Acids Bases and p H Chapter 9 Section

Acids, Bases, and p. H Chapter 9 Section 1

D 12 � D 12 - Explain the chemical composition of acids and bases, and explain the change of p. H in neutralization reactions.

Acids � What do you know about acids? � Can you name some acids? � Have you ever had lemonade? The juice of a lemon is an acid. So, this means that not all acids are alike, since you can safely drink lemonade, but not some other acids.

So, what are acids? � Acids are compounds, which means that they are made of more than one type of element. � One of the elements is always HYDROGEN. � Properties: ◦ ◦ ◦ Taste sour They will turn blue litmus paper red They will conduct electric current They are corrosive They can sting in an open cut (weaker acids), or can damage your skin (stronger acids)
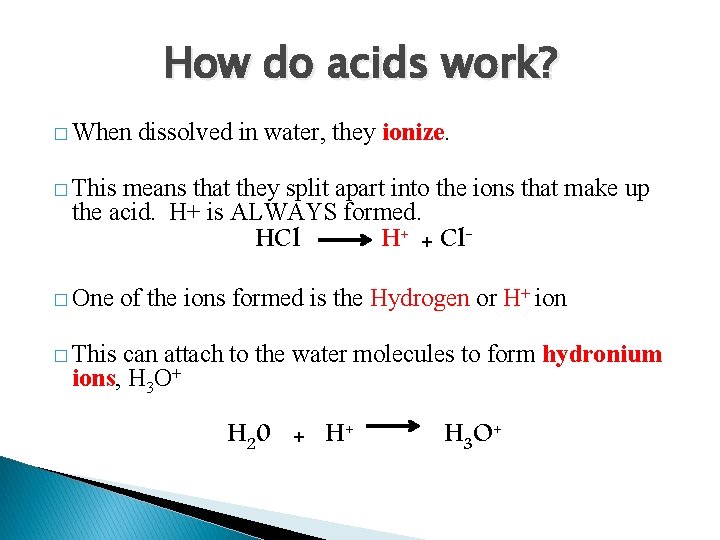
How do acids work? � When dissolved in water, they ionize. � This means that they split apart into the ions that make up the acid. H+ is ALWAYS formed. HCl � One H+ + Cl- of the ions formed is the Hydrogen or H+ ion � This can attach to the water molecules to form hydronium ions, H 3 O+ H 20 + H 3 O+
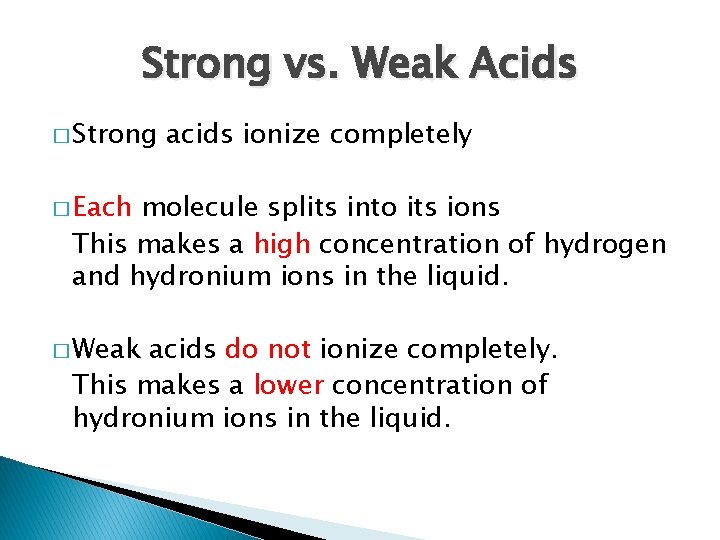
Strong vs. Weak Acids � Strong acids ionize completely � Each molecule splits into its ions This makes a high concentration of hydrogen and hydronium ions in the liquid. � Weak acids do not ionize completely. This makes a lower concentration of hydronium ions in the liquid.
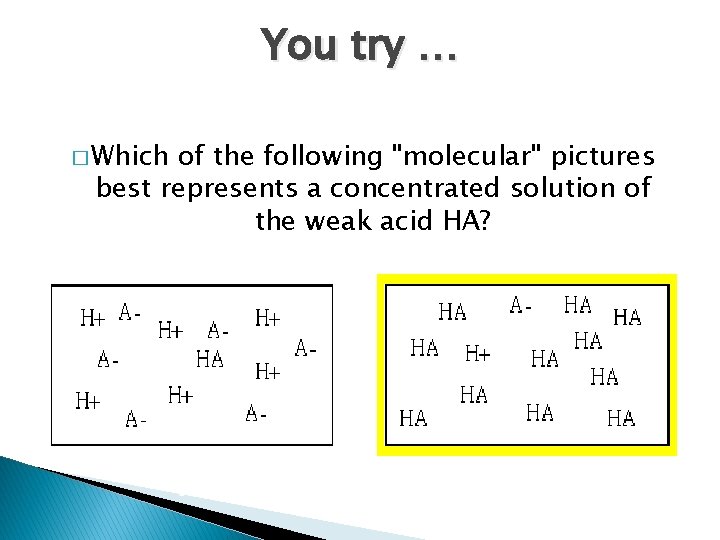
You try … � Which of the following "molecular" pictures best represents a concentrated solution of the weak acid HA? A B

