Acids Bases and p H Lesson 5 Acids

Acids, Bases and p. H Lesson 5

Acids and Bases

Arrhenius Model of Acids and Bases The classical, or Arrhenius, model was developed by Svante Arrhenius in the nineteenth century. n He defined an acid as any substance that liberates or yields hydrogen ions (H+) or protons in water. n
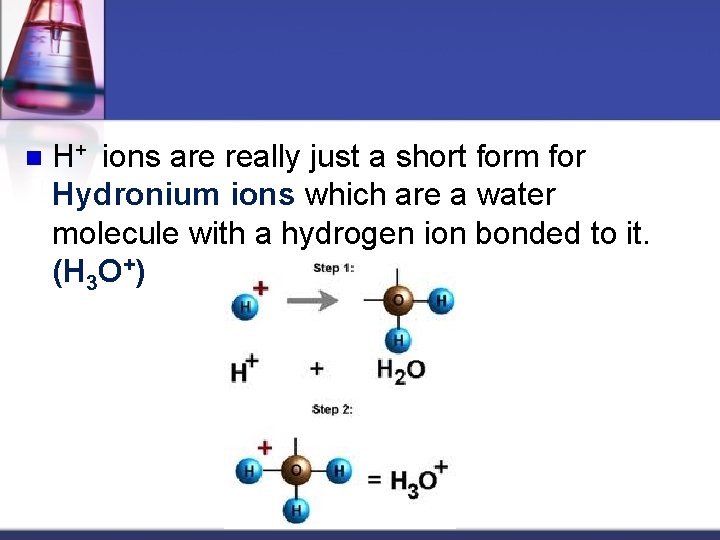
n H+ ions are really just a short form for Hydronium ions which are a water molecule with a hydrogen ion bonded to it. (H 3 O+)
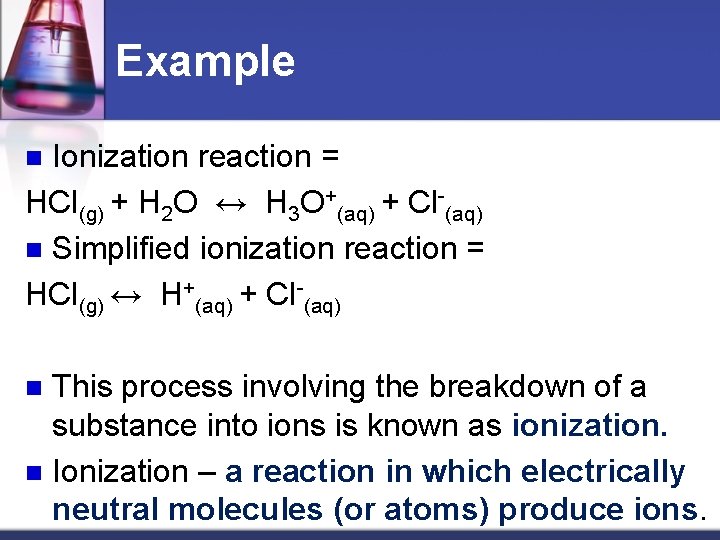
Example Ionization reaction = HCl(g) + H 2 O ↔ H 3 O+(aq) + Cl-(aq) n Simplified ionization reaction = HCl(g) ↔ H+(aq) + Cl-(aq) n This process involving the breakdown of a substance into ions is known as ionization. n Ionization – a reaction in which electrically neutral molecules (or atoms) produce ions. n

