yellow 74) B 75) 1

Handout Answers 1) 2) 3) 4) 5) 6) 7) 2 2 4 2 3 3 4 • p. H=6 • Methyl orange/ Bromthymol blue • Bromocresol green
- Slides: 63