Nitric Acid HNO 3 + H 2 O H 3 O+ NO 3 - � Since there is complete ionization in water, this is a strong acid � An electrolyte is a liquid which can conduct electricity � Nitric Acid conducts electricity well, which means it is a strong electrolyte
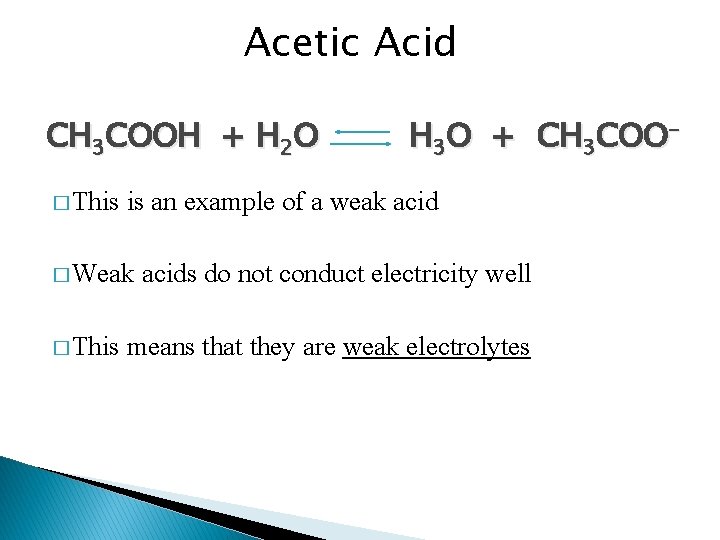
Acetic Acid CH 3 COOH + H 2 O � This is an example of a weak acid � Weak � This H 3 O + CH 3 COO- acids do not conduct electricity well means that they are weak electrolytes

Acid Safety � Acids are corrosive � They can damage living tissues � Acidic vapors can be harmful to eyes, mouth and lungs � Wear safety goggles, gloves, lab apron � Overall, be careful!

What’s next? Bases!

Bases � What do you know about bases? � Can you name some bases? � Do you use soaps or detergents? These are bases. Like acids, not all bases are alike.

So, what are bases? �A base is a compound that increases the number of hydroxide ions (OH-) when mixed with water. � Properties: �A basic solution tastes bitter. � A basic solution feels slippery. � They will turn red litmus paper blue. � Strong bases can also damage the skin.
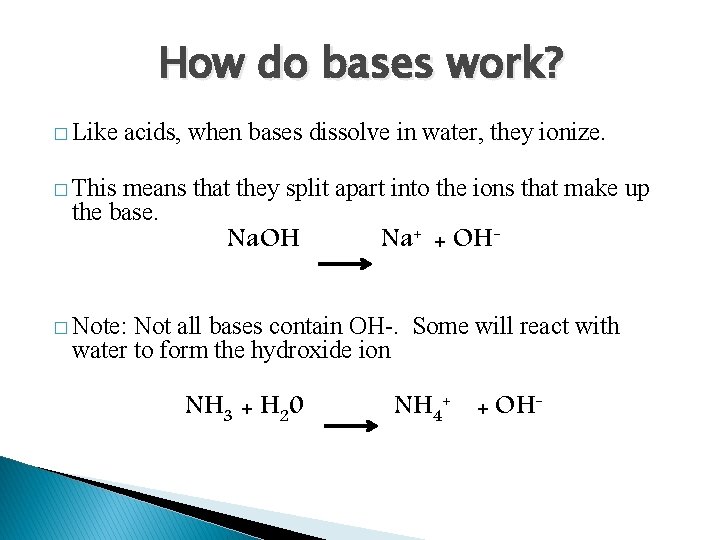
How do bases work? � Like acids, when bases dissolve in water, they ionize. � This means that they split apart into the ions that make up the base. Na. OH Na+ + OH- � Note: Not all bases contain OH-. Some will react with water to form the hydroxide ion NH 3 + H 20 NH 4+ + OH-
Strong and Weak Bases � These work in a way that is similar to acids. � Strong bases cause a high concentration of OH- ions in the solution. � Weak bases cause a low concentration of OHions in the solution.