An Arrhenius base is a substance that dissociates in water to produce hydroxide ions, OH. n Two examples of strong, or almost completely dissociated bases are potassium hydroxide, KOH, and sodium hydroxide, Na. OH or lye. n KOH(s) + H 2 O(l) ↔ K+(aq) + OH-(aq) + H 2 O(l) n

n Most solutions formed by the reaction of polar molecular compounds with water are observed to have either acidic or basic properties.
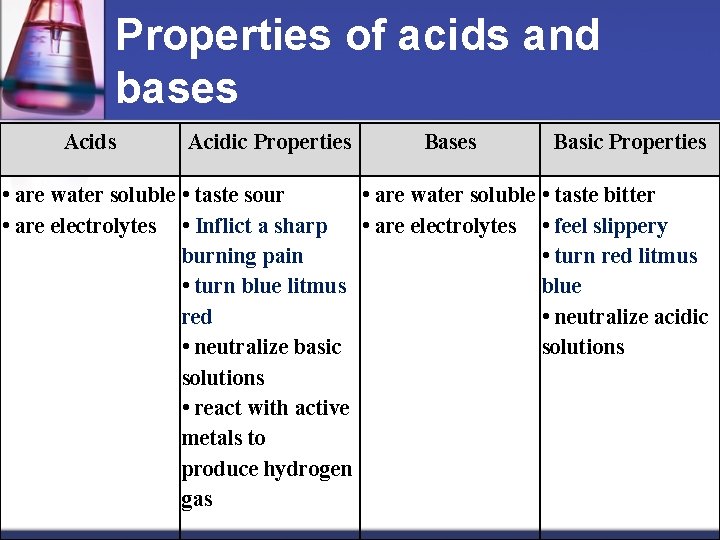
Properties of acids and bases Acidic Properties Basic Properties • are water soluble • taste sour • are water soluble • taste bitter • are electrolytes • Inflict a sharp • are electrolytes • feel slippery burning pain • turn red litmus • turn blue litmus blue red • neutralize acidic • neutralize basic solutions • react with active metals to produce hydrogen gas

Strong versus Weak n Strong acids are ones that dissolve completely into their ions. HCl, and HNO 3 are strong acids. n Strong acid (> 99% ionized) n HCl(aq) + H 2 O(l) H 3 O+(aq) + Cl-(aq) 100% dissociated - all the HCl breaks down into ions.

n Strong and weak acids can be either concentrated or dilute. n Typical strong acids: HCl, HBr, HI, HNO 3, H 2 SO 4, HCl. O 4
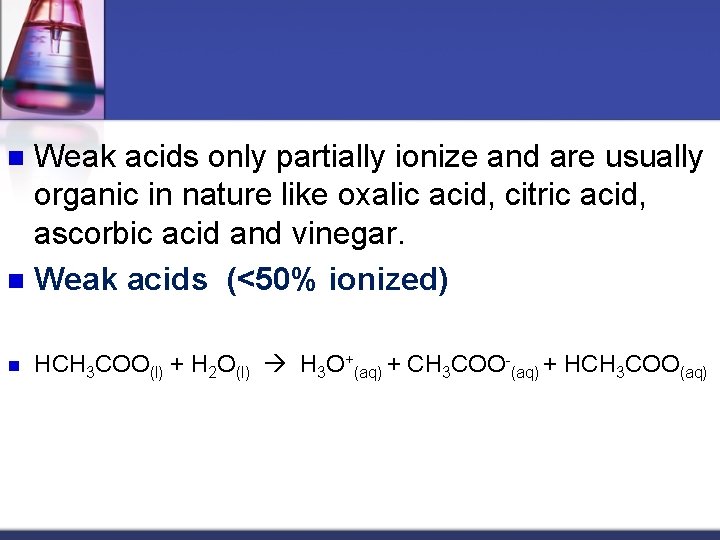
Weak acids only partially ionize and are usually organic in nature like oxalic acid, citric acid, ascorbic acid and vinegar. n Weak acids (<50% ionized) n n HCH 3 COO(l) + H 2 O(l) H 3 O+(aq) + CH 3 COO-(aq) + HCH 3 COO(aq)
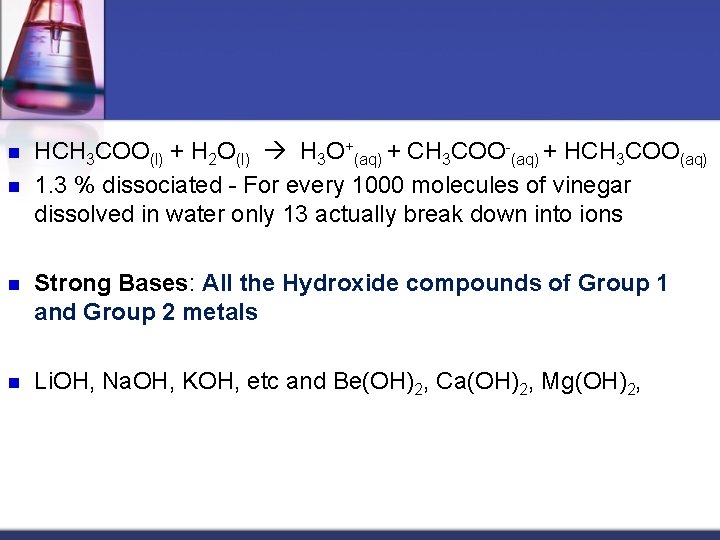
n n HCH 3 COO(l) + H 2 O(l) H 3 O+(aq) + CH 3 COO-(aq) + HCH 3 COO(aq) 1. 3 % dissociated - For every 1000 molecules of vinegar dissolved in water only 13 actually break down into ions n Strong Bases: All the Hydroxide compounds of Group 1 and Group 2 metals n Li. OH, Na. OH, KOH, etc and Be(OH)2, Ca(OH)2, Mg(OH)2,

Hydrogen Ion and Hydroxide Ion Concentrations Determine the concentration of hydrogen or hydroxide ions in each of the following solutions of strong acids or bases. n (Because they are strong we can assume 100% ionization) n
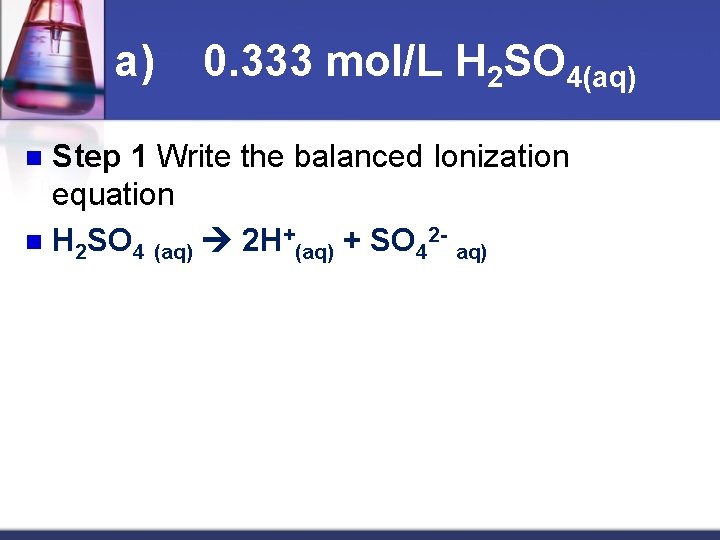
a) 0. 333 mol/L H 2 SO 4(aq) Step 1 Write the balanced Ionization equation n H 2 SO 4 (aq) 2 H+(aq) + SO 42 aq) n
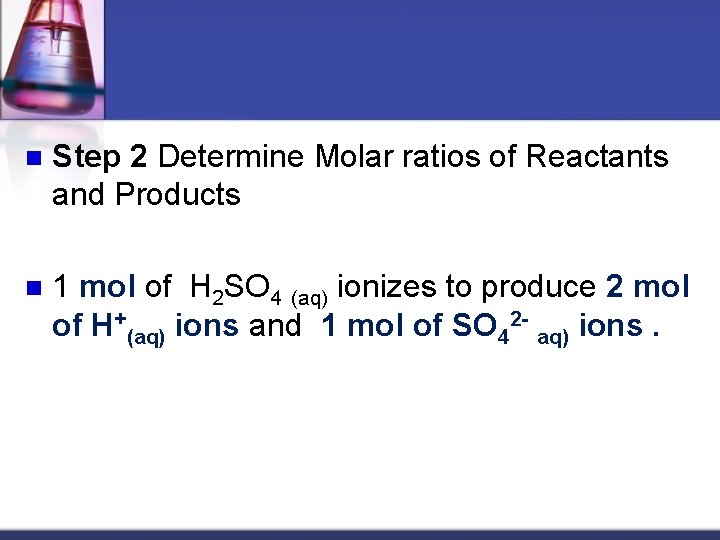
n Step 2 Determine Molar ratios of Reactants and Products n 1 mol of H 2 SO 4 (aq) ionizes to produce 2 mol of H+(aq) ions and 1 mol of SO 42 aq) ions.