Base Safety � Like acids, bases are corrosive and can damage living tissues � Wear safety goggles, gloves, and a lab apron!

What’s next? � How do determine if something is an acid or a base? � How do we measure acid and base concentration or strength?

Homework � Read pages 293 -297.
Using Indicators with Acids and Bases
Detecting Acids and Bases � You can determine if a substance is an acid or base by using an indicator. � INDICATOR - A substance that changes color in the presence of an acid or a base. � Indicators measure the level of hydronium ions. This level determines if a substance is acidic or basic.
Indicators � Cabbage juice can be used as an indicator. � Litmus paper is a common indicator. Litmus strips are small pieces of paper that are treated with a substance that changes color when exposed to acids and bases Ø An acid turns blue litmus paper red Ø A base turns red litmus paper blue
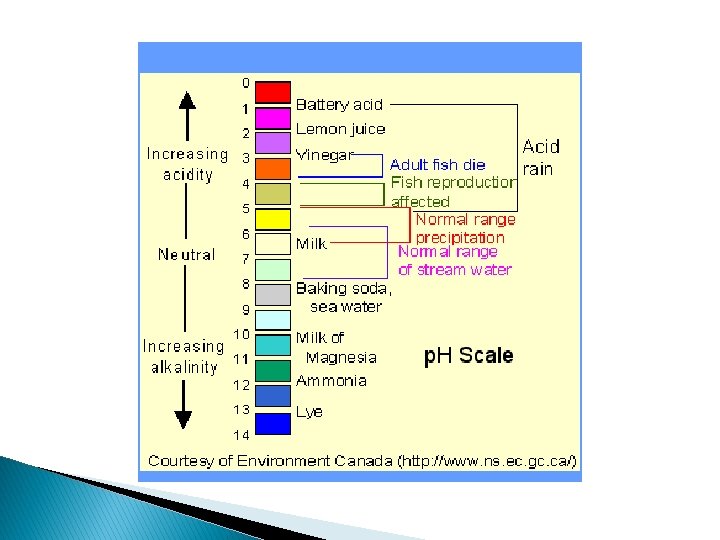
More specific indicators � Litmus papers only function to let you know if a substance is acidic or basic. � They do not indicate strength or weakness of the solution. � To determine how acidic or basic a solution is, we use p. H values. � p. H is the measure of the concentration of hydronium ions in a solution.