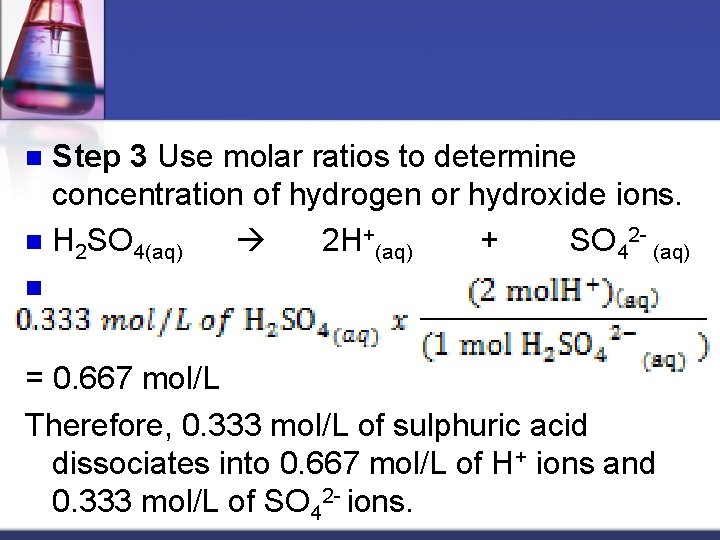
Step 3 Use molar ratios to determine concentration of hydrogen or hydroxide ions. n H 2 SO 4(aq) 2 H+(aq) + SO 42 - (aq) n n = 0. 667 mol/L Therefore, 0. 333 mol/L of sulphuric acid dissociates into 0. 667 mol/L of H+ ions and 0. 333 mol/L of SO 42 - ions.
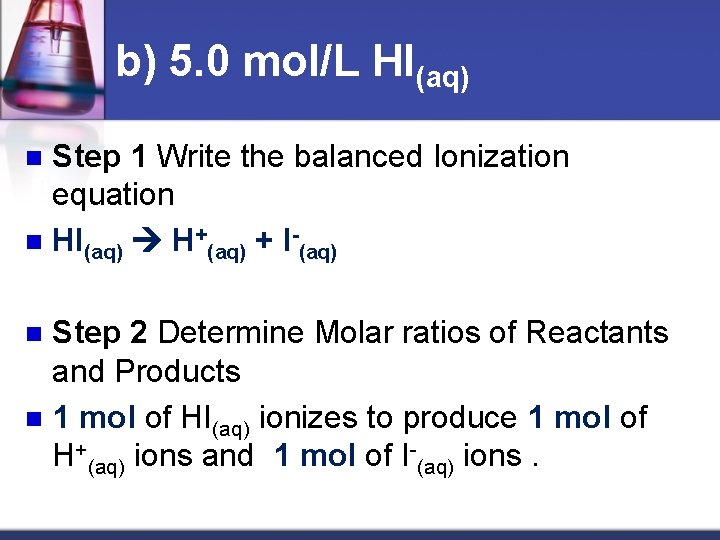
b) 5. 0 mol/L HI(aq) Step 1 Write the balanced Ionization equation n HI(aq) H+(aq) + I (aq) n Step 2 Determine Molar ratios of Reactants and Products n 1 mol of HI(aq) ionizes to produce 1 mol of H+(aq) ions and 1 mol of I-(aq) ions. n

Step 3 Use molar ratios to determine concentration of hydrogen or hydroxide ions. n HI(aq) H+(aq) + I-(aq) n n 5. 0 mol/L of HI(aq) x ( 1 mol H+(aq)) n ( 1 mol HI(aq) ) n = 5. 0 mol/L n

n Therefore, 5. 0 mol/L of HI dissociates into 5. 0 mol/L of H+ ions and 5. 0 mol/L of I- ions.
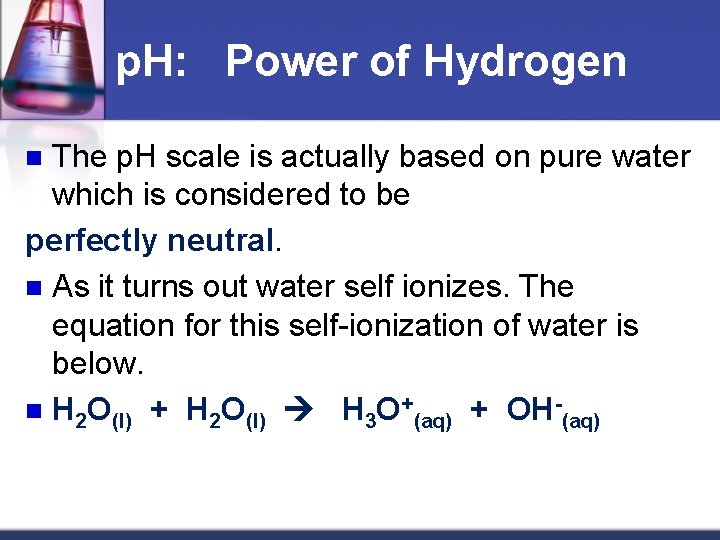
p. H: Power of Hydrogen The p. H scale is actually based on pure water which is considered to be perfectly neutral. n As it turns out water self ionizes. The equation for this self-ionization of water is below. n H 2 O(l) + H 2 O(l) H 3 O+(aq) + OH (aq) n

n The concentration of H 3 O+ and OH- are both 1. 0 X 10 7 mol/L. This can be converted into a p. H of 7.