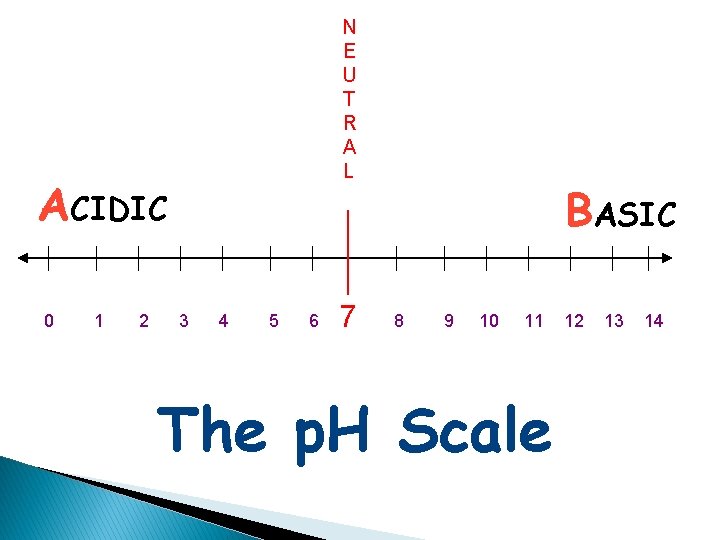
N E U T R A L ACIDIC 0 1 2 3 4 5 6 7 BASIC 8 9 10 11 The p. H Scale 12 13 14
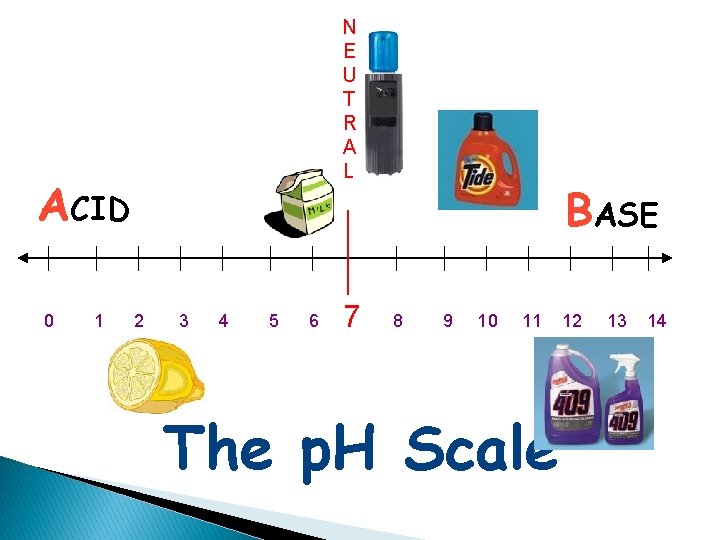
N E U T R A L ACID 0 1 2 3 4 5 6 7 BASE 8 9 10 11 The p. H Scale 12 13 14
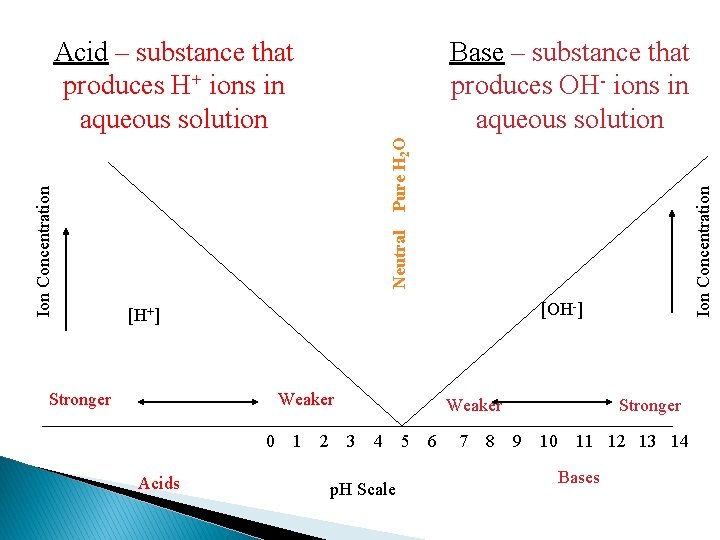
Ion Concentration Pure H 2 O Base – substance that produces OH- ions in aqueous solution Neutral Ion Concentration Acid – substance that produces H+ ions in aqueous solution [OH-] [H+] Stronger Weaker 0 Acids 1 2 Weaker 3 4 p. H Scale 5 6 7 8 Stronger 9 10 11 12 13 14 Bases

Question #1 �Lemon juice has a p. H of 2. 2. Is it an acid or a base? �Which does it contain, hydronium ions or hydroxide ions?

Question #2 �Detergents such as Tide have a p. H of about 10. Is Tide an acid or a base? �Which does it contain, hydronium ions or hydroxide ions?

Question #3 �Seawater has a p. H of 8. 2. Is seawater an acid or a base? �Which does it contain, hydronium ions or hydroxide ions?

Question #4 �The inside of your mouth has a p. H of 7. Is it an acid or a base?

Question #5 �How do you think we came up with the term “acid rain”?

Homework � Read pages 297 -300
- Slides: 32