n This is just proving how it works to DO NOT worry about this or let it confuse you. n The p. H scale is out of 14 and 1. 0 X 10 -7 X 1. 0 X 10 -7 = 1. 0 X 10 -14. This is not a coincidence
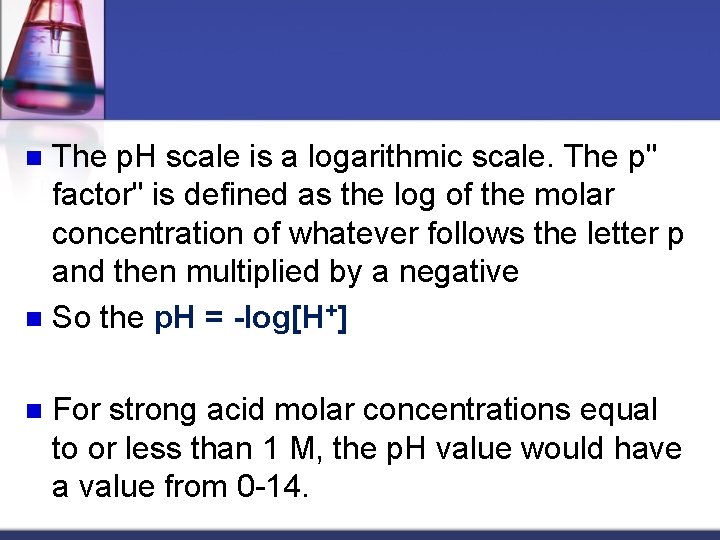
The p. H scale is a logarithmic scale. The p" factor" is defined as the log of the molar concentration of whatever follows the letter p and then multiplied by a negative n So the p. H = log[H+] n n For strong acid molar concentrations equal to or less than 1 M, the p. H value would have a value from 0 -14.
![Example If the Hydrogen ion concentration is 0. 1 mol/L n Then the [OH-] Example If the Hydrogen ion concentration is 0. 1 mol/L n Then the [OH-]](http://slidetodoc.com/presentation_image_h/345ab3370b3ac742a82ed3337dceabac/image-24.jpg)
Example If the Hydrogen ion concentration is 0. 1 mol/L n Then the [OH-] could be found n [OH-] = 1 X 10 -14 / 1 X 10 -1 = 1 X 10 -13 n The p. OH = -log[OH-] = -log(1 X 10 -13) n = -(log 1 + log 10 -13) = -(0 + -13) = -(-13) = 13 n For a [H+] = 0. 1 = 1 X 10 -1 n Then p. H = -log 1 X 10 -1 = -(0 + -1) = 1 n Therefore the p. H + p. OH = 14 n
![Examples: Calculate the p. H of a solution that has a [OH -] = Examples: Calculate the p. H of a solution that has a [OH -] =](http://slidetodoc.com/presentation_image_h/345ab3370b3ac742a82ed3337dceabac/image-25.jpg)
Examples: Calculate the p. H of a solution that has a [OH -] = 1 X 10 -5 M 1. Determine p. OH n p. OH = log [OH ] = [log 1 X 10 5 ] = 5 n 2. Determine the p. H knowing that p. H + p. OH = 14 n p. H = 14 p. OH = 14 5 = 9

Practice Problem n 1. Now here is an example for you to work out. Given 0. 02 M Ba(OH)2 solution: Step 1 Determine the Hydroxide ion molar concentration n Ba(OH)2(aq) Ba 2 (aq) + 2 OH (aq)
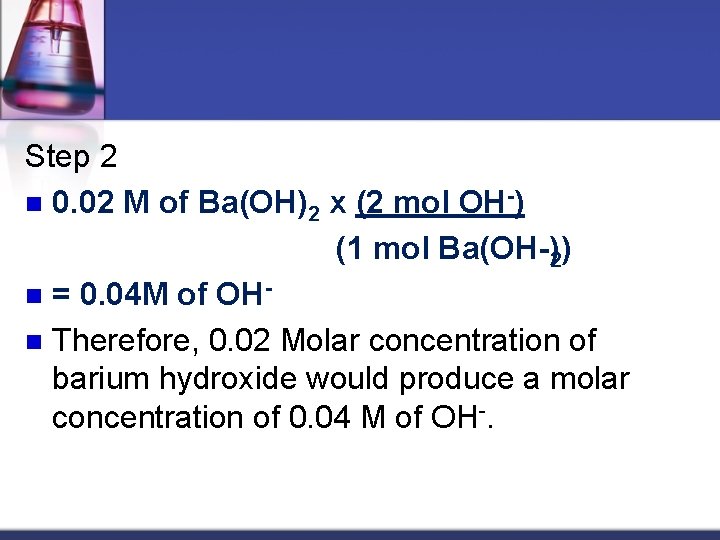
Step 2 n 0. 02 M of Ba(OH)2 x (2 mol OH ) (1 mol Ba(OH )2) n = 0. 04 M of OH n Therefore, 0. 02 Molar concentration of barium hydroxide would produce a molar concentration of 0. 04 M of OH-.
![2. Determine p. OH n p. OH = log [OH ] = [log 4 2. Determine p. OH n p. OH = log [OH ] = [log 4](http://slidetodoc.com/presentation_image_h/345ab3370b3ac742a82ed3337dceabac/image-28.jpg)
2. Determine p. OH n p. OH = log [OH ] = [log 4 X 10 2 ] = 1. 4 n 3. Determine the p. H knowing that p. H + p. OH = 14 n p. H = 14 p. OH n = 14 – 1. 4 = 12. 6
![4. Determine the hydrogen ion concentration n [H+] = 10 -p. H n Substitute 4. Determine the hydrogen ion concentration n [H+] = 10 -p. H n Substitute](http://slidetodoc.com/presentation_image_h/345ab3370b3ac742a82ed3337dceabac/image-29.jpg)
4. Determine the hydrogen ion concentration n [H+] = 10 -p. H n Substitute p. H value into the equation n [H+] = 10 12. 6 n = 2. 5 x 10 13 M

Acid and Bases Reactions (P 393)
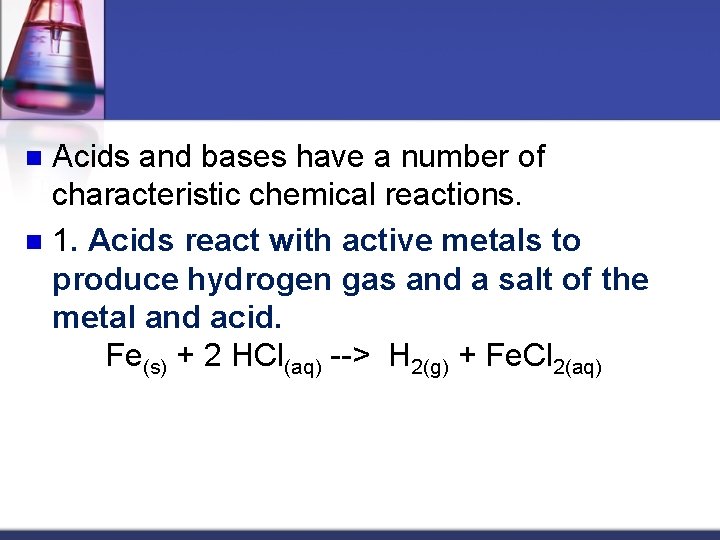
Acids and bases have a number of characteristic chemical reactions. n 1. Acids react with active metals to produce hydrogen gas and a salt of the metal and acid. Fe(s) + 2 HCl(aq) --> H 2(g) + Fe. Cl 2(aq) n

2. Acids react with carbonates to produce a salt and hydrogen carbonate. The hydrogen carbonate immediately decomposes into carbon dioxide and water. 2 HCl(aq) + Na 2 CO 3(aq) 2 Na. Cl(aq) + H 2 CO 3(aq) H 2 O(l) + CO 2(g) 2 HCl(aq)+Na 2 CO 3(aq) 2 Na. Cl(aq)+H 2 O(l) + CO 2(aq) n

3. Acids react with a base to form salt and water. n When an acid and a base of equal strength are mixed they react to form products that have a p. H of near or at 7, this is defined as a neutralization reaction. A Neutralization reaction always produces a salt and water. n The products of a neutralization reaction of an acid and a base are salt and water. n Acid + Base Salt + water

Example: Hydrochloric acid + sodium hydroxide sodium chloride + water n HCl(aq) + Na. OH(aq) Na. Cl + H 2 O n During a Neutralization reaction, the hydrogen ion from the acid reacts with the hydrogen ion from the base to form water. n H+ + OH HOH = H 2 O n

The salt from the reaction is dissolved in the water to form a salt solution which is neutral. n There are many types of salts formed; Na. Cl is just one of many. n

Questions Page 367 # 2, 3 n Page 371 # 2 -6 n Focus on these ones n Page 374 # 9, 10 n Page 375 # 2, 3 5 n
- Slides: 36